Abstract
This study aimed to employed the effects of five thermal processing methods, namely steaming (SM), boiling (BO), frying (FY), roasting (RO), and vacuum sealing (SV), on the sensory, physicochemical properties, and microbial composition of grass carp meat during refrigerated storage, alongside unheated raw meat (RW) as control. The results showed that thermal treatment improved the sensory quality and shelf life of refrigerated grass carp meat, and their shelf life was RW < BO<SM < SV < RO < FY. The moisture content of fish meat decreased significantly with the extension of storage time, the color turned yellow and darkened significantly, and the hardness and chewiness also decreased significantly (p < 0.05). The TVB-N and TBARS suggested that thermal processing could delay the fish meat spoilage. The relative content of saturated fatty acids gradually increased with the extension of refrigeration time, while the relative content of polyunsaturated fats gradually decreased. The diversity and abundance of bacterial flora of grass carp meat from different thermal processing treatments gradually decreased during refrigerated storage, and the Pseudomonas, Acinetobacter, and Exiguobacterium gradually became the dominant microbe. This study provides theoretical basis for people's choice of daily cooking methods.
Keywords: Grass carp, Thermal processing, Refrigeration, Microbial metabolism, Correlation analysis
Highlights
-
•
Shelf life of refrigerated fish meat is greatly affected by heat treatment methods.
-
•
Thermal processing has different effects on fatty acid and colony succession.
-
•
Interaction of bacterial and biochemical drivers significantly affects spoilage.
-
•
Thermal processing slows down the rate of lipid oxidation and spoilage.
1. Introduction
Grass carp (Ctenopharyngodon idella) is one of the most important freshwater farmed fish in China and is widely used as a raw material for food processing due to its short growth cycle, high yield and delicious (Hussin et al., 2019). As delineated in the China Fishery Statistical Yearbook 2023, Chinese aquaculture production of grass carp reached 5.9 million tons in 2022, marking an increase of 150,000 tons compared to 2021 and ranked first in the world aquaculture fish species. Grass carp meat is very popular among consumers because it is rich in nutrients such as high-quality protein, polyunsaturated fatty acids, and vitamins, which has the ability to enhance human immunity and prevent heart disease and night blindness. However, fish meat is prone to quality degradation and microbial spoilage due to their high water content, abundant nutrients and neutral pH (Qin et al., 2020). The microbial metabolic activities significantly affect the degradation of nutrients, texture, and odor, as well as the production of TVB-N and TBARS, which lead to deterioration, oxidization, and spoilage of fish meat (Tan et al., 2023). An increasing number of studies have indicated a close relationship between fish meat quality and microorganisms, and spoilage microorganisms increased rapidly with storage time (Zhuang et al., 2023). Therefore, in order to improve the shelf-life of raw fish, it needs to be heat-treated to kill or inhibit the growth and multiplication of microorganisms.
Heat treatment is a common aquatic product processing method in daily life, including traditional thermal processing methods such as baking, boiling, steaming, deep-frying, and stir-frying, as well as novel thermal processing methods such as microwave heating, ohmic heating, radiofrequency heating and sous-vide cooking (Lian et al., 2022; Liu, Luo, et al., 2023). Various thermal treatments have different heat transfer modes, and this difference further resulting in distinct oxidation degree of meat products, while the oxidation process is also crucial for the formation of food texture and flavor (Cheng et al., 2023). The sous-vide cooking with water as a medium lead to changes in the physicochemical and sensory properties of beef, which further affects its textural and sensory characteristics, especially tenderness and palatability (Gil et al., 2022). Deep-frying and air-frying treatments utilize hot oil and air as heat transfer media, respectively, thereby altering the fatty acid composition of the meat and presenting a pleasing food texture and flavor (Zhou et al., 2022). In addition, heat treatment can reduce pathogenic microorganisms in meat products and increase the preferrance by consumers. Głuchowski et al. (2019) investigated the effect of sous-vide cooking on the quality and storage period of fish, and the results showed that compared with traditional heat treatment methods, sous-vide cooking can significantly extend the storage period of fish meat, maintain the original nutrition, and has a lower rate of cooking loss rate.
The changes in quality, physicochemical indexes and microbial composition of different heat-treated grass carp during refrigerated storage, and their correlation are still unclear. Therefore, the aims of this study were to: (i) investigate the changing pattern in quality of grass carp with different heat treatments during refrigeration; (ii) analyze the bacterial community structure succession of different heat-treated fish meat during storage by using high-throughput sequencing to clarify the key microorganisms and their metabolic activities; and (iii) elucidate the effects of heat treatments on the fatty acid composition of grass carp meat during refrigerated storage. Through the correlation analysis between biochemical changes and microbial colonization of grass carp meat, the effects of heat treatments on the quality of fish meat during refrigerated storage is revealed, and providing guidance for the processing and storage of fish meat.
2. Materials and methods
2.1. Sample preparation
10 fresh grass carp around 1.5 kg and 50.6 cm were purchased from an aquatic product market in Nanchang, China in October 2023. The grass carp were cut into fillets (3 × 2 × 1.5 cm3) after removing scales, viscera and head, and fillets were randomly divided into 6 groups. The fish samples were treated by five thermal processing treatments, namely steam (SM), boil (BO), fry (FY), roast (RO), and sous-vide (SV), the unheated raw meat (RW) was used as a control. In terms of the treatment condition of SM, fillets were steamed in a steamer for 10 min, and cooled, water on the surface was removed with filter paper. For BO, fillets were boiled in boiling water for 10 min and cooled down, water on the surface was removed with filter paper. For FY, fillets were deep-fried in hot oil (edible vegetable blending oil) at about 160 °C for 10 min, and cooled down, the oil on the surface of fillets was removed. For RO, fillets were put in an oven and the temperature was set at 200 °C, was baked for 10 min, after cooling, the grease and water on the surface of the fish fillets were removed. For SV, fish fillets were packed into vacuum packaging bags and then in a water bath at 60 °C and heated for 1 h, and then cooled quickly with cold water at the end of the process, following by the removement of water on the surface.
SV group samples were transferred from vacuum bags to disposable lunch boxes, while the rest of the prepared samples were directly packed in disposable lunch boxes, refrigerated at 4 °C and sampled randomly at different storage times. Due to the different spoilage rates of fish meat treated by different heat methods, the spoilage process of fish meat was divided into prophase (P), medium term (M) and lastphase (L) in the study. The sampling time of RW was after 2, 4 and 7 days of refrigeration, the sampling time of SM, BO, RO, and SV was after 4, 7 and 10 days of refrigeration, and the sampling time of FY was after 7, 10 and 15 days.
2.2. Sensory evaluation
The method of sensory evaluation refers to Zhu et al. (2023), with slight modification. The evaluation team consisted of 6 men and 6 women, aged between 20 and 30 years. All samples were shuffled and renumbered before the evaluation in order to avoid the possible influence. During the evaluation process, the team members judged the freshness and edible quality of grass carp based on the criteria of color, flavor, springiness, tissue morphology and overall acceptability. The score range of each evaluation index is 10 points, of which 0–2.9 points represent complete spoilage, 3–4.9 points represent spoiled quality, 5–6.9 points are considered acceptable quality, 7–8.9 points are considered fresh, and 9–10 points mean exceptional freshness.
2.3. Determination of moisture
The moisture content was determined according to the direct drying method in GB 5009.3–2016. Briefly, 0.5 g of fish sample was taken for the moisture determination using a moisture content tester (Guanya Technology & Science Co., Ltd.).
2.4. Determination of total volatile basic‑nitrogen (TVB-N)
The TVB-N content was extracted and measured as described by the Volatile Salt-Based Nitrogen in Foods of National Standard for Food Safety in GB 5009.228–2016, with slight modification. Briefly, 10 g of fish meat was weighed in a digestion tube, 50 mL of distilled water and 1.0 g of magnesium oxide were then added, the TVB-N content in samples was measured using a Kjeldahl Apparatus (SKD-800, Shanghai Peio Analytical Instruments Co., Ltd., China). The absorption indicator is boric acid solution, bromocresol green and methyl red, after 4 min of distillation, the mixtures were then titrated with 0.05 mol/L HCl standard titration solution. Distilled water (50 mL) was used as blank control. The formula for the calculation of TVB-N content was as follows:
where V1 and V2 indicates the volume of HCl standard titration solution consumed by the sample and blank groups, respectively (mL); c indicates the concentration of HCl standard titration solution (mol/L); m indicates the mass of sample (g); 14 indicates that 1.0 mL of hydrochloric acid standard titration solution equivalent to the mass of nitrogen in grams per mole (g/mol); and 100 is the unit conversion factor.
2.5. Determination of thiobarbituric acid reactive substances (TBARS)
The TBARS value was determined by the method of Ali Ghoflgar Ghasemi et al. (2024), with slight modification. Briefly, 5.0 g of fish meat was weighed, and mixed with 25 mL of 20% trichloroacetic acid solution and 20 mL of distilled water. Then the supernatant was taken after centrifugation (7500g, 10 min) at room temperature for 30 min, and the supernatant was then diluted with distilled water to 50 mL. Finally, 5.0 mL of the above solution was taken and reacted with 2.0 mL of 0.02 mol/L thiobarbituric acid solution in a boiling water bath for 40 min. The absorbance values were measured using a U-2910UV-Visphotometer (Hitachi Co., Ltd., Japan) at 532 and 600 nm, respectively, the results were expressed as mg MDA/kg of sample. The TBARS value was calculated by the following formula:
where TBARS indicates the content of malondialdehyde (MDA) in the sample; A532 is the absorbance of sample at 532 nm; A600 is the absorbance of sample at 600 nm; M is the relative molecular mass of malondialdehyde (72.06); R is the millimolar absorbance coefficient (155).
2.6. Color and texture profile analysis (TPA)
The color was determined according to the method of Yi et al. (2018), with slight modification. Prior to sample determination, the colourimeter undergoes whiteboard calibration, blackboard zeroing. The color of Fish meat coordinates (L*, a*, and b* values) were measured using a Hunterlab Agera colorimeter (Hunter Associates Laboratory Inc., Reston, VA, USA).
Texture profile was measured as described by Ye et al. (2020), with slight modification. A texture analyzer TA-XT Plus (Stable Micro Systems Ltd., Godalming, UK) was used for the determination. The program of the texture instrument was set to TPA mode, probe model: P/36R; trigger force: 5.0 g; displacement: 15.0 mm; pre-test speed: 3.0 mm/s; test speed: 1.0 mm/s; post-test speed: 10.0 mm/s; the time interval between two compressions was 5.0 s, and 50% compression was performed. The indexes of hardness, elasticity, adhesion, cohesion and chewability were selected for data analysis.
2.7. Determination of fatty acid composition
Lipid extraction was performed according to the method of Cao et al. (2019) and Liu, Piao, et al. (2023) with slight modifications. Briefly, 25.0 g fish sample was added into 200 mL of chloroform-methanol-water mixture (V:V:V, 1:2:1), homogenized at 8000 g for 3 min at 4 °C in an ice-water bath, followed by homogenization for 30 s with 50 mL of chloroform. Then, distilled water (50 mL) was added to homogenize for 30 s prior to centrifugation at 7800 rpm, 4 °C for 10 min. The supernatant was separated by a separatory funnel, and the lower chloroform layer was transferred to a conical flask, 5.0 g of anhydrous sodium sulfate was added and filtered with neutral filter paper. The filtrate was concentrated by vacuum rotary evaporation to obtain crude lipid.
The methyl esterification of sample was carried out based on the method of Lian et al. (2023) with slight modification. Briefly, 30.00 mg lipid sample was reacted with 2.0 mL of n-hexane and 200 μL of 4% NaOH-methanol solution at 37 °C for methyl esterification reaction for 35 min. After centrifugation, the n-hexane layer (1.0 mL) was taken and filtered through a 0.22 μm organic membrane for gas chromatography analysis.
The fatty acids were separated on a CP-Si188 fused silica capillary column (100 m × 0.25 mm × 0.2 μm) with an inlet temperature of 250 °C, pressure of 24.52 psi, and total gas flow rate of 29.4 mL/min. The injection volume was 1.0 μL. Hydrogen (99.99%) was used as the carrier gas. The programmed temperature was as follows: 45 °C, 4–43 min; increased to 175 °C in 27 min at a speed of 13 °C/min; then increased to 215 °C in 35 min at a speed of 5 °C/min. The temperature for FID detector was 250 °C, the flow rate of hydrogen and air was 30 mL/min and 300 mL/min, respectively. Nitrogen (99.99%) was used as the combustion gas at a flow rate of 30 mL/min.
2.8. Microbial diversity analysis
Three pieces of fish meat were randomly taken each time and entrusted to Shanghai Meiji Bio-pharmaceutical Technology Co., Ltd. for 16S rRNA high-throughput sequencing. The total DNA was extracted from the fish sample and detected by 1% agarose gel electrophoresis. According to the designated sequencing region, specific primers with barcode for PCR amplification were synthesized, which was followed by replication in triplicate for each sample. The PCR products of the same sample were mixed and detected by 2% agarose gel electrophoresis, and the gels were cutted to recover the PCR products by using the AxyPrep DNA gel recovery kit. The primers used for sequencing were universal primers for the V3-V4 region of 16S rRNA gene: 338F 5’-ACTCCTACGGGGAGGCAGCAG-3′ and 806R 5’-GCACTACHVGGGTWTCTAAT-3′. The NEXTflex™ Rapid DNA-Seq Kit (Bioo Scientific, USA) was used to build the database, and the gene sequencing was done on a Miseq PE300/NovaSeq PE250 platform (Illumina, USA). The sequencing results were analyzed using the Bioinformatics Cloud Platform of company (https://cloud.majorbio.com/).
2.9. Statistical analysis
The results were expressed as mean ± standard deviation, the experimental data were plotted using Origin 2021 and the genes cloud platform (https://www.genescloud.cn). The one-way analysis of variance (ANOVA) and significant difference were performed with Duncan's test using SPSS software version 21.0, p < 0.05 indicates significant difference.
3. Results and discussion
3.1. Sensory evaluation
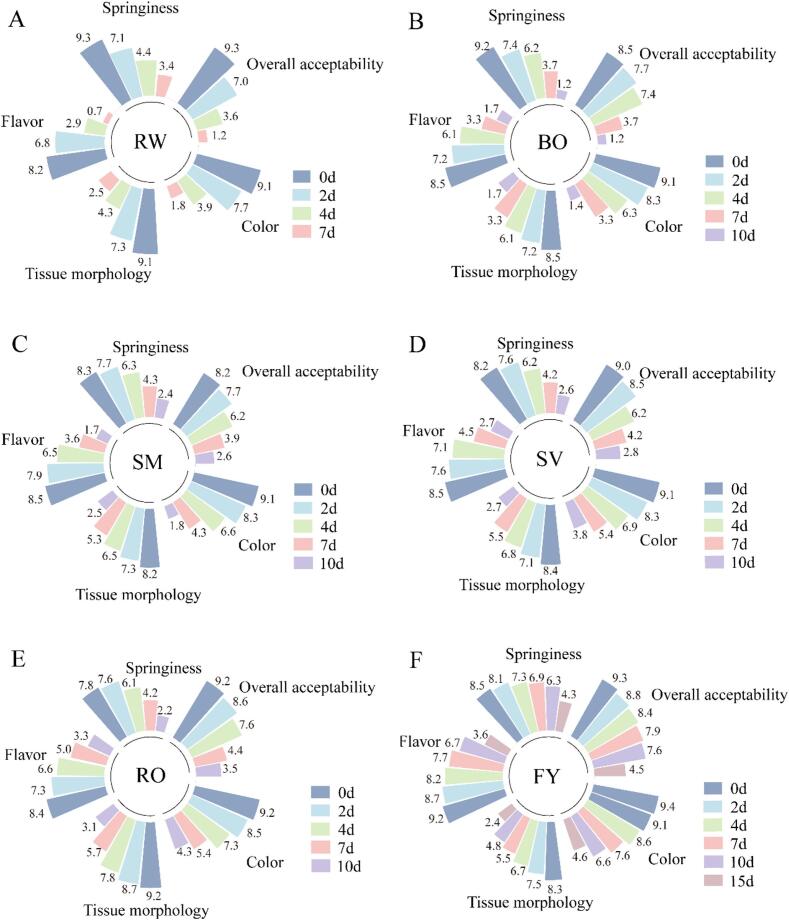
Sensory evaluation is a key indicator in determining the freshness and quality of grass carp, which directly affects consumer acceptance. As shown in Fig. 1, the color, flavor, tissue morphology, springiness, and overall acceptability of all sample groups gradually decreased with the extension of refrigeration time, and with the most significant changes observed in the RW group (p < 0.05). Based on the sensory evaluation, the RW group showed noticeable signs of spoilage (<5 points) at 4 days (Fig. 1A). In contrast, the FY group only exhibited signs of spoilage on the 15th day, with an overall acceptability score of 4.46 (Fig. 1F). This might be due to the fact that the frying process leads to the evaporation of a large amount of water, which inhibits the growth and reproduction of microorganisms. The activity of spoilage bacteria serves as a pivotal factor in the degradation of grass carp quality (Liang et al., 2024). However, the shelf-life (unacceptable quality) of grass carp meat in SM, BO, RO and SV groups was >4 days and <7 days, and their shelf life was BO<SM < SV < RO (Fig. 1B-E).
Fig. 1.
Sensory evaluation of grass carp meat treated by different thermal processing methods during refrigerated storage.
3.2. Changes in physicochemical properties
3.2.1. Moisture
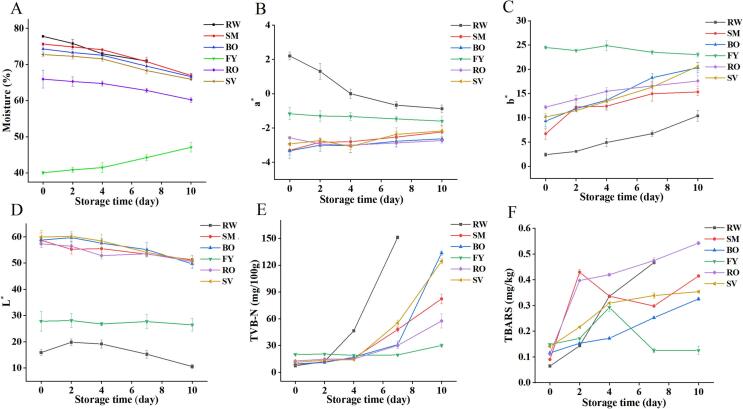
Fish spoilage is often accompanied by water loss, which in turn can promote the growth of spoilage microorganisms (Tan et al., 2022). Studies have shown that the degradation of fish proteins induced by spoilage bacteria, which leads to the reduction of the water holding capacity of myofibrillar proteins, is the main reason for the promotion of muscle water loss (Bahrami Feridoni & Khademi Shurmasti, 2020). Changes in the moisture of grass carp meat with different thermal processing treatments during refrigeration were shown in Fig. 2A. The moisture content of RW, SM, BO, RO and SV were higher than that of FY after heat treatment, the results were consistent with the results of Oppong et al. (2021). This is attributed to the high temperature of food during frying caused changes in the structure of fish meat, and the evaporation of the water on fish filles, which resulted in lower moisture content in the fried fish fillets compared to the other heat methods. In addition, the moisture content of FY gradually increased as the refrigeration process progressed, and opposite trend was observed for fish meat from other heat treatments. These may be due to the fact that the moisture on the outside of fried fish fillets was very low, and as the refrigeration proceeded, the fish fillets absorbed the moisture from the air slowly, resulting in a higher moisture content of the fish meat. The moisture content of RW, SM, BO, RO, and SV were all gradually reduced, among which RW loses its water the fastest. This might be that the surface of raw meat contains a large number of spoilage bacteria, and the fish meat itself contains certain endogenous proteases, with the prolongation of refrigeration time, microorganisms and endogenous proteases begin to destroy and decompose muscle proteins (Bao et al., 2020). And the water bound to proteins was converted into free water and slowly exudes, resulting in a gradual loss of water. The loss of water from SM, BO, RO and SV samples during 0–4 days of refrigeration was slower, which was due to the fact that fish meats have been heat treated before refrigeration. Thermal processing killed most of the microorganisms and caused the fish meat to spoilage at a slower rate than RW. However, on the 4th to 7th day, the water loss of fish meat accelerated, indicating that with the extension of storage time, microorganisms on the surface of SM, BO, RO and SV fish fillets multiplied in large numbers, causing the increase of fish meat spoilage rate to a certain extent.
Fig. 2.
Changes in the physicochemical properties of grass carp meat treated by different thermal processing methods during refrigerated storage. (A) moisture, (B) a* value, (C) b* value, (D) L* value, (E) TVB-N content, (F) TBARS content.
3.2.2. Color
The changes in a* value (redness) of grass carp meat with different heat treatments during refrigeration were shown in Fig. 2B. Compared with RW, the a* values of SM, BO, FY, RO and SV changed less, and the redness of RW gradually decreased. The study of Sáez et al. (2021) pointed out that this was due to the destroy of hemoglobin during refrigeration, and Fe2+ was oxidized to Fe3+, and thereby led the fish meat to brown. The changes in b* value (yellowness) of fish meat during refrigeration were shown in Fig. 2C. The yellowness of FY changed less during refrigeration and showed only a slight decrease, while RW, SM, BO, RO, and SV increased during refrigeration. However, there was slight increase in TVB-N content in the fish meat of SM, BO, RO, and SV at 0–4 d and RW at 0–2 day of storage. Therefore, the increase of yellowness during the pre-refrigeration period could be caused by protein and lipid oxidation. The changes in L* value (brightness) of fish meat were shown in Fig. 2D. The brightness of SM, BO, RO and SV gradually decreased during refrigeration, which may be caused by protein and lipid oxidation, and microbial growth in fish meat. The L* value of FY changed less during refrigeration due to its slower spoilage rate and its own lower brightness. The brightness of RW first increased and then decreased during refrigeration. This due to the brightness of raw meat was low before refrigeration, and the surface began to become bright with the exudation of moisture after refrigeration. On the other hand, this exuded moisture promoted the growth of microorganisms on the surface of the fish fillets, and with the action of microorganisms and the oxidation of proteins and lipids, the brightness of the fish meat gradually decreased and the color became darker. In summary, FY showed the least change in color during refrigeration, RW showed the most obvious color change, SM, BO, RO and SV showed less change in redness, but the color of fish meat was obviously darker and yellower.
3.2.3. Texture profile
During the refrigeration process, the loss of water, protein degradation, and microbial action can lead to changes in the textural properties and affect the taste of fish meat. In this study, hardness, elasticity, cohesion, adhesion, and chewiness of fish meat were determined. As shown in Table 1, the hardness of RW decreased significantly during refrigerated storage, this could be attributed to the action of endogenous enzymes and microorganisms in the raw meat, which destroyed the protein structure. The hardness of FY gradually decreased due to the increase in moisture content of fish meat. And the hardness of RO and BO decreased gradually in the later stages of storage, but the hardness of SM increased. The elasticity of FY increased after 10 days of refrigeration, but there was no significant difference (p < 0.05). Similar to the trend of hardness, RW showed a decrease in elasticity, cohesion, and chewability after 4 days of refrigeration, but adhesion increased. In addition to FY, the viscosity of SM, BO, RO, and SV also increased after refrigeration, which may be attributed to the secretion of extracellular enzymes and polysaccharides by microorganisms to form a mucus layer during fish spoilage, and the simultaneous binding of spoiled muscle tissues to biofilms produced by bacteria, resulting in increased viscosity (Katiyo et al., 2020). Overall, the texture of FY was relatively stable during refrigeration, while the texture of RW was the most unstable.
Table 1.
Texture of grass carp meat prepared by different thermal processing treatments during refrigerated storage.
| Cooking methods | Time (d) | Hardness | Adhesiveness | Springiness | Cohesiveness | Chewiness |
|---|---|---|---|---|---|---|
| RW | 0 | 7129 ± 232a | −21.2 ± 4.0a | 0.62 ± 0.05a | 0.47 ± 0.03a | 2067 ± 156a |
| 2 | 4402 ± 224b | −45.9 ± 14.9a | 0.50 ± 0.03b | 0.32 ± 0.05b | 707 ± 57b | |
| 4 | 2502 ± 317c | −172.1 ± 57.8b | 0.47 ± 0.03b | 0.35 ± 0.03b | 417 ± 37c | |
| SM | 0 | 1091 ± 79d | −4.0 ± 3.3a | 0.48 ± 0.03b | 0.33 ± 0.07a | 170 ± 37b |
| 2 | 1427 ± 134d | −0.4 ± 0.4a | 0.76 ± 0.14a | 0.43 ± 0.08a | 474 ± 172ab | |
| 4 | 1852 ± 98c | −1.3 ± 0.8a | 0.62 ± 0.06ab | 0.41 ± 0.04a | 475 ± 86ab | |
| 7 | 2285 ± 131b | −154.8 ± 85.1b | 0.64 ± 0.17ab | 0.37 ± 0.05a | 559 ± 250a | |
| 10 | 2757 ± 458a | −111.3 ± 14.6b | 0.67 ± 0.12ab | 0.43 ± 0.03a | 796 ± 259a | |
| BO | 0 | 1499 ± 238bc | −0.4 ± 0.2a | 0.70 ± 0.18a | 0.40 ± 0.04a | 416 ± 138a |
| 2 | 1466 ± 197bc | −0.8 ± 0.7a | 0.67 ± 0.03a | 0.31 ± 0.09a | 299 ± 94ab | |
| 4 | 2017 ± 234a | −0.2 ± 0.3a | 0.72 ± 0.08a | 0.28 ± 0.08a | 404 ± 114a | |
| 7 | 1801 ± 215ab | −5.4 ± 6.4a | 0.64 ± 0.02ab | 0.36 ± 0.04a | 421 ± 75a | |
| 10 | 1248 ± 349c | −189.6 ± 53.3b | 0.42 ± 0.17b | 0.29 ± 0.06a | 152 ± 75.1b | |
| FY | 0 | 3316 ± 258a | −0.6 ± 0.1a | 0.78 ± 0.07a | 0.46 ± 0.06a | 1180 ± 122a |
| 2 | 2931 ± 252ab | −0.0 ± 0.0a | 0.74 ± 0.13a | 0.31 ± 0.01b | 678 ± 119a | |
| 4 | 2515 ± 338bc | −0.5 ± 0.8a | 0.81 ± 0.02a | 0.32 ± 0.03b | 655 ± 134a | |
| 7 | 2104 ± 332c | −0.4 ± 0.6a | 0.83 ± 0.05a | 0.46 ± 0.09a | 806 ± 252a | |
| 10 | 2285 ± 62c | −0.5 ± 0.4a | 0.85 ± 0.08a | 0.48 ± 0.02a | 930 ± 88a | |
| RO | 0 | 1341 ± 63b | −1.9 ± 0.9a | 0.58 ± 0.04a | 0.34 ± 0.06a | 269 ± 59c |
| 2 | 2557 ± 208a | 0.0 ± 0.0a | 0.61 ± 0.18a | 0.21 ± 0.04b | 352 ± 203bc | |
| 4 | 2853 ± 46a | −0.3 ± 0.2a | 0.73 ± 0.12a | 0.41 ± 0.07a | 865 ± 284a | |
| 7 | 2401 ± 216a | −0.6 ± 0.6a | 0.79 ± 0.03a | 0.41 ± 0.06a | 773 ± 119a | |
| 10 | 2420 ± 613a | −87.2 ± 25.6b | 0.74 ± 0.05a | 0.36 ± 0.02a | 663 ± 201ab | |
| SV | 0 | 750 ± 57c | −23.0 ± 18.2a | 0.57 ± 0.07 | 0.37 ± 0.02a | 158 ± 31b |
| 2 | 1136 ± 171ab | −1.5 ± 1.1a | 0.50 ± 0.05a | 0.26 ± 0.01c | 148 ± 42b | |
| 4 | 1095 ± 161b | −2.4 ± 2.6a | 0.52 ± 0.04a | 0.30 ± 0.02bc | 171 ± 40ab | |
| 7 | 1408 ± 237a | −119.5 ± 38.5b | 0.57 ± 0.11a | 0.32 ± 0.05b | 260 ± 86a | |
| 10 | 1405 ± 128a | −138.3 ± 86.0b | 0.31 ± 0.03b | 0.29 ± 0.01bc | 124 ± 18b |
Note: Different lowercase letters indicated significant differences at P < 0.05 between days within groups.
3.2.4. TVB-N and TBARS values
TVB-N is mainly ammonia and amines produced through the degradation of animal proteins by endogenous enzymes and spoilage bacteria, and it is widely recognized as an important indicator of meat spoilage (Cai et al., 2011). As shown in Fig. 2E, the TVB-N content of all treatment groups gradually increased with storage time during refrigeration. Among them, the most significant increase was observed in RW, where the content rose to 46.7 mg/100 g at the 4th day. This due to that RW was not treated with high temperature, the autolytic enzyme activity was high, which acted together with spoilage bacteria, resulting in a significant increase in TVB-N content (Yu et al., 2017). However, the TVB-N content of BO, RO, SV, and SM increased slowly and below 30 mg/100 g from 0 to 4 days, but increased to 30.99, 30.15, 55.16 and 47.97 mg/100 g, respectively, on the 7th day, which were above the limit values. This might be due to the loss of autolysin activity after high temperature treatment. It well-known that high temperature can kill some microorganisms that are not resistant to high temperature, while the microorganisms that have not been killed gradually begin to multiply with the extension of time thus leading to the spoilage of fish meat and the increase of TVB-N content (Zhang et al., 2021). In addition, the content of FY changed little during refrigeration, with only a slight increase on the 7th to 10th day. This may be related to its high fat content and low moisture content, which is not favorable for microbial growth. In the later stages of refrigeration, microorganisms began to multiply slowly as the moisture increased. It can be seen that the spoilage rate of fish meat in all groups was RW > BO>SV > SM > RO > FY.
Food oxidation of lipids occurs during refrigeration and may promote deterioration of food flavor, meat spoilage, and nutrient loss (Zhang et al., 2021). In contrast, malondialdehyde (MDA) is a peroxide product produced by the oxidative decomposition of unsaturated fatty acids in animal lipids. Therefore, the TBARS value can be determined by measuring the content of MDA as a chemical indicator of lipid oxidation. The changes in TBARS values of grass carp meat prepared by different heat treatments during the refrigeration process were shown in Fig. 2F. The TBA values of RW, BO, RO, and SV all increased with the extension of storage time, indicating that the lipids in the fish meat were continuously oxidized during refrigeration. During the first 2 days of refrigeration, the TBARS values of grass carp meat in heat-treated groups increased more rapidly than those of RW, which was related to the fact that heat treatment of fish meat promoted lipid oxidation to produce aroma compounds. The opposite was true after 2 days, which might be attributed to the release of oxidative enzymes and pro-oxidants from various ruptured organelles that accelerated the oxidation of lipids in fish meat (Calanche et al., 2019). However, the TBARS values of grass carp meat from SM and FY groups showed a tendency to increase and then decrease, which may be attributed to the reaction between MDA and other reactive lipid carbonyl compounds, resulting in a decrease in MDA content, and consequently a decrease in the TBARS values (Ventanas et al., 2007). In this study, grass carp meat from RW had higher oxidative rancidity than that of heat-treated groups, and the degree of lipid oxidation of fish meat from heat treatment group during refrigeration was RO > SM > SV > BO>FY.
3.3. Changes in fatty acid composition
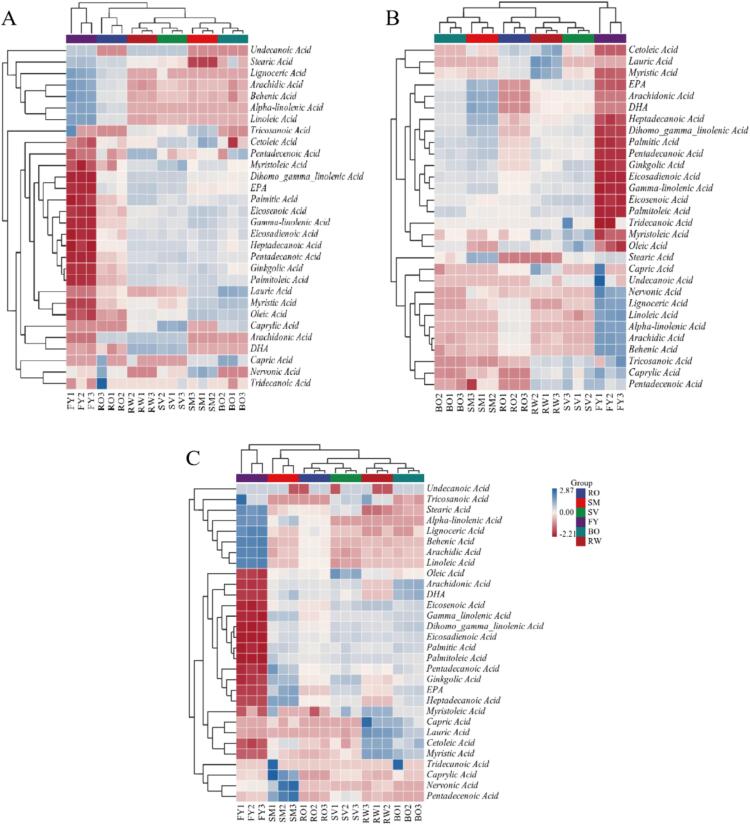
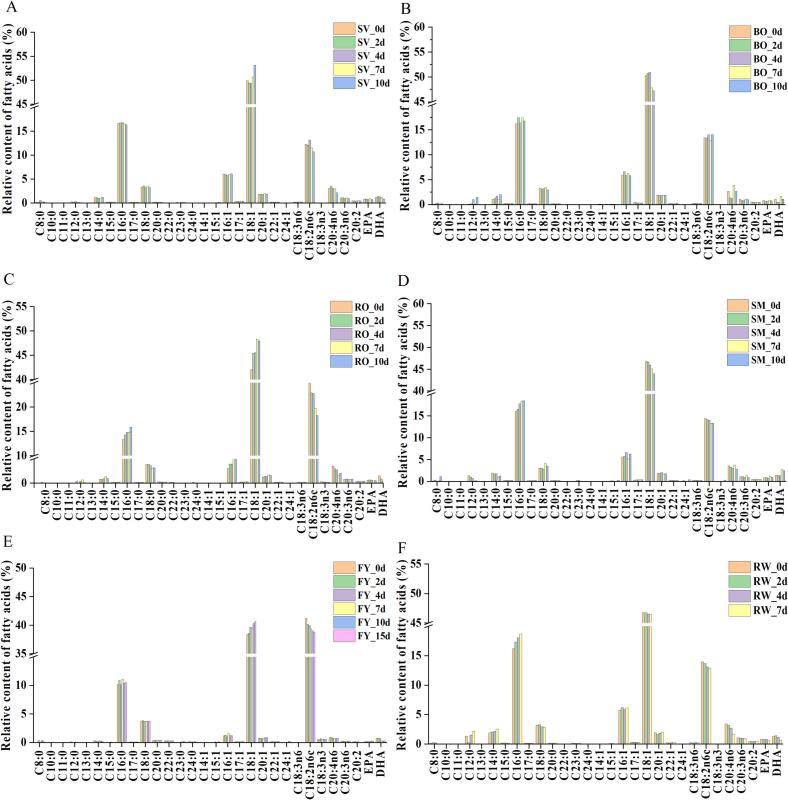
Fatty acids are important determinants of meat quality during storage. Different thermal processing treatments had significant effects on the type and content of fatty acid composition of grass carp meat during refrigerated storage (Fig. 3). The fatty acid composition of the RW grass carp meat was significantly different from that in the FY and RO groups, while there was little difference with the fatty acid composition of the other thermal processing methods, suggesting that frying and baking not only changed the fatty acid composition of fish meat, but also influenced the fatty acid changes during storage. As shown in Fig. 4, a total of 30 fatty acids were detected in the different heat treatment groups, among which palmitic acid (C16:0), oleic acid (C18:1), and linoleic acid (C18:2) were the most abundant, this consistent with the findings of Sağglık et al. (2003) and Özogul et al. (2007). The relative content of palmitic acid showed an increasing trend in RW, RO, and SM fish meat during the refrigeration, while the trend was not significant in the other groups (p > 0.05). And the relative content of oleic acid gradually increased in RO and FY, and decreased in the SM and RW groups. In all thermal processed fish meat, the relative content of linoleic acid (C18:2) showed a decreasing trend.
Fig. 3.
Heat map of fatty acid composition in P (A), M (B) and L (C) phase of grass carp meat treated by different thermal processing methods during refrigerated storage.
Fig. 4.
Fatty acid composition of grass carp meat treated by different thermal processing methods during refrigerated storage. The A, B, C, D, and E represent steam (SM), boil (BO), fry (FY), roast (RO), and sous-vide (SV) samples, respectively.
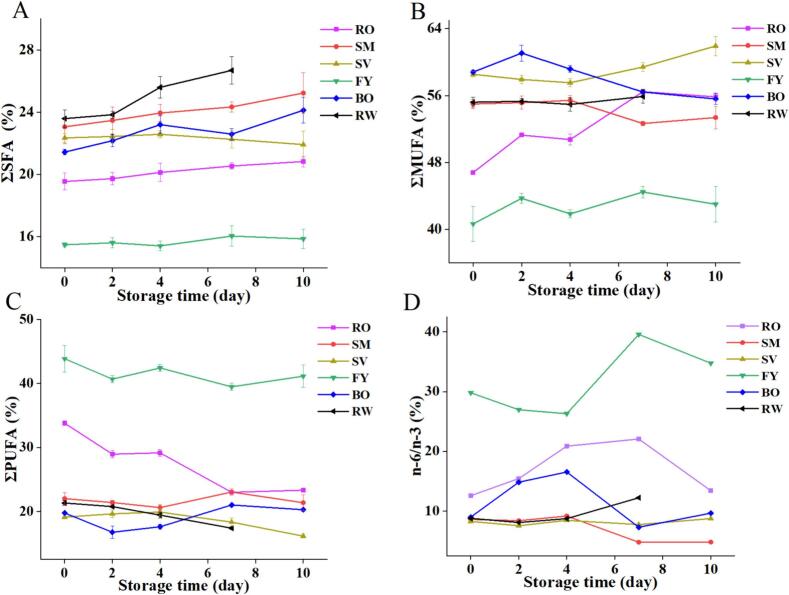
The unsaturated fatty acid content of fish meat with different heat methods accounted for about 75%–85% of the total fatty acid content, which was much higher than that of pork, lamb and chicken. However, the unsaturated fatty acids were susceptible to oxidation during storage, which led to increased food spoilage. As shown in Fig. 5A and C, the relative content of ΣSFA showed an increasing trend, while the relative content of ΣPUFA showed a decreasing trend, especially in the RO and RW groups. This may be due to the greater oxidation rate of unsaturated fatty acids than saturated fatty acids, thus changing the fatty acid composition. Among all treatment groups, the relative content of ΣSFA and ΣMUFA were the lowest in the FY group, while the relative content of ΣPUFA was the highest (Fig. 5A, B, C). Sabetian et al. (2014) obtained similar findings in refrigerated rainbow trout fillets at −18 °C. This change could be attributed to the conversion of ΣPUFA to ΣSFA after oxidation, leading to double bond breakage, thus altering the fatty acid composition. The n-6/n-3 in all groups showed a trend of first increasing and then decreasing, as shown in Fig. 5D, which is consistent with the results of Kim et al. (2020). Except for the FY and RO groups, the n-6/n-3 was lower than the recommended value of the so-called “typical Western diet” (20:1) during the whole storage process (Husted & Bouzinova, 2016), but higher than that of the nutritional perspective (4:1) (Buckland et al., 2022).
Fig. 5.
The fatty acid relative content of grass carp meat treated by different thermal processing methods during refrigerated storage.
3.4. Changes in bacterial community during refrigeration of grass carp meat
Microbial spoilage is the main reason for the quality degradation of fish meat during storage. Therefore, it is necessary to characterize the flora in different heat-treated fish meat to investigate the bacterial flora composition and bacterial community succession during refrigeration of grass carp meat treated with different heat treatments.
3.4.1. Alpha diversity analysis
The sequences and corresponding operational taxonomic units (OTUs) were obtained by random sampling sequencing. The coverages of all treatment groups exceeded 0.998, indicating that the sequencing data were reliable (Table 2). Chao and ACE represent bacterial abundance, the Shannon and Simpson indices represent bacterial diversity. Compared with the pre-refrigeration period, the Shannon, Chao and ACE indices of the flora in all fish meat decreased and the Simpson index increased in the late refrigeration period, indicating that the richness and diversity of fish meat flora decreased during refrigeration, and some bacteria gradually adapted to the environment to became the dominant bacteria in the later period. Pan et al. (2021) also found that the changes of bacterial communities in tea polyphenol-treated crispy grass carp under the refrigerated conditions were similar to those in the later period, the diversity of bacterial flora gradually decreased. In the pre-refrigeration period, the flora diversity of RWP, SMP, BOP, ROP, and SVP were all much higher than that of FYP. In the medium-term refrigeration, the flora diversity of FY had a small increase, but in the later period, the diversity decreased dramatically, and the same trend was observed in BO. The flora diversity of fish meat was basically unchanged in the middle period, while it had a large decrease in the pre-refrigeration period. The bacterial diversity of RW, SM, and SV decreased significantly in the middle period and remained basically reduced in the later period, while RO showed a significant decrease in diversity in both middle and later periods. These results suggested that RO, BO, RW, SM, and SV treatments changed the species of the dominant flora in the fish meat and the time period in which they appeared.
Table 2.
Alpha diversity index of microflora in different heat-treated fish samples during refrigerated storage.
| Samples | Sobs | Shannon | Simpson | ACE | Chao | |
|---|---|---|---|---|---|---|
| P | RWP | 198 ± 48cd | 2.58 ± 0.21b | 0.154 ± 0.016ab | 273 ± 90bc | 273 ± 66b |
| SMP | 311 ± 53abc | 2.56 ± 0.36b | 0.162 ± 0.054ab | 353 ± 75ab | 358 ± 74ab | |
| BOP | 272 ± 137bc | 2.10 ± 0.46bc | 0.278 ± 0.131abcd | 313 ± 147ab | 311 ± 142ab | |
| FYP | 72 ± 32de | 0.99 ± 0.28defg | 0.563 ± 0.075def | 139 ± 39cde | 96 ± 12d | |
| ROP | 415 ± 90a | 4.23 ± 0.48a | 0.057 ± 0.050a | 428 ± 90a | 430 ± 90a | |
| SVP | 339 ± 54ab | 3.93 ± 0.20a | 0.046 ± 0.006a | 352 ± 57ab | 356 ± 59ab | |
| M | RWM | 75 ± 7de | 2.07 ± 0.14bc | 0.223 ± 0.054abc | 89 ± 13e | 87 ± 12d |
| SMM | 17 ± 7e | 1.05 ± 0.37def | 0.471 ± 0.183bcdef | 100 ± 121de | 29 ± 18d | |
| BOM | 266 ± 137bc | 1.61 ± 1.09cde | 0.462 ± 0.360bcdef | 386 ± 40ab | 341 ± 107ab | |
| FYM | 85 ± 54de | 1.09 ± 0.18def | 0.500 ± 0.019bcdef | 125 ± 57cde | 111 ± 62cd | |
| ROM | 196 ± 130cd | 0.71 ± 0.54fg | 0.775 ± 0.172fg | 239 ± 131bcd | 234 ± 131bc | |
| SVM | 29 ± 2e | 0.56 ± 0.21fg | 0.741 ± 0.144fg | 76 ± 32e | 87 ± 49d | |
| L | RWL | 61 ± 4e | 1.80 ± 0.10bcd | 0.253 ± 0.032abcd | 80 ± 8e | 74 ± 9d |
| SML | 14 ± 4e | 0.58 ± 0.09fg | 0.679 ± 0.057efg | 17 ± 8e | 15 ± 5d | |
| BOL | 37 ± 8e | 1.63 ± 0.71cde | 0.367 ± 0.281abcde | 46 ± 124e | 52 ± 20d | |
| FYL | 19 ± 8e | 0.54 ± 0.37fg | 0.741 ± 0.212fg | 22 ± 11e | 20 ± 9d | |
| ROL | 27 ± 12e | 0.81 ± 0.53efg | 0.576 ± 0.293def | 124 ± 87de | 48 ± 26d | |
| SVL | 18 ± 2e | 0.11 ± 0.02g | 0.965 ± 0.010g | 27 ± 15e | 22 ± 6d | |
Note:Different lower case letters represent significance differences at p < 0.05 between five thermal processing treatments in P, M and L periods.
3.4.2. Analysis of microbial composition
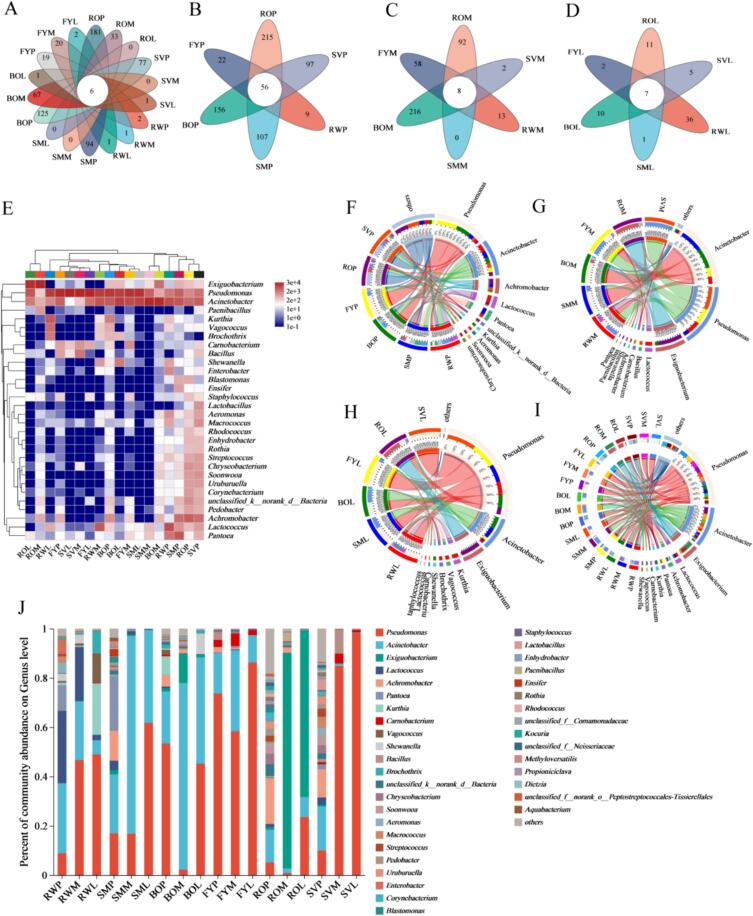
Further analysis of the flora composition in fish meat treated with different thermal processing methods were presented in Fig. 6A. The venn diagrams showed that six genera were considered as the core genera throughout the refrigerated storage. And there were 56, 8, and 7 core genera in different treatments in pre-, middle, and later refrigeration, respectively (Fig. 6B, C, and D). The diversity results were consistent with the Venn plots that all samples gradually decreased with the increase of storage time. The similarity and differences in the composition of spoilage flora of grass carp meat during refrigeration are shown in the heat map (Fig. 6E). At the genus level, the genera with relative abundance in the top 30 were selected for community dynamics analysis, with red and blue colors representing higher and lower relative abundance. The relative abundance of most of the genera in grass carp meat treated by different thermal processing methods in the middle and later refrigeration period was lower than that in the pre-refrigeration period, especially for the RO treatment group, which reached a relatively stable level of bacterial composition in the middle period. In addition, there were also some differences in the microbial composition of fish meat from different heat treatments during the same refrigeration period. And these differences would further lead to variation in texture, color, flavor, protein degradation, and fatty acid composition of fish meat treated by different thermal processing methods during refrigeration.
Fig. 6.
Analysis of changes in microbial composition of grass carp meat treated by different thermal processing methods during refrigerated storage. (A ∼ D) Venn diagrams of different treatments, (E) community heatmap analysis on genus level, (F ∼ I) structure and dynamic succession of the bacterial community in different treatment samples, (J) the relative abundance of community analysis on genus level.
High-quality sequencing reads were classified at the genus and species levels to investigate the structure and dynamic succession of the bacterial community in different heat treatments of fish meat during refrigeration (Fig. 6F-I). At the genus level, the top 10 dominant bacteria (Pseudomonas, Acinetobacter and Achromobacter, etc.) in three periods of fish meat accounted for 75.91%, 96.86% and 99.53% of the total bacterial population, respectively. The relative abundance of Pseudomonas and Acinetobacter was higher during refrigeration. Among the bacterial communities in the pre-spoilage phase, Pseudomonas was the most predominant microbe in FY and BO, which accounted for 73.71% and 53.35%, respectively (Fig. 6F). In addition, the relative abundance of Acinetobacter was essentially the same in all groups. However, the relative abundance of Pseudomonas (98.26%) had the highest percentage in SV at the end of refrigeration (Fig. 6H). And Acinetobacter was the most predominant microbe in SM and BO (Fig. 6H). Acinetobacter and Acinetobacter were common spoilage bacteria in food, such as braised duck meat (Li et al., 2023) and refrigerated pork meat (Wang et al., 2021).
In order to study the succession of microbial communities in grass carp meat with different heat treatments during refrigeration, 16S rRNA gene sequences were analyzed at the genus level. As shown in Fig. 6J, the flora composition of each fish sample showed significant differences at the genus level. In this study, the dominant genera were higher in the pre-refrigeration period than in the middle and later period in all treatment groups, and the RWL group was richer in dominant genera than the heat-treated group. These indicated that heat treatment reduced the diversity of the grass carp meat flora, and the diversity of bacterial flora was reduced during the storage process. Different from fish meat in other treatment groups, RO had a higher number of bacterial species in the pre-refrigeration period, and the spoilage bacteria were dominated by the genus Exiguobacterium in the middle and later period, with a relative abundance of 87.52% and 67.54%, respectively. However, the abundance of this genus was lower in the fish meat prepared by all other heat treatments, indicating that baking-treated fish meat was more favorable for the growth of Exiguobacterium. Higher abundance of Pseudomonas and Actinobacteria was observed in all heat-treated groups of fish meat during refrigeration, except for the RO group. Pseudomonas was recognized as one of the main bacteria responsible for meat spoilage (Wickramasinghe et al., 2019), with the relative abundance in FYL and SVL samples reaching 86.32% and 98.27%, respectively. Acinetobacter was also a common genus in spoiled meat and was the dominant genus in fresh fish fillets. The relative abundance of this genus exceeded 10% in all samples, but with the prolongation of storage time, the Acinetobacter abundance significantly decreased, which is consistent with the results of Pan et al. (2018). The fish meat in heat-treated group was differed from RW, Acinetobacter became the dominant genus in SMM, SML, BOM, and BOL during refrigeration, while its abundance decreased to <1% in SVM and SVL. However, Lactococcus, Kurthia, and Vagococcus only appeared in higher abundance in RW, which suggests that these genera had less spoilage effect on heat-treated fish meat. However, Barakat et al. (2000) found Lactococcus was the dominant genera during refrigeration process of cooked chicken. Achromobacter was detected in SMP, BOP, FYP, ROP, and SVP groups, but its abundance gradually decreased with refrigeration time, whereas it was not detected in RW. Results suggested that the bacteria of this genus may be better adapted to the growth environment of heat-treated fish meat. In conclusion, different thermal processing methods have different effects on the dominant flora of fish meat, but the effects and mechanisms of these flora on the quality of heat-treated grass carp meat need further investigation.
3.5. Correlation between microbiota and biochemical changes
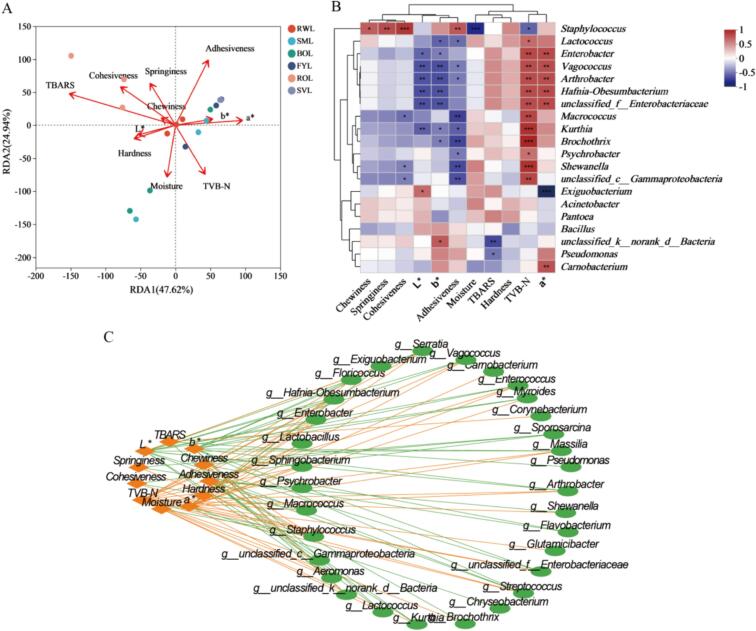
The structure of microorganisms is influenced by the physicochemical indicators of fish meat, and conversely, microorganisms in fish meat affect its physicochemical properties. Physicochemical indicators such as moisture content, TBARS value and TVB-N content are considered as key influences on the microbial community succession during refrigerated storage of grass carp meat (Huang et al., 2022). Therefore, the correlation between microbiota and biochemical changes of grass carp meat with different heat treatments was analyzed through Spearman's correlation analysis (Fig. 7). The specific values of Spearman's correlation coefficient and p-value were shown in Supplementary Table S1 and Table S2, respectively. The results of Redundancy analysis (RDA) of the fish samples is shown in Fig. 7A, ROL was positively correlated with TBARS, cohesiveness and springiness, SVL and FYL were positively correlated with adhesiveness, a* and b*. The microbial abundance of BOL was mainly influenced by moisture.
Fig. 7.
The correlation between physicochemical properties and microbiota of grass carp meat treated by different thermal processing methods during refrigerated storage. (A) RDA analysis, (B) correlation heatmap, and (C) network diagram of microorganisms and physicochemical properties.
To further investigate the relationship between physicochemical indicators and microbial community succession, we selected the top 20 microorganisms with the highest relative abundance in the later refrigeration period of grass carp meat, and analyzed their correlation with physicochemical indicators. As shown in Fig. 7B, the relative abundance of Brochothrix, Shewanella, Lactococcus, and Psychrobacter showed a positive correlation with TVB-N, they were identified as specific spoilage flora (Sun et al., 2020; Wang et al., 2021). The network diagram of microorganisms and physicochemical properties was given in Fig. 7C. Exiguobacterium had a strong negative correlation with a* (Coefficient < −0.8, p < 0.05), while Brochothrix showed a strong positive correlation with TVB-N (Coefficient > 0.8, p < 0.05), consistent with the above results.
4. Conclusion
This study investigated the changes in physicochemical properties and microbial metabolic activities of five heat-treated grass carp meat during refrigerated storage. Results suggested that different treating methods had different effects on the storage quality of grass carp meat, the RW group spoiled after 4-day storage, while the FY group did not display any signs of spoilage until the 15th day. During cold storage, the values of TVB-N, TBARS and b* of fish meat in each treatment group were increasing, while the values of moisture content, L* and a* were decreasing. The hardness, cohesiveness, chewiness and elasticity of the fish meat gradually decreased, while the viscosity increased with the prolongation of cold storage time, except in the FY group. RO and FY significantly changed the fatty acid composition of fish meat. The changes in microbial metabolic activities showed that dominant bacteria such as Pseudomonas, Bacillus immobilis, and Microbacterium were the main causes of spoilage of refrigerated grass carp meat. Prolonged refrigeration leads to lipid oxidation and spoilage of fish meat, but different treating methods retard this spoilage. However, this study did not further explore how these heat treatments affect the above characteristics and the mechanism of their interaction in grass carp meat. In the future, we propose to use gas chromatography-ion mobility spectrometry (GC-IMS), electronic nose technology (E-nose) and high performance liquid chromatography (HPLC) to monitor and analyze the changes of volatile and non-volatile flavor components of grass carp during cold storage with different heat treatments. Through the application of these advanced technologies, the effects of heat treatment on the flavor of grass carp can be more accurately evaluated, and the scientific basis for selecting appropriate cooking techniques and evaluating the shelf life of grass carp can be provided.
CRediT authorship contribution statement
Lu Zhang: Writing – review & editing, Supervision, Resources, Methodology, Funding acquisition, Formal analysis. Yaqin Yu: Writing – original draft, Investigation, Formal analysis, Data curation. Chunming Tan: Writing – review & editing, Validation, Investigation, Formal analysis, Data curation. Shi Nie: Investigation, Formal analysis. Qinghui Wen: Validation, Software. Zongcai Tu: Writing – review & editing, Supervision, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the National Key Research and Development Program of China, grant number 2022YFD2100902.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101662.
Contributor Information
Chunming Tan, Email: cmtan_ouc@163.com.
Zongcai Tu, Email: tuzc_mail@aliyun.com.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Ali Ghoflgar Ghasemi M., Hamishehkar H., Javadi A., Homayouni-Rad A., Jafarizadeh-Malmiri H. Natural-based edible nanocomposite coating for beef meat packaging. Food Chemistry. 2024;435 doi: 10.1016/j.foodchem.2023.137582. [DOI] [PubMed] [Google Scholar]
- Bahrami Feridoni S., Khademi Shurmasti D. Effect of the nanoencapsulated sour tea (Hibiscus sabdariffa L.) extract with carboxymethylcellulose on quality and shelf life of chicken nugget. Food Science & Nutrition. 2020;8(7):3704–3715. doi: 10.1002/fsn3.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y., Wang K., Yang H., Regenstein J.M., Ertbjerg P., Zhou P. Protein degradation of black carp (Mylopharyngodon piceus) muscle during cold storage. Food Chemistry. 2020;308 doi: 10.1016/j.foodchem.2019.125576. [DOI] [PubMed] [Google Scholar]
- Barakat R.K., Griffiths M.W., Harris L.J. Isolation and characterization of Carnobacterium, Lactococcus, and Enterococcus spp. from cooked, modified atmosphere packaged, refrigerated, poultry meat. International Journal of Food Microbiology. 2000;62(1–2):83–94. doi: 10.1016/S0168-1605(00)00381-0. [DOI] [PubMed] [Google Scholar]
- Buckland G., De Silva Johnson S., Johnson L., Taylor C.M., Jones L.R., Emmett P.M. The relationship between dietary intakes and plasma concentrations of PUFA in school-age children from the Avon longitudinal study of parents and children (ALSPAC) cohort. British Journal of Nutrition. 2022;127(9):1367–1377. doi: 10.1017/S0007114521002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Chen Q., Wan X., Zhao J. Determination of total volatile basic nitrogen (TVB-N) content and Warner–Bratzler shear force (WBSF) in pork using Fourier transform near infrared (FT-NIR) spectroscopy. Food Chemistry. 2011;126(3):1354–1360. doi: 10.1016/j.foodchem.2010.11.098. [DOI] [Google Scholar]
- Calanche J., Tomas A., Martinez S., Jover M., Alonso V., Roncalés P., Beltrán J.A. Relation of quality and sensory perception with changes in free amino acids of thawed seabream (Sparus aurata) Food Research International. 2019;119:126–134. doi: 10.1016/j.foodres.2019.01.050. [DOI] [PubMed] [Google Scholar]
- Cao Q., Du H., Huang Y., Hu Y., You J., Liu R., Xiong S., Manyande A. The inhibitory effect of chlorogenic acid on lipid oxidation of grass carp (Ctenopharyngodon idellus) during chilled storage. Food and Bioprocess Technology. 2019;12(12):2050–2061. doi: 10.1007/s11947-019-02365-0. [DOI] [Google Scholar]
- Cheng L., Li X., Tian Y., Wang Q., Li X., An F., Luo Z., Shang P., Liu Z., Huang Q. Mechanisms of cooking methods on flavor formation of Tibetan pork. Food Chemistry: X. 2023;19 doi: 10.1016/j.fochx.2023.100873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil M., Rudy M., Stanisławczyk R., Duma-Kocan P. Effect of traditional cooking and sous vide heat treatment, cold storage time and muscle on physicochemical and sensory properties of beef meat. Molecules. 2022;27(21):7307. doi: 10.3390/molecules27217307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Głuchowski A., Czarniecka-Skubina E., Wasiak-Zys G., Nowak D. Effect of various cooking methods on technological and sensory quality of Atlantic salmon (Salmo salar) Foods. 2019;8(8):323. doi: 10.3390/foods8080323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q., Jiao X., Yan B., Zhang N., Huang J., Zhao J., Zhang H., Chen W., Fan D. Changes in physicochemical properties of silver carp (Hypophthalmichthys molitrix) surimi during chilled storage: The roles of spoilage bacteria. Food Chemistry. 2022;387 doi: 10.1016/j.foodchem.2022.132847. [DOI] [PubMed] [Google Scholar]
- Hussin S.N., Azlan A., Khoo H.E., Abdul Kadir N.A.A., Razman M.R. Comparison of fat composition and chemical properties of fat extracts between fish fillets of selected warm-water and cold-water fish. Bioscience Journal. 2019;35(6) doi: 10.14393/BJ-v35n6a2019-42782. [DOI] [Google Scholar]
- Husted K.S., Bouzinova E.V. The importance of n-6/n-3 fatty acids ratio in the major depressive disorder. Medicina. 2016;52(3):139–147. doi: 10.1016/j.medici.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Katiyo W., Kock H.L.D., Coorey R., Buys E. Sensory implications of chicken meat spoilage in relation to microbial and physicochemical characteristics during refrigerated storage. LWT- Food Science and Technology. 2020;128 [Google Scholar]
- Kim H.-J., Kim H.-J., Jeon J., Nam K.-C., Shim K.-S., Jung J.-H.…Jang A. Comparison of the quality characteristics of chicken breast meat from conventional and animal welfare farms under refrigerated storage. Poultry Science. 2020;99(3):1788–1796. doi: 10.1016/j.psj.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Qu S., Ma P., Zhang J., Zhao K., Chen L.…Tang H. Effects of chitosan coating combined with thermal treatment on physicochemical properties, bacterial diversity and volatile flavor of braised duck meat during refrigerated storage. Food Research International. 2023;167:112627. doi: 10.1016/j.foodres.2023.112627. [DOI] [PubMed] [Google Scholar]
- Lian F., Cheng J.-H., Sun D.-W. Insight into the effect of microwave treatment on fat loss, fatty acid composition and microstructure of pork subcutaneous back fat. LWT- Food Science and Technology. 2023;187 doi: 10.1016/j.lwt.2023.115297. [DOI] [Google Scholar]
- Lian F., Sun D.-W., Cheng J.-H., Ma J. Improving modification of structures and functionalities of food macromolecules by novel thermal technologies. Trends in Food Science & Technology. 2022;129:327–338. doi: 10.1016/j.tifs.2022.10.001. [DOI] [Google Scholar]
- Liang Q., Hu X., Zhong B., Huang X., Wang H., Yu C., Tu Z., Li J. Regulating effects of low salt dry-curing pre-treatment on microbiota, biochemical changes and flavour precursors of grass carp (Ctenopharyngodon idella) fillets during storage at 4 °C. Food Chemistry: X. 2024;21 doi: 10.1016/j.fochx.2024.101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Luo X., Huang Y., Zhao M., Liu T., Wang J., Feng F. Influence of cooking techniques on food quality, digestibility, and health risks regarding lipid oxidation. Food Research International. 2023;167 doi: 10.1016/j.foodres.2023.112685. [DOI] [PubMed] [Google Scholar]
- Liu X., Piao C., Ju M., Zhang J., Zhang W., Cui F., Li G., Cui M. Effects of low salt on lipid oxidation and hydrolysis, fatty acids composition and volatiles flavor compounds of dry-cured ham during ripening. LWT- Food Science and Technology. 2023;187 doi: 10.1016/j.lwt.2023.115347. [DOI] [Google Scholar]
- Oppong D., Panpipat W., Cheong L.-Z., Chaijan M. Comparative effect of frying and baking on chemical, physical, and microbiological characteristics of frozen fish nuggets. Foods. 2021;10(12):3158. doi: 10.3390/foods10123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özogul Y., Özogul F., Alagoz S. Fatty acid profiles and fat contents of commercially important seawater and freshwater fish species of Turkey: A comparative study. Food Chemistry. 2007;103(1):217–223. doi: 10.1016/j.foodchem.2006.08.009. [DOI] [Google Scholar]
- Pan Z., Li L., Shen Z., Chen Y., Li M. Characterization of the microbiota in air- or vacuum-packed crisp grass carp (Ctenopharyngodon idella C. et V.) fillets by 16S rRNA PCR–denaturing gradient gel electrophoresis and high-throughput sequencing. Journal of Food Protection. 2018;81(6):1022–1029. doi: 10.4315/0362-028X.JFP-17-498. [DOI] [PubMed] [Google Scholar]
- Pan Z., Li L., Shen Z., Chen Y., Li M. Effects of tea polyphenol treatments on the quality and microbiota of crisp grass carp fillets during storage at 4 °C. Applied Sciences. 2021;11(10):4370. doi: 10.3390/app11104370. [DOI] [Google Scholar]
- Qin J., Wang Z., Wang X., Shi W. Effects of microwave time on quality of grass carp fillets processed through microwave combined with hot-air drying. Food Science & Nutrition. 2020;8(8):4159–4171. doi: 10.1002/fsn3.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabetian M., Torabi Delshad S., Moini S., Rajabi Islami H., Beglaryan R., Motalebi A. Identification and changes in fatty acid profile of rainbow trout (Oncorhynchus mykiss) fillet during frozen storage (−18 °C) Journal of Aquatic Food Product Technology. 2014;23(4):321–332. doi: 10.1080/10498850.2012.717592. [DOI] [Google Scholar]
- Sáez M.I., Suárez M.D., Alarcón F.J., Martínez T.F. Assessing the potential of algae extracts for extending the shelf life of rainbow trout (Oncorhynchus mykiss) fillets. Foods. 2021;10(5):910. doi: 10.3390/foods10050910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sağglık S., Alpaslan M., Gezgin T., Çetintürkc K., Tekinay A., Güven K.C. Fatty acid composition of wild and cultivated gilthead seabream (Sparus aurata) and sea bass (Dicentrarchus labrax) European Journal of Lipid Science and Technology. 2003;105(2):104–107. doi: 10.1002/ejlt.200390013. [DOI] [Google Scholar]
- Sun X., Hong H., Jia S., Liu Y., Luo Y. Effects of phytic acid and lysozyme on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets stored at 4 °C. Food Microbiology. 2020;86 doi: 10.1016/j.fm.2019.103313. [DOI] [PubMed] [Google Scholar]
- Tan C., Hu J., Gao B., Zhang B., Li P., Shang N. Effects of the interaction between Aeromonas sobria and Macrococcus caseolyticus on protein degradation of refrigerated sturgeon fillets: Novel perspective on fish spoilage. LWT- Food Science and Technology. 2023;183 doi: 10.1016/j.lwt.2023.114908. [DOI] [Google Scholar]
- Tan C., Xiao M., Wu R., Li P., Shang N. Unraveling the effects of biochemical drivers on the bacterial communities and volatile profiles in refrigerated sturgeon fillets at 4 °C. Frontiers in Microbiology. 2022;2022(13) doi: 10.3389/fmicb.2022.849236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventanas S., Estévez M., Delgado C.L., Ruiz J. Phospholipid oxidation, non-enzymatic browning development and volatile compounds generation in model systems containing liposomes from porcine longissimus dorsi and selected amino acids. European Food Research and Technology. 2007;225(5–6):665–675. doi: 10.1007/s00217-006-0462-2. [DOI] [Google Scholar]
- Wang D., Zhou F., Lai D., Zhang Y., Hu J., Lin S. Curcumin-mediated sono/photodynamic treatment preserved the quality of shrimp surimi and influenced its microbial community changes during refrigerated storage. Ultrasonics Sonochemistry. 2021;78 doi: 10.1016/j.ultsonch.2021.105715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe N.N., Ravensdale J., Coorey R., Chandry S.P., Dykes G.A. The predominance of Psychrotrophic Pseudomonads on aerobically stored chilled red meat. Comprehensive Reviews in Food Science and Food Safety. 2019;18(5):1622–1635. doi: 10.1111/1541-4337.12483. [DOI] [PubMed] [Google Scholar]
- Ye Z., Shang Z., Li M., Qu Y., Long H., Yi J. Evaluation of the physiochemical and aromatic qualities of pickled Chinese pepper (Paojiao) and their influence on consumer acceptability by using targeted and untargeted multivariate approaches. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109535. [DOI] [PubMed] [Google Scholar]
- Yi J., Kebede B., Kristiani K., Buvé C., Van Loey A., Grauwet T., Hendrickx M. The potential of kiwifruit puree as a clean label ingredient to stabilize high pressure pasteurized cloudy apple juice during storage. Food Chemistry. 2018;255:197–208. doi: 10.1016/j.foodchem.2018.02.052. [DOI] [PubMed] [Google Scholar]
- Yu D., Xu Y., Jiang Q., Yang F., Xia W. Freshness assessment of grass carp (Ctenopharyngodon idellus) fillets during storage at 4 °C by physicochemical, microbiological and sensorial evaluations. Journal of Food Safety. 2017;37(2) doi: 10.1111/jfs.12305. [DOI] [Google Scholar]
- Zhang J., Zhang Y., Zou Y., Zhang W. Effects of ultrasound-assisted cooking on quality characteristics of spiced beef during cold storage. LWT- Food Science and Technology. 2021;136 doi: 10.1016/j.lwt.2020.110359. [DOI] [Google Scholar]
- Zhou M., Shi G., Deng Y., Wang C., Qiao Y., Xiong G., Wang L., Wu W., Shi L., Ding A. Study on the physicochemical and flavor characteristics of air frying and deep frying shrimp (crayfish) meat. Frontiers in Nutrition. 2022;9:1022590. doi: 10.3389/fnut.2022.1022590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Yang C., Song Y., Qiang Y., Han D., Zhang C. Changes provoked by altitudes and cooking methods in physicochemical properties, volatile profile, and sensory characteristics of yak meat. Food Chemistry: X. 2023;20 doi: 10.1016/j.fochx.2023.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S., Tian L., Liu Y., Wang L., Hong H., Luo Y. Amino acid degradation and related quality changes caused by common spoilage bacteria in chill-stored grass carp (Ctenopharyngodon idella) Food Chemistry. 2023;399 doi: 10.1016/j.foodchem.2022.133989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.