Abstract
We used CD4 lymphocyte clones from herpes simplex virus type 2 (HSV-2) lesions or the cervix and molecular libraries of HSV-2 DNA to define HSV-2 major capsid protein VP5 and glycoprotein E (gE) as T-cell antigens. Responses to eight HSV-2 glycoprotein, tegument, nonstructural, or capsid antigens were compared in 19 donors. Recognition of VP5 and tegument VP22 were similar to that of gB2 and gD2, currently under study as vaccines. These prevalence data suggest that HSV capsid and tegument proteins may also be candidate vaccine antigens.
CD4 responses to herpes simplex virus (HSV) may have several functional roles, including secretion of cytokines with antiviral and immunostimulatory activity, cytotoxic T-lymphocyte (CTL) activity, inhibition of viral replication, and B-cell help (37–39). CD4 reactivity with glycoproteins B (gB) and D is therefore one rationale for their use as HSV vaccines (40, 41). Responses to gB and gD are prevalent in peripheral blood mononuclear cells (PBMC) (31, 41), but few lesion-derived CD4 clones recognize these proteins (13). Recognition of gB or gD occurs in about 40 to 50% of bulk skin-infiltrating lymphocyte cell lines expanded from recurrent genital HSV type 2 (HSV-2) lesions (15).
The spectrum of HSV T-cell antigens is expanding. Tegument protein UL48 (VP16) contains at least eight CD4 T-cell epitopes (14, 16). Tegument proteins UL21 and UL49 (VP22), nonstructural protein UL50 (dUTPase), and gC2 are antigens for HSV-2 lesion-derived CD4 T-cell clones (13, 14). Type-common epitopes in UL21 and UL49 are recognized by potentially pathogenic local CD4 T cells in herpes simplex keratitis (15a). CD4 clones from HSV-1 retinitis are stimulated by tegument proteins UL46 and UL47 (33), and gH and gL of HSV-1 are also antigenic for PBMC (36). We now add the first virion capsid protein and an additional glycoprotein to the set of known HSV T-cell antigens. We have also begun to compare the prevalence of CD4 responses to specific HSV-2 proteins in PBMC.
T-cell clone ESL2.2 (104/well), derived from a recurrent HSV-2 lesion and expanded as previously described (14), is CD4+ (13) and reacts with a 1:100 dilution of UV-treated sonicates of HSV-2 333 (11) (Table 1) but not with HSV-1 E115 (31). Autologous irradiated PBMC (105/well) were used as antigen-presenting cells (APC) in triplicate proliferation assays (12). To make genetic libraries, HSV-2 HG52 (5) genomic DNA was digested separately with SmaI or AluI and fragments were mixed and ligated into each blunt-end-digested pUEX vector, as described previously (2, 14). Bulk preparations of β-galactosidase fusion protein inclusion bodies (14, 22) were tested (1:100) with PBMC as APC. Library pUEX3-HG52-SmaI-AluI stimulated proliferation. This library was interrogated as pools and derivative single colonies. The pUEX3 plasmid in active colony A7A4A7 contained an AluI fragment with HSV-2 nucleotides 37.341 to 36.616 encoding amino acids 1078 to 1319 of UL19 (5), which were in frame with β-galactosidase. The UL19 gene encodes the major capsid protein VP5. A SmaI fragment (150,831 to 150,596) from within ICP4, in reverse orientation, followed the UL19 fragment in A7A4A7. Reactivity with UL19 was confirmed by expressing full-length PCR-amplified UL19 (15a).
TABLE 1.
Specificity and HLA restriction of lesion- and cervix-derived CD4+ T-cell clones
| Clone | Source | Resultsa of reactions with:
|
Predicted HSV-2 protein | |||||
|---|---|---|---|---|---|---|---|---|
| Whole HSV-1 protein | Whole HSV-2 protein | pUEX1-based libraryb | pUEX2-based library | pUEX3-based library | Active library clone | |||
| ESL2.2 | Recurrent day 3 genital HSV-2 lesion | 0 | 66,905 | 17 | 192 | 1,092 | 94,760 | UL19 amino acids 1078–1319 |
| EA.17 | Cervix | 3,298 | 53,283 | 5,860 | 272 | 13 | 15,686 | US8 amino acids 1–259 |
Results are Δcpm from triplicate [3H]thymidine incorporation assays.
DNA used in library was from full-length HSV-2 HG52 for ESL2.2 and from HG52 plasmids covering US for EA.17.
An HSV-2 type-specific CD4+ clone (EA.17) from the cervix of a subject with recurrent genital herpes was studied in a similar fashion. Clone derivation, documentation of CTL activity, and mapping of the epitope to the US region of HSV-2 DNA have been previously described (16). Libraries were made with plasmids BB40 and HindIII “l,” containing most of HSV-2 HG52 US (provided by A. Davison), by using the same restriction endonucleases and expression vectors described above. Library pUEX1-US-SmaI-AluI and derivative clone US19 were positive (Table 1). US19 contains an AluI fragment with HSV-2 nucleotides 143,798 to 144,662, predicted to encode a short region (143,798 to 143,843) of nonsense peptide 5′ to the viral ATG, which is followed in frame by amino acids 1 to 259 of US8 (gE).
These results show for the first time that the clonal human HSV-specific cellular immune responses include cells reactive with a viral capsid protein. Earlier observations of human PBMC proliferative responses to whole HSV capsid preparations (10, 19) could be due to reactivity with VP5 alone or in combination with other capsid proteins. T-cell reactivity with gE in humans has not been reported; mice develop probable CD4 responses against the HSV-1 homologue (8). Having previously defined several other CD4 antigens using lesion-derived cells, we next compared reactivity with these new antigens to that of previously known proteins.
Full-length open reading frames for UL19, UL21, UL49, UL50, and US8 were each amplified by PCR from genomic HSV-2 HG52 DNA or derivative plasmids, as described elsewhere (15a), by using a proofreading DNA polymerase. Protein expression required the pcDNA3.1/His series (Invitrogen), except that of UL49, which used pEGFP-C1 (Clontech). Proteins were tested as sonicates of transiently transfected (Fugene-6; Boehringer Mannheim) Cos-7 cells. Each (except US8, for which APC were unavailable) was highly antigenic for the index T-cell clone at 1:100 (15a) and was used at this dilution. Purified gB2 and gD2 (missing the signal and transmembrane regions) and UL48 (provided by R. L. Burke and M. A. Tigges) were used at 1 μg/ml as described previously (15). This dose was previously found to be optimal for stimulating HSV antigen-specific CD4 T-cell clones.
Responder cell lines were Ficoll-purified PBMC (2 × 106 in 24-well plates in T-cell medium [13]) stimulated for 12 to 14 days with whole HSV-2 antigen with growth supported by interleukin 2 (Hemagen) (32 U/ml) from day 5. Donors were 19 HSV-2-infected, HIV-uninfected adults with symptomatic genital herpes caused by HSV-2 (specimens were kindly provided by A. Wald and the clinical staff at the Virology Research Clinic, Seattle, Wash.). Responder cells (104/well) and autologous irradiated PBMC serving as APC (105/well) were incubated in triplicate 3-day proliferation assays. Positive control was whole HSV-2 333 antigen; negative controls were media, mock virus, and sonicates of Cos-7 cells transfected with an empty vector. Results are expressed in counts per minute (cpm) as Δcpm = mean experimental cpm − mean control cpm.
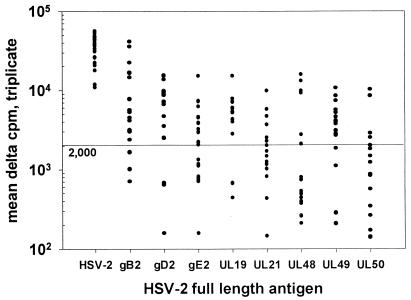
All donors had a positive response to whole HSV-2 antigen (mean Δcpm, 35,374; range 11,831 to 56,488) (Fig. 1). None had a significant response to mock Vero cell preparation in comparison to media (mean Δcpm, 132; range, 328 to 518) or to an empty vector in comparison to media. We set the Δcpm criterion for a positive response to an HSV-2 antigen at 2,000. Responses to glycoproteins ranged from 58% for gE2 (11 of 19) and 68% for truncated gD2 (13 of 19) to 84% for truncated gB2 (16 of 19). Among the tegument proteins, responses to UL49 (14 of 19 [74%]) were more prevalent than responses to VP16 (UL48) (6 of 17 [35%]) or UL21 (8 of 19 [42%]). For the nonstructural protein UL50 (dUTPase), responses were seen in 5 of 19 persons (26%), while for major capsid protein UL19, responses were seen in 10 of 12 persons (83%). The diversity of the proliferative response to HSV varied considerably. The median number of antigens recognized was four, with a range of one to eight (Fig. 2). We sought to determine if the diversity of the response as measured in the HSV-2-specific line correlated with the proliferative response in fresh PBMC. PBMC from our panel of donors were stimulated for 5 days with whole HSV-2 and net [3H]thymidine incorporation in comparison to mock Vero antigen measured in triplicate. The Spearman rank correlation coefficient (Instat; Graphpad Software) is 0.48, which has a two-tailed P value of 0.044 for the relationship between the magnitude of the proliferative response among fresh PBMC and the diversity of the response.
FIG. 1.
Proliferative responses of HSV-2-stimulated PBMC lines for whole HSV-2 and defined HSV-2 antigens. Nineteen immunocompetent adults with symptomatic genital HSV-2 infection served as donors. Results are net Δcpm compared to mock virus (HSV-2), media (gB2, gD2, UL48), or lysates of cells transfected with empty vectors (other antigens). Data points with values of <102 are not visible.
FIG. 2.
Number of distinct HSV-2 protein antigens to which proliferative responses were detected in short-term PBMC lines from 19 HSV-2-infected adults.
Both subjective and objective spectrums of infection and disease severity exist for genital HSV-2 infection. Only a minority of persons with HSV-2 infection are aware of this (3), and even after patient education and repeated examinations correlated with viral cultures, some individuals do not have recognizable lesions (6, 18, 35). The rate of HSV-2 shedding from the anogenital tract varies up to 10-fold among immunocompetent women, as measured by serial specimens from multiple anatomic sites and sensitive PCR testing (34). Higher shedding is likely to result in an increased risk of transmission of this medically important virus. Little is known concerning the viral, host immune, or other host correlates of either objective viral shedding rates or subjective disease severity.
It is recognized that CD4 responses are required to prime and maintain many CD8 responses (9, 20) as well as antibody responses and are important in genital tract defense against HSV-2 in animals (21). Local and systemic gamma interferon, a product of several lymphocyte subsets, may be correlated with disease severity in humans (4, 32). CD4 responder cells are present early in herpetic lesions and contribute to local CTL activity (15). Different HSV antigens administered in an identical fashion (likely to elicit mainly CD4 responses) may elicit unique cytokine profiles in animals after viral challenge (7). Animal models have also shown the importance of CD8 T cells in ganglionic control of HSV (23, 24, 28, 29), and CD8 responses were correlated with disease severity in human immunodeficiency virus- and HSV-2-coinfected humans (25). The specificity, breadth, evolution, and magnitude of both CD4 and CD8 responses to HSV are therefore of interest.
Our results indicate that major capsid protein VP5 and tegument protein VP22 frequently elicit CD4 responses in PBMC. Reactivity with VP5 is perhaps not surprising, as this protein is quite abundant in virions (27), is long, might therefore contain many HLA class II binding motifs (27), and elicits a strong antibody response (1). We used HSV-restimulated cell lines as responders for this initial screen, as fresh PBMC were found to proliferate to the unpurified mock antigen preparations made with empty vectors. Possibly, responses to some HSV proteins may have been differentially amplified or lost during this cycle of enrichment of HSV-specific cells. Future studies will use more highly purified antigens suitable for direct interrogation of panels of PBMC in proliferation and cytokine stimulation formats. In this way, the possible correlations between CD4 responses and the severity of infection or disease can be rigorously explored in defined patient populations.
Acknowledgments
This work was supported in part by NIH grants AI30731 and CA70017 and the Howard Hughes Pilot Research Initiative (D.M.K.).
A. Davison, R. L. Burke, and M. A. Tigges supplied reagents. L. Corey and A. Wald generously supplied clinical specimens.
REFERENCES
- 1.Ashley R L, Dalessio J, Burchett S, Brown Z, Berry S, Mohan K, Corey L. Herpes simplex virus-2 (HSV-2) type-specific antibody correlates of protection in infants exposed to HSV-2 at birth. J Clin Investig. 1992;90:511–514. doi: 10.1172/JCI115888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bressan G M, Stanley K K. pUEX, a bacterial expression vector related to pEX with universal host specificity. Nucleic Acids Res. 1987;15:10056. doi: 10.1093/nar/15.23.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corey L, Wald A. Genital herpes. In: Holmes K K, Sparling P F, Mardh P A, Lemon S M, Stamm W E, Piot P, Wasserheit J M, editors. Sexually transmitted diseases. 3rd ed. New York, N.Y: McGraw-Hill, Inc.; 1999. pp. 285–312. [Google Scholar]
- 4.Cunningham A L, Merigan T C. Gamma interferon production appears to predict time of recurrence of herpes labialis. J Immunol. 1983;130:2397–2400. [PubMed] [Google Scholar]
- 5.Dolan A, Jamieson F E, Cunningham C, Barnett B C, McGeoch D J. The genome sequence of herpes simplex virus type 2. J Virol. 1998;72:2010–2021. doi: 10.1128/jvi.72.3.2010-2021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frenkel L M, Garratty E M, Shen J P, Wheeler N, Clark O, Bryson Y J. Clinical reactivation of herpes simplex virus type 2 infection in seropositive pregnant women with no history of genital herpes. Ann Intern Med. 1993;118:414–418. doi: 10.7326/0003-4819-118-6-199303150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Ghiasi H, Hofman F M, Cai S, Perng G C, Nesburn A B, Wechsler S L. Vaccination with different HSV-1 glycoproteins induces different patterns of ocular cytokine responses following HSV-1 challenge of vaccinated mice. Vaccine. 1999;17:2576–2582. doi: 10.1016/s0264-410x(99)00056-0. [DOI] [PubMed] [Google Scholar]
- 8.Ghiasi H, Kaiwar R, Nesburn A B, Slanina S, Wechsler S L. Baculovirus-expressed glycoprotein E (gE) of herpes simplex virus type-1 (HSV-1) protects mice against lethal intraperitoneal and lethal ocular HSV-1 challenge. Virology. 1992;188:469–476. doi: 10.1016/0042-6822(92)90500-o. [DOI] [PubMed] [Google Scholar]
- 9.Kalams S A, Walker B D. The critical need for CD4 help in maintaining effective cytolytic T lymphocyte responses. J Exp Med. 1998;188:2199–2204. doi: 10.1084/jem.188.12.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalimo K O K, Joronen I A, Havu V K. Cell-mediated immunity against herpes simplex virus envelope, capsid, excreted, and crude antigens. Infect Immun. 1983;39:24–28. doi: 10.1128/iai.39.1.24-28.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kit S, Kit M, Qavi H, Trkula D, Otsuka H. Nucleotide sequence of the herpes simplex virus type 2 (HSV-2) thymidine kinase gene and predicted amino acid sequence of thymidine kinase polypeptide and its comparison with the HSV-1 thymidine kinase gene. Biochim Biophys Acta. 1983;741:158–170. doi: 10.1016/0167-4781(83)90056-8. [DOI] [PubMed] [Google Scholar]
- 12.Koelle D M, Abbo H, Peck A, Ziegweid K, Corey L. Direct recovery of HSV-specific T lymphocyte clones from human recurrent HSV-2 lesions. J Infect Dis. 1994;169:956–961. doi: 10.1093/infdis/169.5.956. [DOI] [PubMed] [Google Scholar]
- 13.Koelle D M, Corey L, Burke R L, Eisenberg R J, Cohen G H, Pichyangkura R, Triezenberg S J. Antigenic specificity of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J Virol. 1994;68:2803–2810. doi: 10.1128/jvi.68.5.2803-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koelle D M, Frank J M, Johnson M L, Kwok W W. Recognition of herpes simplex virus type 2 tegument proteins by CD4 T cells infiltrating human genital herpes lesions. J Virol. 1998;72:7476–7483. doi: 10.1128/jvi.72.9.7476-7483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koelle D M, Posavad C M, Barnum G R, Johnson M L, Frank J M, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Investig. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Koelle D M, Reymond S N, Chen H, Kwok W W, McClurkan C, Gyaltsong T, Petersdorf E W, Rotkis W, Talley A R, Harrison D A. Tegument-specific, virus-reactive CD4 T cells localize to the cornea in herpes simplex virus interstitial keratitis in humans. J Virol. 2000;74:10930–10938. doi: 10.1128/jvi.74.23.10930-10938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koelle D M, Schomogyi M, Corey L. Antigen-specific T-cells localize to the uterine cervix in women with genital herpes simplex virus type 2 virus infection. J Infect Dis. 2000;182:662–670. doi: 10.1086/315749. [DOI] [PubMed] [Google Scholar]
- 17.Kwok W W, Liu A W, Novak E J, Gebe J A, Ettinger R A, Nepom G T, Reymond S N, Koelle D M. HLA-DQ tetramers identify epitope-specific T cells in peripheral blood of herpes simplex virus type 2-infected individuals: direct detection of immunodominant antigen-responsive cells. J Immunol. 2000;164:4244–4249. doi: 10.4049/jimmunol.164.8.4244. [DOI] [PubMed] [Google Scholar]
- 18.Langenberg A, Benedetti J, Jenkins J, Ashley R, Winter C, Corey L. Development of clinically recognizable genital lesions among women previously identified as having asymptomatic herpes simplex virus type 2 infection. Ann Intern Med. 1989;110:882–887. doi: 10.7326/0003-4819-110-11-882. [DOI] [PubMed] [Google Scholar]
- 19.Ljungman P, Zetterqvist L, Sundqvist V, Jeansson S, Heimdahl A, Wahren B. Lymphocyte and IgG responses to different herpes simplex virus antigens in patients with frequent HSV-1 recurrences. Clin Exp Immunol. 1988;72:207–210. [PMC free article] [PubMed] [Google Scholar]
- 20.Matloubian M, Concepcion R J, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milligan G N, Bernstein D I, Bourne N. T lymphocytes are required for protection of the vaginal mucosa and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093–6100. [PubMed] [Google Scholar]
- 22.Neophytou P I, Roep B O, Arden S D, Muir E M, Duinkerken G, Kallan A, de Vries R R, Hutton J C. T-cell epitope analysis using subtracted expression libraries (TEASEL): application to a 38 kDa autoantigen recognized by T cells from an insulin-dependent diabetic patient. Proc Natl Acad Sci USA. 1996;93:2014–2018. doi: 10.1073/pnas.93.5.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pereira R A, Simmons A. Cell surface expression of H2 antigens on primary sensory neurons in response to acute but not latent herpes simplex virus infection in vivo. J Virol. 1999;73:6484–6489. doi: 10.1128/jvi.73.8.6484-6489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira R A, Simon M M, Simmons A. Granzyme A, a noncytolytic component of CD8+ cell granules, restricts the spread of herpes simplex virus in the peripheral nervous systems of experimentally infected mice. J Virol. 2000;74:1029–1032. doi: 10.1128/jvi.74.2.1029-1032.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posavad C M, Koelle D M, Shaughnessy M F, Corey L. Severe genital herpes infections in HIV-infected individuals with impaired HSV-specific CD8+ cytotoxic T lymphocyte responses. Proc Natl Acad Sci USA. 1997;94:10289–10294. doi: 10.1073/pnas.94.19.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rammansee H G. Chemistry of peptides associated with MHC class I and class II molecules. Curr Opin Immunol. 1995;7:85–96. doi: 10.1016/0952-7915(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 27.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2231–2295. [Google Scholar]
- 28.Simmons A, Tscharke D C. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speck P, Simmons A. Precipitous clearance of herpes simplex virus antigens from the peripheral nervous systems of experimentally infected C57BL/10 mice. J Gen Virol. 1998;79:561–564. doi: 10.1099/0022-1317-79-3-561. [DOI] [PubMed] [Google Scholar]
- 30.Spruance S L, Chow F S. Pathogenesis of herpes simplex virus in cultures of epidermal cells from subjects with frequent recurrences. J Infect Dis. 1980;142:671–675. doi: 10.1093/infdis/142.5.671. [DOI] [PubMed] [Google Scholar]
- 31.Torseth J W, Cohen G H, Eisenberg R J, Berman P W, Lasky L A, Cerini C P, Heilman C J, Kerwar S, Merigan T C. Native and recombinant herpes simplex virus type 1 envelope proteins induce human immune T-lymphocyte responses. J Virol. 1987;61:1532–1539. doi: 10.1128/jvi.61.5.1532-1539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torseth J W, Merigan T C. Significance of local gamma interferon in recurrent herpes simplex infection. J Infect Dis. 1986;153:979–983. doi: 10.1093/infdis/153.5.979. [DOI] [PubMed] [Google Scholar]
- 33.Verjans G M, Dings M E, McLauchlan J, van Der Kooi A, Hoogerhout P, Brugghe H F, Timmermans H A, Baarsma G S, Osterhaus A D. Intraocular T cells of patients with herpes simplex virus (HSV)-induced acute retinal necrosis recognize HSV tegument proteins VP11/12 and VP13/14. J Infect Dis. 2000;182:923–927. doi: 10.1086/315759. [DOI] [PubMed] [Google Scholar]
- 34.Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women: effect of acyclovir treatment. J Clin Investig. 1997;99:1092–1097. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wald A, Zeh J, Selke S, Warren T, Ryncarz A J, Ashley R, Krieger J N, Corey L. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342:844–850. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 36.Westra D F, Verjans G M, Osterhaus A D, van Kooij A, Welling G W, Scheffer A J, The T H, Welling-Wester S. Natural infection with herpes simplex virus type 1 (HSV-1) induces humoral and T cell responses to the HSV-1 glycoprotein H:L complex. J Gen Virol. 2000;81:2011–2015. doi: 10.1099/0022-1317-81-8-2011. [DOI] [PubMed] [Google Scholar]
- 37.Yasukawa M, Inatsuki A, Horiuchi T, Kobayashi Y. Functional heterogeneity among herpes simplex virus-specific human CD4+ T cells. J Immunol. 1991;146:1341–1347. [PubMed] [Google Scholar]
- 38.Yasukawa M, Inatsuki A, Kobayashi Y. Helper activity in antigen-specific antibody production mediated by CD4+ human cytotoxic T cell clones directed against herpes simplex virus. J Immunol. 1988;140:3419–3425. [PubMed] [Google Scholar]
- 39.Yasukawa M, Kobayashi Y. Inhibition of herpes simplex virus replication in vitro by human cytotoxic T cell clones and natural killer cell clones. J Gen Virol. 1985;66:2225–2229. doi: 10.1099/0022-1317-66-10-2225. [DOI] [PubMed] [Google Scholar]
- 40.Zarling J M, Moran P A, Brewer L, Ashley R, Corey L. Herpes simplex virus (HSV)-specific proliferative and cytotoxic T-cell responses in humans immunized with an HSV type 2 glycoprotein subunit vaccine. J Virol. 1988;62:4481–4485. doi: 10.1128/jvi.62.12.4481-4485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarling J M, Moran P A, Burke R L, Pachl C, Berman P W, Lasky L A. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. IV. Recognition and activation by cloned glycoproteins gB and gD-1. J Immunol. 1986;136:4669–4673. [PubMed] [Google Scholar]