Abstract
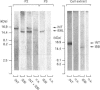
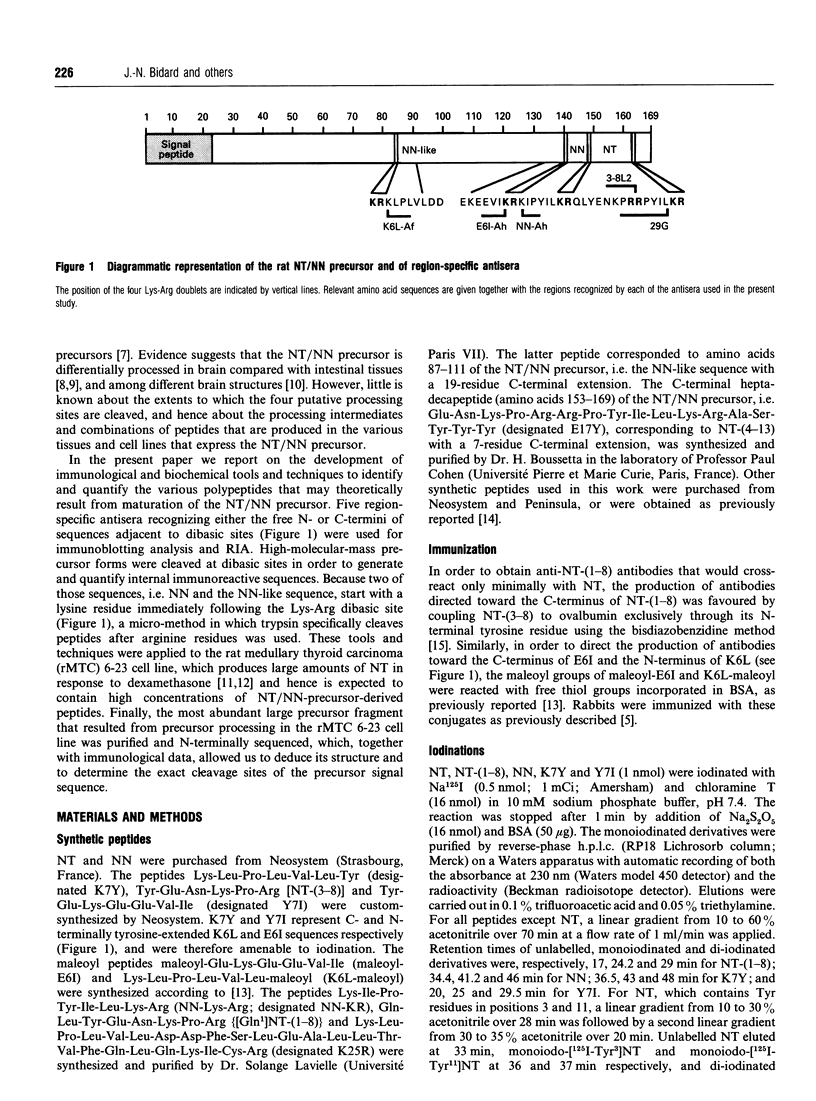
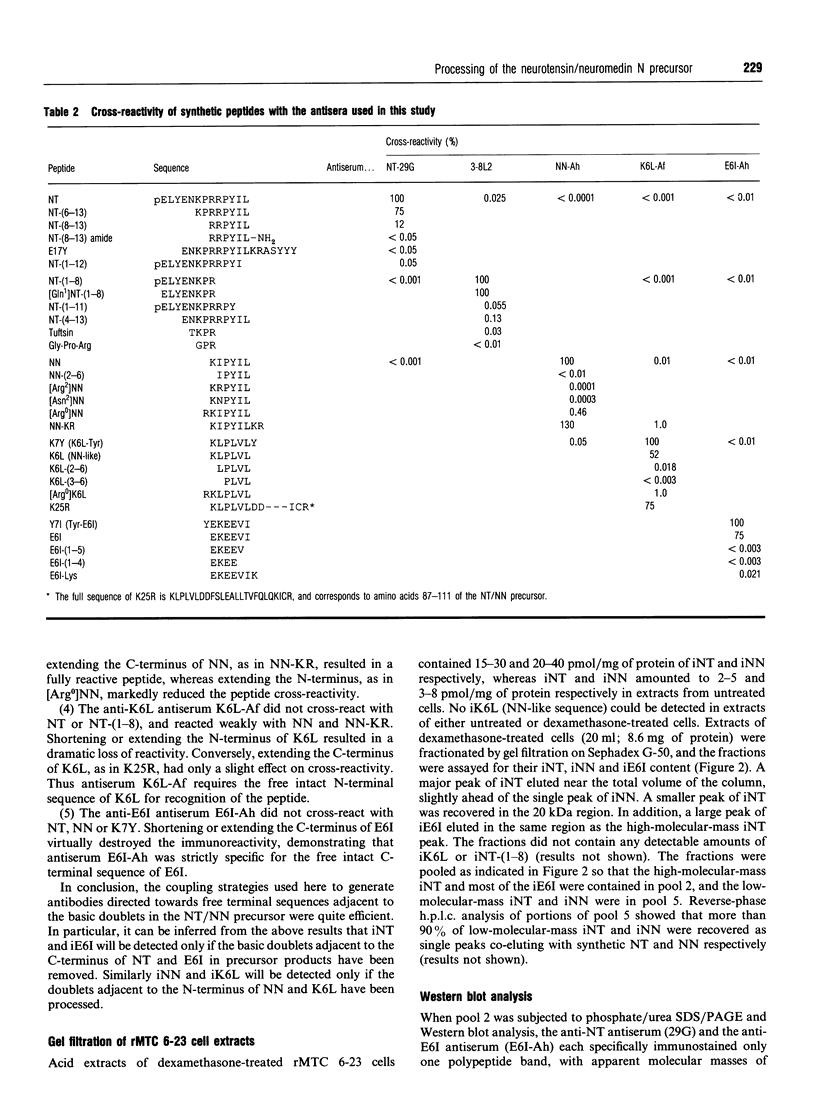
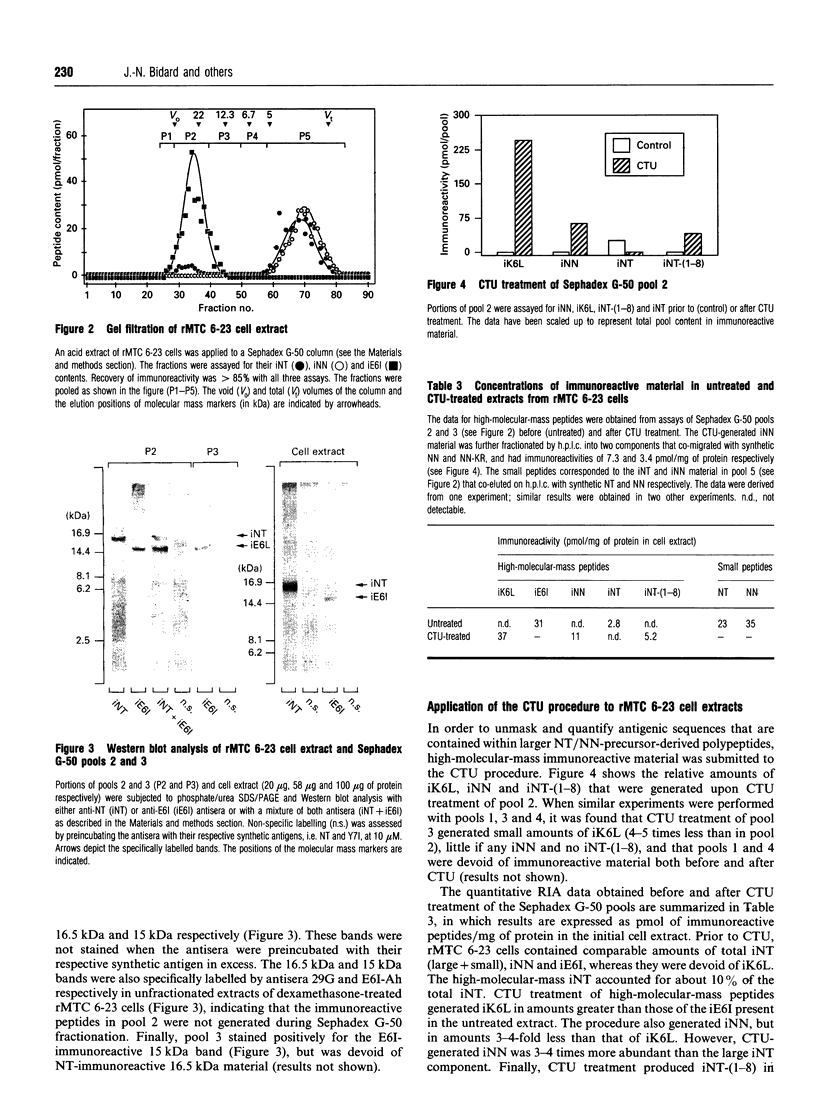
Neurotensin (NT) and neuromedin N (NN) are two related biologically active peptides that are encoded in the same precursor molecule. In the rat, the precursor consists of a 169-residue polypeptide starting with an N-terminal signal peptide and containing in its C-terminal region one copy each of NT and NN. NN precedes NT and is separated from it by a Lys-Arg sequence. Two other Lys-Arg sequences flank the N-terminus of NN and the C-terminus of NT. A fourth Lys-Arg sequence occurs near the middle of the precursor and is followed by an NN-like sequence. Finally, an Arg-Arg pair is present within the NT moiety. The four Lys-Arg doublets represent putative processing sites in the precursor molecule. The present study was designed to investigate the post-translational processing of the NT/NN precursor in the rat medullary thyroid carcinoma (rMTC) 6-23 cell line, which synthesizes large amounts of NT upon dexamethasone treatment. Five region-specific antisera recognizing the free N- or C-termini of sequences adjacent to the basic doublets were produced, characterized and used for immunoblotting and radioimmunoassay studies in combination with gel filtration, reverse-phase h.p.l.c. and trypsin digestion of rMTC 6-23 cell extracts. Because two of the antigenic sequences, i.e. NN and the NN-like sequence, start with a lysine residue that is essential for recognition by their respective antisera, a micromethod by which trypsin specifically cleaves at arginine residues was developed. The results show that dexamethasone-treated rMTC 6-23 cells produced comparable amounts of NT, NN and a peptide corresponding to a large N-terminal precursor fragment lacking the NN and NT moieties. This large fragment was purified. N-Terminal sequencing revealed that it started at residue Ser23 of the prepro-NT/NN sequence, and thus established the Cys22-Ser23 bond as the cleavage site of the signal peptide. Two other large N-terminal fragments bearing respectively the NN and NT sequences at their C-termini were present in lower amounts. The NN-like sequence was internal to all the large fragments. There was no evidence for the presence of peptides with the NN-like sequence at their N-termini. This shows that, in rMTC 6-23 cells, the precursor is readily processed at the three Lys-Arg doublets that flank and separate the NT and NN sequences. In contrast, the Lys-Arg doublet that precedes the NN-like sequence is not processed in this system.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blache P., Kervran A., Martinez J., Bataille D. Development of an oxyntomodulin/glicentin C-terminal radioimmunoassay using a "thiol-maleoyl" coupling method for preparing the immunogen. Anal Biochem. 1988 Aug 15;173(1):151–159. doi: 10.1016/0003-2697(88)90172-8. [DOI] [PubMed] [Google Scholar]
- Brakch N., Boussetta H., Rholam M., Cohen P. Processing endoprotease recognizes a structural feature at the cleavage site of peptide prohormones. The pro-ocytocin/neurophysin model. J Biol Chem. 1989 Sep 25;264(27):15912–15916. [PubMed] [Google Scholar]
- Carraway R. E., Mitra S. P. Differential processing of neurotensin/neuromedin N precursor(s) in canine brain and intestine. J Biol Chem. 1990 May 25;265(15):8627–8631. [PubMed] [Google Scholar]
- Carraway R. E., Mitra S. P. Purification of large neuromedin N (NMN) from canine intestine and its identification as NMN-125. Biochem Biophys Res Commun. 1991 Aug 30;179(1):301–308. doi: 10.1016/0006-291x(91)91369-n. [DOI] [PubMed] [Google Scholar]
- Carraway R. E., Mitra S. P. The use of radioimmunoassay to compare the tissue and subcellular distributions of neurotensin and neuromedin N in the cat. Endocrinology. 1987 May;120(5):2092–2100. doi: 10.1210/endo-120-5-2092. [DOI] [PubMed] [Google Scholar]
- Carraway R., Leeman S. E. Radioimmunoassay for neurotensin, a hypothalamic peptide. J Biol Chem. 1976 Nov 25;251(22):7035–7044. [PubMed] [Google Scholar]
- Cuber J. C., Herrmann C., Kitabgi P., Bosshard A., Bernard C., De Nadai F., Chayvialle J. A. Neuromedin-N is not released with neurotensin from rat ileum. Endocrinology. 1990 Mar;126(3):1584–1592. doi: 10.1210/endo-126-3-1584. [DOI] [PubMed] [Google Scholar]
- Cupo A., Pontarotti P. A., Jarry T., Delaage M. A new immunological approach to the detection and the quantitation of the Met5-enkephalin precursors in rat brain. Neuropeptides. 1984 Sep;4(5):375–387. doi: 10.1016/0143-4179(84)90113-6. [DOI] [PubMed] [Google Scholar]
- Cupo A., Rougon-Rapuzzi G., Delaage M. A. Immunochemical detection of vasopressin precursors: artificial processing and quantification along the hypothalamo-hypophysial axis. Eur J Biochem. 1981 Mar 16;115(1):169–174. doi: 10.1111/j.1432-1033.1981.tb06213.x. [DOI] [PubMed] [Google Scholar]
- Dobner P. R., Barber D. L., Villa-Komaroff L., McKiernan C. Cloning and sequence analysis of cDNA for the canine neurotensin/neuromedin N precursor. Proc Natl Acad Sci U S A. 1987 May;84(10):3516–3520. doi: 10.1073/pnas.84.10.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Granier C., van Rietschoten J., Kitabgi P., Poustis C., Freychet P. Synthesis and characterization of neurotensin analogues for structure/activity relationship studies. Acetyl-neurotensin-(8--13) is the shortest analogue with full binding and pharmacological activities. Eur J Biochem. 1982 May;124(1):117–124. doi: 10.1111/j.1432-1033.1982.tb05913.x. [DOI] [PubMed] [Google Scholar]
- Kislauskis E., Bullock B., McNeil S., Dobner P. R. The rat gene encoding neurotensin and neuromedin N. Structure, tissue-specific expression, and evolution of exon sequences. J Biol Chem. 1988 Apr 5;263(10):4963–4968. [PubMed] [Google Scholar]
- Kitabgi P., De Nadai F., Cuber J. C., Dubuc I., Nouel D., Costentin J. Calcium-dependent release of neuromedin N and neurotensin from mouse hypothalamus. Neuropeptides. 1990 Feb;15(2):111–114. doi: 10.1016/0143-4179(90)90047-3. [DOI] [PubMed] [Google Scholar]
- Kitabgi P., Masuo Y., Nicot A., Berod A., Cuber J. C., Rostène W. Marked variations of the relative distributions of neurotensin and neuromedin N in micropunched rat brain areas suggest differential processing of their common precursor. Neurosci Lett. 1991 Mar 11;124(1):9–12. doi: 10.1016/0304-3940(91)90810-g. [DOI] [PubMed] [Google Scholar]
- Odum L., Rehfeld J. F. Expression and processing of procholecystokinin in a rat medullary thyroid carcinoma cell line. Biochem J. 1990 Oct 1;271(1):31–36. doi: 10.1042/bj2710031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw C., McKay D., Johnston C. F., Halton D. W., Fairweather I., Kitabgi P., Buchanan K. D. Differential processing of the neurotensin/neuromedin N precursor in the mouse. Peptides. 1990 Mar-Apr;11(2):227–235. doi: 10.1016/0196-9781(90)90075-g. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Zeytinoğlu F. N., Brazeau P., Mougin C. Regulation of neurotensin secretion in a mammalian C cell line: effect of dexamethasone. Regul Pept. 1983 May;6(2):147–154. doi: 10.1016/0167-0115(83)90007-1. [DOI] [PubMed] [Google Scholar]
- Zeytinoğlu F. N., Gagel R. F., Tashjian A. H., Jr, Hammer R. A., Leeman S. E. Characterization of neurotensin production by a line of rat medullary thyroid carcinoma cells. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3741–3745. doi: 10.1073/pnas.77.6.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nadai F., Cuber J. C., Kitabgi P. The characterization and regional distribution of neuromedin N-like immunoreactivity in rat brain using a highly sensitive and specific radioimmunoassay. Comparison with the distribution of neurotensin. Brain Res. 1989 Oct 23;500(1-2):193–198. doi: 10.1016/0006-8993(89)90313-2. [DOI] [PubMed] [Google Scholar]