Abstract
Purpose
To report the observation that the efficacy of topical glaucoma treatment improved after surgical correction of ectropion in a 71-year-old male with a known history of glaucoma.
Observations
The patient initially presented for tearing and lid malposition and was found to have bilateral elevated intraocular pressures (IOP) in addition to bilateral lower eyelid ectropion. IOP control was initially prioritized over ectropion repair, with IOP remaining elevated despite topical glaucoma treatment and selective laser trabeculoplasty. Sequential unilateral ectropion repair was then carried out, with topical glaucoma treatment resumed after the first repair. It was observed that the IOP improved with topical glaucoma treatment on each side after ectropion repair, despite no changes to medications nor dosing.
Conclusions and importance
The efficacy of topical glaucoma treatment is dependent on drop availability and absorption. While recent efforts to increase drop efficacy have been focused on engineering formulations that increase retention or corneal penetration, our case highlights that in selected glaucoma patients, correction of lid malposition may serve as an effective way to improve drop efficacy.
Keywords: Eyelid positioning, Ectropion, Topical drop efficacy, Glaucoma management
1. Introduction
The incidence of both glaucoma and eyelid malposition increases with age.1 Additionally, patients with glaucoma can develop lid abnormalities following glaucoma procedures or as a side-effect of topical ocular antihypertensives.2 Thus, it is common to see glaucoma and lid malposition occur in the same patient. However, the literature contains little information regarding how eyelid abnormalities may impact glaucoma management and drop absorption. Here we report a case where efficacy of topical glaucoma treatment improved after surgical correction of ectropion.
2. Case report
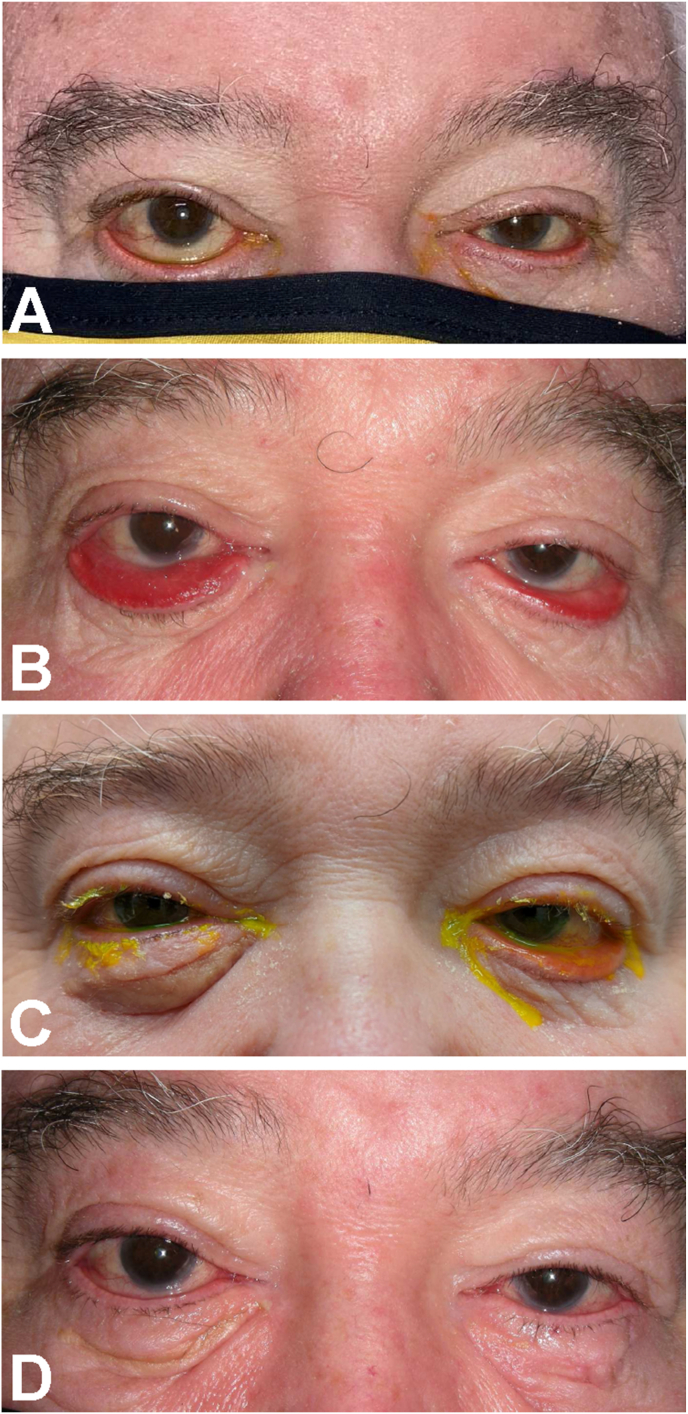
A 71-year-old man, with a known history of glaucoma, presented to the oculoplastic clinic for evaluation of tearing and lid malposition in the setting of recent Bell's palsy as well as CN III and VI palsy of unclear etiology (Fig. 1A). He was found to have bilateral lower eyelid cicatricial ectropion and elevated intraocular pressures (IOP) (32 mmHg OD, 26 mmHg OS). Ectropion repair was initially deferred given his elevated IOP and multiple cranial neuropathies. After neuro-ophthalmology evaluation and extensive unrevealing work up, a decision was made to observe him. Latanoprost was started for IOP control. Despite initial improvement to 20 mmHg OD and 22 mmHg OS, the patient noted that he felt the eye drops roll out of his eyes and there was concern for worsening ectropion. In an attempt for him to be drop-independent, he underwent selective laser trabeculoplasty (SLT) which reduced the IOP to 16 mmHg OU. During this time, his cicatricial ectropion continued to worsen due to a presumed combination of medication-induced toxicity, rosacea, and eye rubbing (Fig. 1B). Additionally, IOP began to increase post-SLT to 22–24 mmHg OU. Punctal occlusion or sustained eyelid closure was not attempted for better drop absorption pre-operatively given the extent of ectropion preventing drop retention in the inferior cul de sac. The decision was made to proceed with ectropion repair given worsening of the lid malposition despite improvement of other cranial neuropathies; per patient preference for unilateral surgery, the worse right side was repaired first via rotational sutures and a skin graft. Post-operatively, latanoprost was re-started bilaterally and pressures were noted to be 16 mmHg OD on the reopposed right side and 26 mmHg OS where ectropion remained. The patient reported feeling drops were better retained on his right side (Fig. 1C). He then underwent left sided cicatricial ectropion repair via a lateral tarsal strip approach with skin graft (Fig. 1D). Following this procedure, the IOP improved to 13 mmHg OD and 16 mmHg OS on latanoprost monotherapy.
Fig. 1.
Progression and surgical correction of the patient's ectropion. A. External photo demonstrating bilateral ectropion at presentation to our oculoplastic clinic. B. External photo demonstrating progression of the patient's ectropion and irritation. C. External photo after surgical repair of right ectropion. This photo demonstrates better retention of a fluorescein eye drop on the right compared to the left that has not yet undergone repair. D. External photo after repair of ectropion of both eyes.
3. Discussion and conclusions
This patient received the same topical glaucoma medication before and after ectropion repair, and significant IOP improvement in each eye was only seen after each side's ectropion repair. The ectropion repair was not accompanied by punctal occlusion, which has been shown to increase glaucoma topical drop efficacy3,4, or by temporary tarsorrhaphy, which theoretically could increase topical drop efficacy by increasing its retention. Thus, the increased glaucoma topical drop efficacy seen in this case is most likely due to surgical correction of ectropion. Two factors from the procedure could have contributed to the better pressure control: the eyelid surgery itself and/or the improved eyelid positioning after surgery. It has been reported that eyelid surgery alone has negligible effect on the IOP.5 Meanwhile, lower lid laxity has been shown to negatively correlate with response to eye drops in dry eye treatment.6 While exposure of the eye surface and poor natural tear film distribution inherent to patients with lower lid laxity likely contribute to baseline dry eye symptoms irrelevant of medical treatment, lower lid laxity likely also worsens the dry eye symptoms by impairing absorption of eye drops. In light of the above literature, our interpretation of the case is that correction of the eyelid malposition, and not the surgery itself, leads to increased drop efficacy.
Despite it being a seemingly intuitive process, the delivery of a drug molecule from an eye drop into the eye is a multi-step process and one with a low efficiency. The majority of an eye drop does not make it into the desired region inside the eye (which is the anterior chamber in our case) due to various anatomical and biochemical barriers.7,8 Upon touching the surface of the eye, the medication encounters dilution and protein-binding by tears, followed by loss of the medication via leakage through the nasolacrimal duct and/or spillage over eyelids. The remaining medication is retained in the inferior cul-de-sac. From there, drug molecules are captured by the upper eyelid as part of the tear film and delivered to the precorneal area,9, 10, 11 where a small percentage of drug molecules with the correct biochemical configuration finally enters the eye via the cornea following concentration gradient principles. Thus, maximum eye drop efficacy requires not only an optimized eye drop formulation, but also proper lid positioning and movement.
In ectropion, both lid positioning and lid movement are compromised. In this case, drop retention was poor and precorneal spread of the drops were inefficient prior to the ectropion repair. Following the repair, both problems were addressed, which likely underlies the better response to topical glaucoma therapy. While recent efforts to increase drop efficacy have been focused on engineering formulations that increase retention or corneal penetration and drug delivery options that bypass the ocular surface such as intracameral injections,12 we hope this case can encourage correction of lid malposition as another way to improve drop efficacy in selected glaucoma patients.
Patient consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editor of this journal.
Acknowledgments and disclosures
Funding
No funding or grant support
Conflicts of Interest
The authors have no financial disclosures.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
CRediT authorship contribution statement
Yu Xia: Writing – original draft, Data curation. Nathaniel A. Blecher: Writing – original draft, Data curation. Philip L. Custer: Writing – review & editing. Erin G. Sieck: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
References
- 1.Golan S., Rabina G., Kurtz S., Leibovitch I. The prevalence of glaucoma in patients undergoing surgery for eyelid entropion or ectropion. Clin Interv Aging. 2016;11:1429–1432. doi: 10.2147/CIA.S97694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan P., Malhotra R. Oculoplastic considerations in patients with glaucoma. Surv Ophthalmol. 2016;61(6):718–725. doi: 10.1016/j.survophthal.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Opitz D.L., Tung S., Jang U.S., Park J.J. Silicone punctal plugs as an adjunctive therapy for open-angle glaucoma and ocular hypertension. Clin Exp Optom. 2011;94(5):438–442. doi: 10.1111/j.1444-0938.2010.00587.x. [DOI] [PubMed] [Google Scholar]
- 4.Huang T.C., Lee D.A. Punctal occlusion and topical medications for glaucoma. Am J Ophthalmol. 1989;107(2):151–155. doi: 10.1016/0002-9394(89)90214-6. [DOI] [PubMed] [Google Scholar]
- 5.Sackel D.J., Palu R. The impact of eyelid surgery on intraocular pressure. Invest Ophthalmol Vis Sci. 2012;53(14):1035. 1035. [Google Scholar]
- 6.Oh S.H., Lyu B., Yim H.B., Lee N.Y. Lower lid laxity is negatively correlated with improvement of the ocular surface disease index in dry eye treatment. Curr Eye Res. 2016;41(2):165–170. doi: 10.3109/02713683.2015.1015142. [DOI] [PubMed] [Google Scholar]
- 7.Gaudana R., Ananthula H.K., Parenky A., Mitra A.K. Ocular drug delivery. AAPS J. 2010;12(3):348–360. doi: 10.1208/s12248-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel A., Cholkar K., Agrahari V., Mitra A.K. Ocular drug delivery systems: an overview. World J Pharmacol. 2013;2(2):47–64. doi: 10.5497/wjp.v2.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maurice D.M. Ophthalmic Drug Delivery. Springer; New York: 1987. Kinetics of topically applied ophthalmic drugs; pp. 19–26. [Google Scholar]
- 10.Ahmed I., Patton T.F. Disposition of timolol and inulin in the rabbit eye following corneal versus non-corneal absorption. Int J Pharm. 1987;38(1):9–21. [Google Scholar]
- 11.Agrahari V., Mandal A., Agrahari V., et al. A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res. 2016;6(6):735–754. doi: 10.1007/s13346-016-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medeiros F.A., Walters T.R., Kolko M., et al. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1) Ophthalmology. 2020;127(12):1627–1641. doi: 10.1016/j.ophtha.2020.06.018. [DOI] [PubMed] [Google Scholar]