Abstract
Metallothionein (MT) is a potent hydroxyl radical scavenger but its antioxidant properties in vivo have not been defined. Most of the recent results indicate that it does not afford protection to cells against the lethal action of oxidative stress. However, the possibility that MT confers protection against oxidative damage to a specific cellular target, such as DNA, had not been considered. We compared V79 Chinese hamster cells enriched in and depleted of MT in terms of DNA-strand scission. Zinc induces an increase in MT content of V79 Chinese hamster cells, without concomitant increase in the GSH level. These induced cells are more resistant to the production of DNA-strand scission by H2O2 than the parental cells. Conversely, cells rendered partially deprived of MT, by transfection with a plasmid vector in which the MT-I cDNA is antisense oriented in relation to a simian virus 40 promoter, became more susceptible to the DNA-damaging action of H2O2. The transfected cells did not exhibit alterations of GSH, superoxide dismutase- and H2O2-destroying enzymes. Indirect immunofluorescence indicated that most of the MT was concentrated in the cell nucleus. Neither overexpression nor lower expression of MT resulted in differential resistance to the killing action of H2O2. However, the combined high nuclear concentration of MT and its excellent hydroxyl scavenger properties confer protection to DNA from hydroxyl radical attack.
Full text
PDF

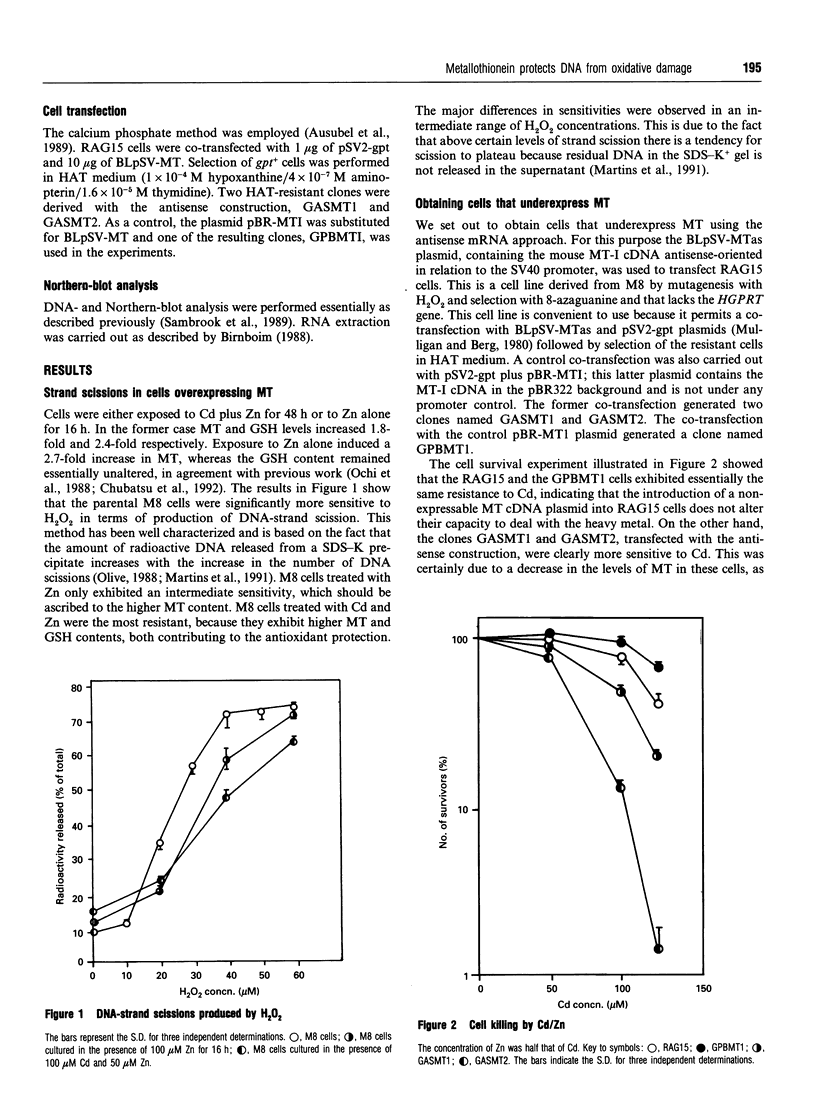
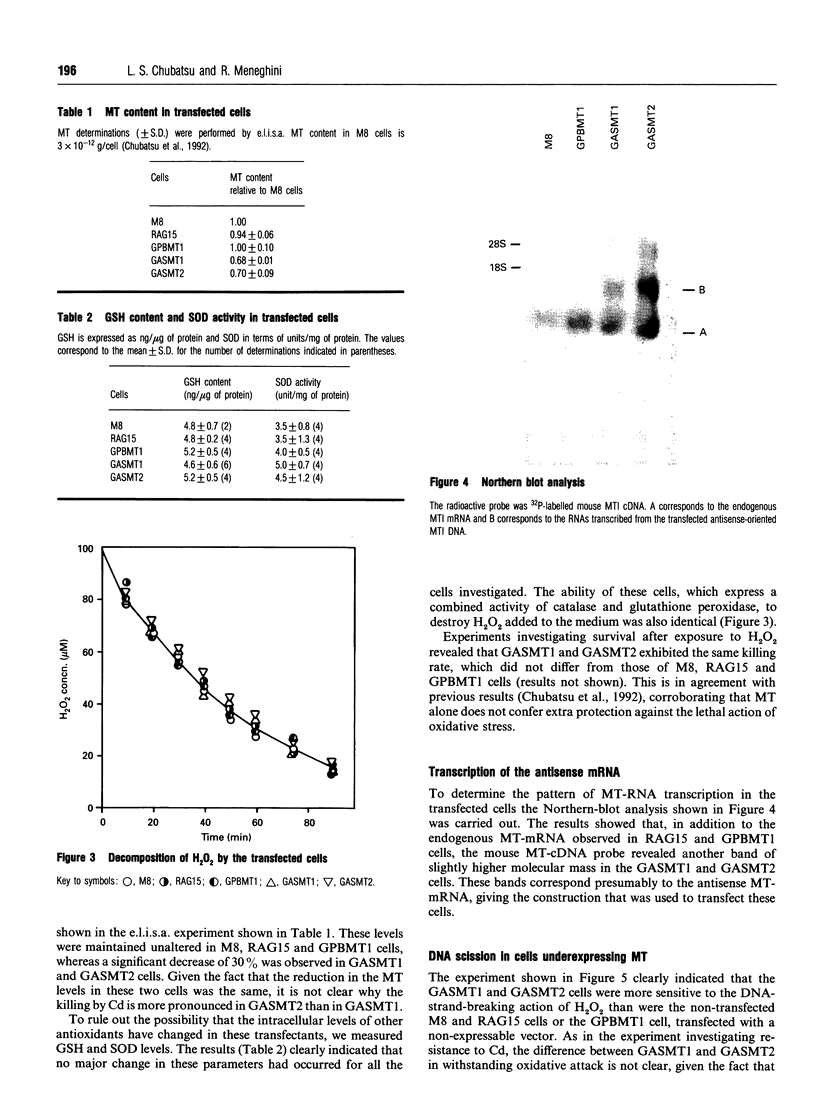
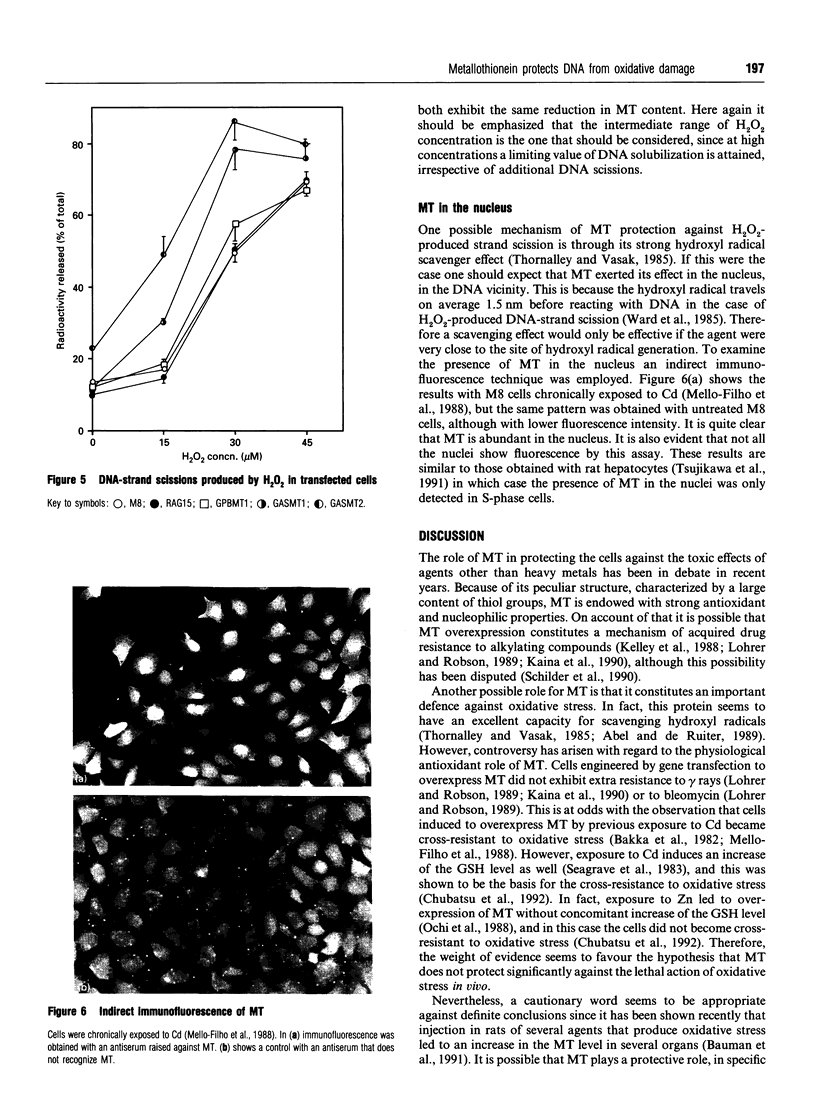

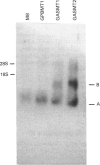
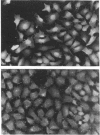
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel J., de Ruiter N. Inhibition of hydroxyl-radical-generated DNA degradation by metallothionein. Toxicol Lett. 1989 May;47(2):191–196. doi: 10.1016/0378-4274(89)90075-1. [DOI] [PubMed] [Google Scholar]
- Akerboom T. P., Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Dizdaroglu M. Iron ion-dependent modification of bases in DNA by the superoxide radical-generating system hypoxanthine/xanthine oxidase. J Biol Chem. 1989 Aug 5;264(22):13024–13028. [PubMed] [Google Scholar]
- Bakka A., Johnsen A. S., Endresen L., Rugstad H. E. Radioresistance in cells with high content of metallothionein. Experientia. 1982 Mar 15;38(3):381–383. doi: 10.1007/BF01949406. [DOI] [PubMed] [Google Scholar]
- Banerjee D., Onosaka S., Cherian M. G. Immunohistochemical localization of metallothionein in cell nucleus and cytoplasm of rat liver and kidney. Toxicology. 1982;24(2):95–105. doi: 10.1016/0300-483x(82)90048-8. [DOI] [PubMed] [Google Scholar]
- Bauman J. W., Liu J., Liu Y. P., Klaassen C. D. Increase in metallothionein produced by chemicals that induce oxidative stress. Toxicol Appl Pharmacol. 1991 Sep 1;110(2):347–354. doi: 10.1016/s0041-008x(05)80017-1. [DOI] [PubMed] [Google Scholar]
- Bellomo G., Vairetti M., Stivala L., Mirabelli F., Richelmi P., Orrenius S. Demonstration of nuclear compartmentalization of glutathione in hepatocytes. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4412–4416. doi: 10.1073/pnas.89.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. Rapid extraction of high molecular weight RNA from cultured cells and granulocytes for Northern analysis. Nucleic Acids Res. 1988 Feb 25;16(4):1487–1497. doi: 10.1093/nar/16.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubatsu L. S., Gennari M., Meneghini R. Glutathione is the antioxidant responsible for resistance to oxidative stress in V79 Chinese hamster fibroblasts rendered resistant to cadmium. Chem Biol Interact. 1992 Mar;82(1):99–110. doi: 10.1016/0009-2797(92)90017-f. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Perrin F., Gannon F., Palmiter R. D. Isolation and characterization of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6511–6515. doi: 10.1073/pnas.77.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M., Vasák M. Iron(II)-substituted metallothionein: evidence for the existence of iron-thiolate clusters. Biochemistry. 1986 Dec 30;25(26):8353–8356. doi: 10.1021/bi00374a003. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Kaina B., Lohrer H., Karin M., Herrlich P. Overexpressed human metallothionein IIA gene protects Chinese hamster ovary cells from killing by alkylating agents. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2710–2714. doi: 10.1073/pnas.87.7.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. Metallothioneins: proteins in search of function. Cell. 1985 May;41(1):9–10. doi: 10.1016/0092-8674(85)90051-0. [DOI] [PubMed] [Google Scholar]
- Kelley S. L., Basu A., Teicher B. A., Hacker M. P., Hamer D. H., Lazo J. S. Overexpression of metallothionein confers resistance to anticancer drugs. Science. 1988 Sep 30;241(4874):1813–1815. doi: 10.1126/science.3175622. [DOI] [PubMed] [Google Scholar]
- Knight S. A., Sunde R. A. The effect of progressive selenium deficiency on anti-glutathione peroxidase antibody reactive protein in rat liver. J Nutr. 1987 Apr;117(4):732–738. doi: 10.1093/jn/117.4.732. [DOI] [PubMed] [Google Scholar]
- Korbashi P., Katzhendler J., Saltman P., Chevion M. Zinc protects Escherichia coli against copper-mediated paraquat-induced damage. J Biol Chem. 1989 May 25;264(15):8479–8482. [PubMed] [Google Scholar]
- Lohrer H., Robson T. Overexpression of metallothionein in CHO cells and its effect on cell killing by ionizing radiation and alkylating agents. Carcinogenesis. 1989 Dec;10(12):2279–2284. doi: 10.1093/carcin/10.12.2279. [DOI] [PubMed] [Google Scholar]
- Martins E. A., Chubatsu L. S., Meneghini R. Role of antioxidants in protecting cellular DNA from damage by oxidative stress. Mutat Res. 1991 Sep-Oct;250(1-2):95–101. doi: 10.1016/0027-5107(91)90166-l. [DOI] [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Mello-Filho A. C., Chubatsu L. S., Meneghini R. V79 Chinese-hamster cells rendered resistant to high cadmium concentration also become resistant to oxidative stress. Biochem J. 1988 Dec 1;256(2):475–479. doi: 10.1042/bj2560475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Nassi-Calò L., Mello-Filho C., Meneghini R. o-phenanthroline protects mammalian cells from hydrogen peroxide-induced gene mutation and morphological transformation. Carcinogenesis. 1989 Jun;10(6):1055–1057. doi: 10.1093/carcin/10.6.1055. [DOI] [PubMed] [Google Scholar]
- Ochi T., Otsuka F., Takahashi K., Ohsawa M. Glutathione and metallothioneins as cellular defense against cadmium toxicity in cultured Chinese hamster cells. Chem Biol Interact. 1988;65(1):1–14. doi: 10.1016/0009-2797(88)90026-9. [DOI] [PubMed] [Google Scholar]
- Olive P. L. DNA precipitation assay: a rapid and simple method for detecting DNA damage in mammalian cells. Environ Mol Mutagen. 1988;11(4):487–495. doi: 10.1002/em.2850110409. [DOI] [PubMed] [Google Scholar]
- Oyanagui Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem. 1984 Nov 1;142(2):290–296. doi: 10.1016/0003-2697(84)90467-6. [DOI] [PubMed] [Google Scholar]
- Schilder R. J., Hall L., Monks A., Handel L. M., Fornace A. J., Jr, Ozols R. F., Fojo A. T., Hamilton T. C. Metallothionein gene expression and resistance to cisplatin in human ovarian cancer. Int J Cancer. 1990 Mar 15;45(3):416–422. doi: 10.1002/ijc.2910450306. [DOI] [PubMed] [Google Scholar]
- Seagrave J., Hildebrand C. E., Enger M. D. Effects of cadmium on glutathione metabolism in cadmium sensitive and cadmium resistant Chinese hamster cell lines. Toxicology. 1983 Dec;29(1-2):101–107. doi: 10.1016/0300-483x(83)90042-2. [DOI] [PubMed] [Google Scholar]
- Thomas J. P., Bachowski G. J., Girotti A. W. Inhibition of cell membrane lipid peroxidation by cadmium- and zinc-metallothioneins. Biochim Biophys Acta. 1986 Dec 10;884(3):448–461. doi: 10.1016/0304-4165(86)90195-9. [DOI] [PubMed] [Google Scholar]
- Thornalley P. J., Vasák M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta. 1985 Jan 21;827(1):36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- Tsujikawa K., Imai T., Kakutani M., Kayamori Y., Mimura T., Otaki N., Kimura M., Fukuyama R., Shimizu N. Localization of metallothionein in nuclei of growing primary cultured adult rat hepatocytes. FEBS Lett. 1991 Jun 3;283(2):239–242. doi: 10.1016/0014-5793(91)80597-v. [DOI] [PubMed] [Google Scholar]
- Ward J. F., Blakely W. F., Joner E. I. Mammalian cells are not killed by DNA single-strand breaks caused by hydroxyl radicals from hydrogen peroxide. Radiat Res. 1985 Sep;103(3):383–392. [PubMed] [Google Scholar]
- de Mello Filho A. C., Meneghini R. Protection of mammalian cells by o-phenanthroline from lethal and DNA-damaging effects produced by active oxygen species. Biochim Biophys Acta. 1985 Oct 30;847(1):82–89. doi: 10.1016/0167-4889(85)90156-9. [DOI] [PubMed] [Google Scholar]