Abstract
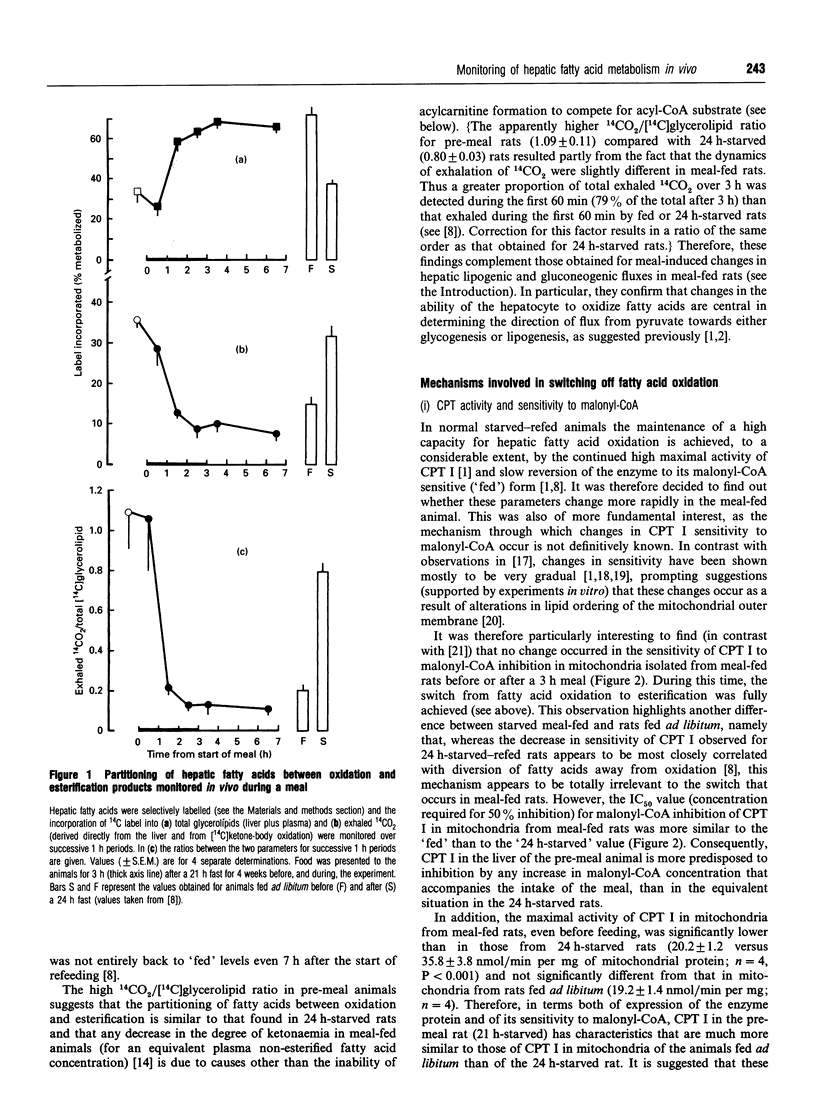
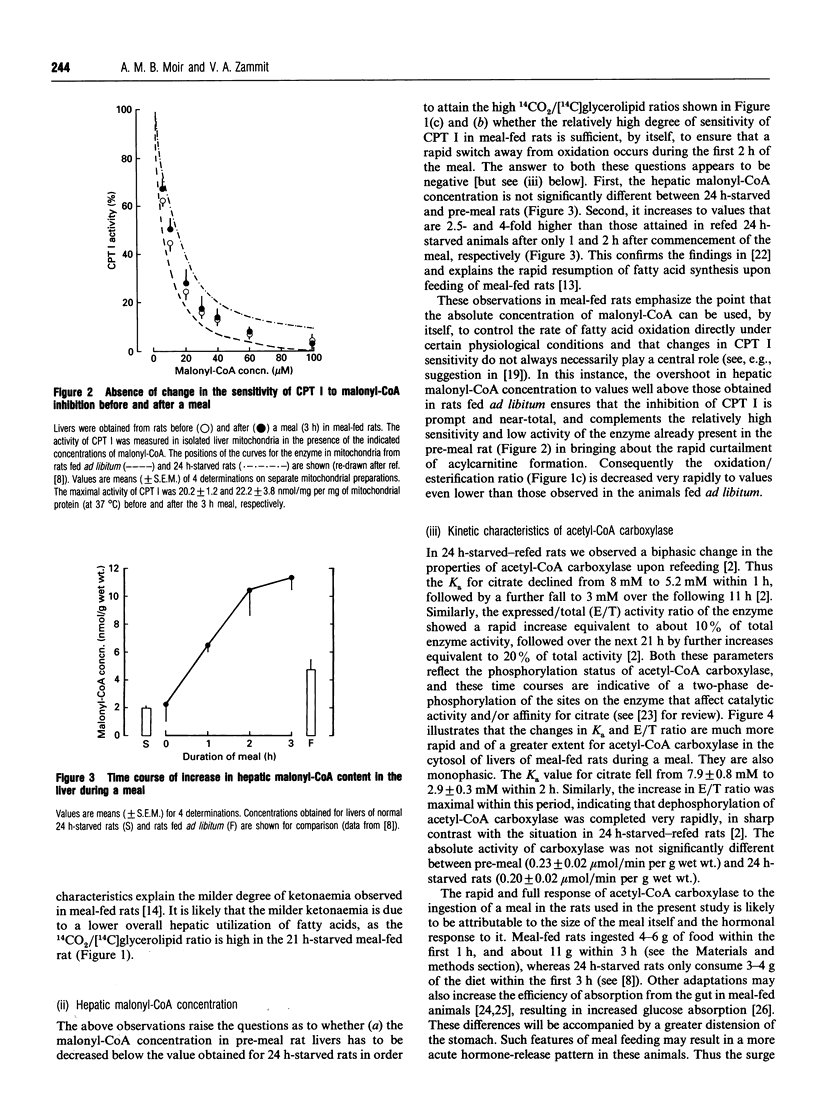
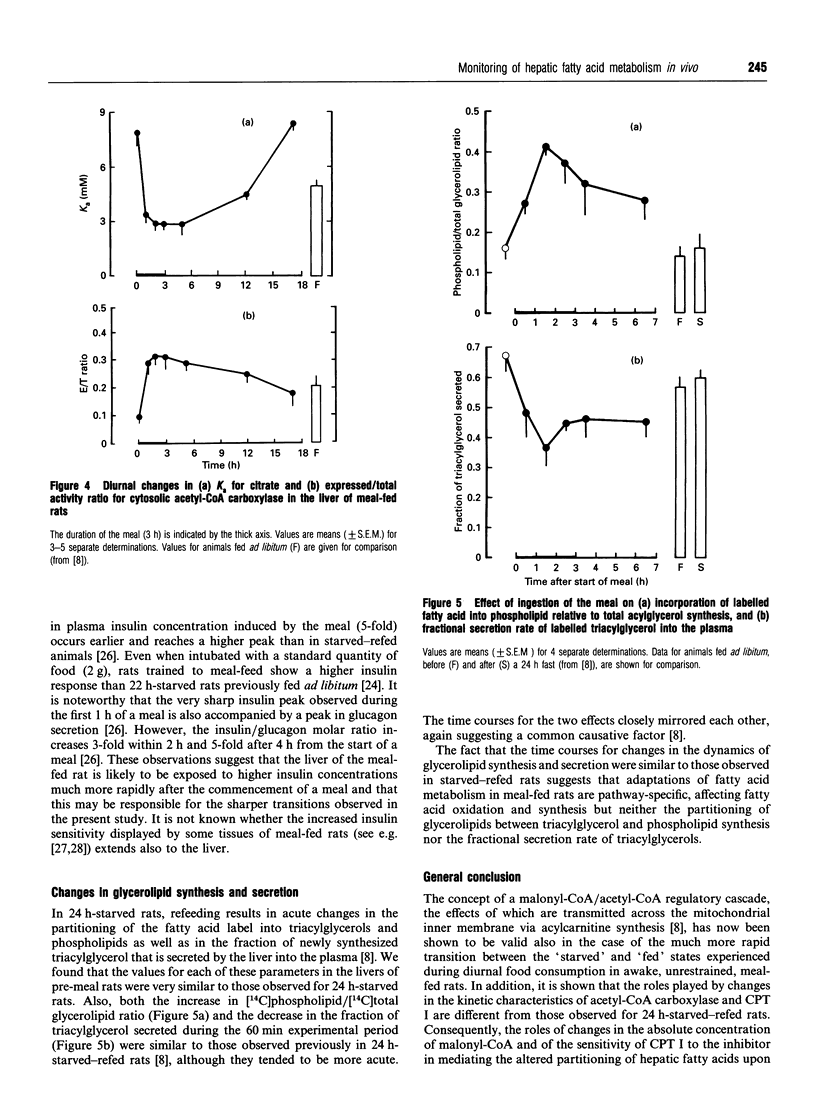
The effects of the ingestion of a meal on the partitioning of hepatic fatty acids between oxidation and esterification were studied in vivo for meal-fed rats. The time course for the reversal of the starved state was extremely rapid and the process was complete within 2 h, in marked contrast with the reversal of the effects of starvation in rats fed ad libitum [A. M. B. Moir and V. A. Zammit (1993) Biochem. J. 289, 49-55]. This rapid reversal occurred in spite of the fact that, in the liver of the meal-fed animals before feeding, a similar degree of partitioning of fatty acids in favour of oxidation was observed as in 24 h-starved rats (previously fed ad libitum). This suggested that the lower degree of ketonaemia observed in meal-fed rats before a meal is not due to the inability of acylcarnitine formation to compete successfully with esterification of fatty acids to the glycerol moiety. Investigation of the possible mechanisms that could contribute towards the rapid switching-off of fatty acid oxidation revealed that this was correlated with a very rapid rise and overshoot in hepatic malonyl-CoA concentration, but not with any change in the activity, or sensitivity to malonyl-CoA, of the mitochondrial overt carnitine palmitoyltransferase (CPT I). The role of these two parameters in the reversal of fasting-induced hepatic fatty acid oxidation was thus the inverse of that observed previously for refed 24 h-starved rats. The rapid increase in [malonyl-CoA] was accompanied by an immediate and complete reversion of the kinetic characteristics (Ka for citrate, expressed/total activity ratio) of acetyl-CoA carboxylase to those found in the post-meal animals, again in contrast with the time course observed in refed 24 h-starved rats [A. M. B. Moir and V. A. Zammit (1990) Biochem. J. 272, 511-517]. The rapidity with which these changes occurred was specific to the partitioning of acyl-CoA; the meal-induced diversion of glycerolipids towards phospholipid synthesis and the acute inhibition of the fractional rate of triacylglycerol secretion occurred with very similar time courses to those observed upon refeeding of 24 h-starved rats. The results confirm the central role played by differences in the dynamics of changes in hepatic malonyl-CoA concentration, and CPT I sensitivity to it, in determining the route through which ingested glucose is converted into hepatic glycogen upon refeeding of starved rats which had previously been meal-fed or fed ad libitum.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius L., Williamson D. H. The utilization of ketone bodies by the interscapular brown adipose tissue of the rat. Biochim Biophys Acta. 1981 Oct 23;666(1):127–132. doi: 10.1016/0005-2760(81)90098-9. [DOI] [PubMed] [Google Scholar]
- Dobson G. P., Veech R. L., Passonneau J. V., Huang M. T. In vivo portal-hepatic venous gradients of glycogenic precursors and incorporation of D-[3-3H]glucose into liver glycogen in the awake rat. J Biol Chem. 1990 Sep 25;265(27):16350–16357. [PubMed] [Google Scholar]
- Easom R. A., Zammit V. A. A cold-clamping technique for the rapid sampling of rat liver for studies on enzymes in separate cell fractions. Suitability for the study of enzymes regulated by reversible phosphorylation-dephosphorylation. Biochem J. 1984 Jun 15;220(3):733–738. doi: 10.1042/bj2200733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escrivá F., Pascual-Leone A. M., Hernández J., Ferré P., Girard J. Effect of feeding pattern on the sensitivity of hepatic carnitine palmitoyl-transferase to inhibition by malonyl-CoA in the rat. Comp Biochem Physiol A Comp Physiol. 1987;87(4):1041–1043. doi: 10.1016/0300-9629(87)90035-1. [DOI] [PubMed] [Google Scholar]
- Gamble M. S., Cook G. A. Alteration of the apparent Ki of carnitine palmitoyltransferase for malonyl-CoA by the diabetic state and reversal by insulin. J Biol Chem. 1985 Aug 15;260(17):9516–9519. [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Restoration of the properties of carnitine palmitoyltransferase I in liver mitochondria during re-feeding of starved rats. Biochem J. 1986 Oct 15;239(2):485–488. doi: 10.1042/bj2390485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Role of carnitine palmitoyltransferase I in the regulation of hepatic ketogenesis during the onset and reversal of chronic diabetes. Biochem J. 1988 Jan 15;249(2):409–414. doi: 10.1042/bj2490409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie D. G. Regulation of fatty acid synthesis via phosphorylation of acetyl-CoA carboxylase. Prog Lipid Res. 1989;28(2):117–146. doi: 10.1016/0163-7827(89)90010-6. [DOI] [PubMed] [Google Scholar]
- Holness M. J., French T. J., Sugden M. C. Hepatic glycogen synthesis on carbohydrate re-feeding after starvation. A regulatory role for pyruvate dehydrogenase in liver and extrahepatic tissues. Biochem J. 1986 Apr 15;235(2):441–445. doi: 10.1042/bj2350441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M. J., Sugden M. C. Comparison of tissue pyruvate dehydrogenase activities on re-feeding rats fed ad libitum or meal-fed rats with a chow-diet meal. Biochem J. 1989 Aug 15;262(1):321–325. doi: 10.1042/bj2620321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. T., Veech R. L. Role of the direct and indirect pathways for glycogen synthesis in rat liver in the postprandial state. J Clin Invest. 1988 Mar;81(3):872–878. doi: 10.1172/JCI113397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip M. M., Ip C., Tepperman H. M., Tepperman J. Effect of adaptation to meal-feeding on insulin, glucagon and the cyclic nucleotide-protein kinase system in rats. J Nutr. 1977 May;107(5):746–757. doi: 10.1093/jn/107.5.746. [DOI] [PubMed] [Google Scholar]
- Kolodziej M. P., Zammit V. A. Sensitivity of inhibition of rat liver mitochondrial outer-membrane carnitine palmitoyltransferase by malonyl-CoA to chemical- and temperature-induced changes in membrane fluidity. Biochem J. 1990 Dec 1;272(2):421–425. doi: 10.1042/bj2720421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A. M., Zammit V. A. Changes in the properties of cytosolic acetyl-CoA carboxylase studied in cold-clamped liver samples from fed, starved and starved-refed rats. Biochem J. 1990 Dec 1;272(2):511–517. doi: 10.1042/bj2720511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A. M., Zammit V. A. Monitoring of changes in hepatic fatty acid and glycerolipid metabolism during the starved-to-fed transition in vivo. Studies on awake, unrestrained rats. Biochem J. 1993 Jan 1;289(Pt 1):49–55. doi: 10.1042/bj2890049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A. M., Zammit V. A. Selective labelling of hepatic fatty acids in vivo. Studies on the synthesis and secretion of glycerolipids in the rat. Biochem J. 1992 Apr 1;283(Pt 1):145–149. doi: 10.1042/bj2830145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard C. B., Foster D. W., McGarry J. D. Evidence for suppression of hepatic glucose-6-phosphatase with carbohydrate feeding. Diabetes. 1984 Feb;33(2):192–195. doi: 10.2337/diab.33.2.192. [DOI] [PubMed] [Google Scholar]
- O'Dea K., Kaplovitz H., Marino A. Effect of meal-feeding on insulin sensitivity and incorporation of [U-14C] glucose into lipids in rat aorta. J Nutr. 1977 Oct;107(10):1896–1901. doi: 10.1093/jn/107.10.1896. [DOI] [PubMed] [Google Scholar]
- Pallardo F. V., Williamson D. H. Comparison of the flux of carbon to hepatic glycogen deposition and fatty acid and cholesterol synthesis on refeeding rats fed ad libitum or meal-fed rats with a chow-diet meal. Biochem J. 1989 Jan 15;257(2):607–610. doi: 10.1042/bj2570607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prip-Buus C., Pegorier J. P., Duee P. H., Kohl C., Girard J. Evidence that the sensitivity of carnitine palmitoyltransferase I to inhibition by malonyl-CoA is an important site of regulation of hepatic fatty acid oxidation in the fetal and newborn rabbit. Perinatal development and effects of pancreatic hormones in cultured rabbit hepatocytes. Biochem J. 1990 Jul 15;269(2):409–415. doi: 10.1042/bj2690409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Stakkestad J. A., Bremer J., Borrebaek B. Determination of malonyl-coenzyme A in rat heart, kidney, and liver: a comparison between acetyl-coenzyme A and butyryl-coenzyme A as fatty acid synthase primers in the assay procedure. Anal Biochem. 1984 Apr;138(1):107–111. doi: 10.1016/0003-2697(84)90776-0. [DOI] [PubMed] [Google Scholar]
- Sugden M. C., Watts D. I., Palmer T. N., Myles D. D. Direction of carbon flux in starvation and after refeeding: in vitro and in vivo effects of 3-mercaptopicolinate. Biochem Int. 1983 Sep;7(3):329–337. [PubMed] [Google Scholar]
- Vrána A., Fábry P., Braun T. Insulin sensitivity of adipose tissue and of diaphragm in rats adapted to periodic hyperphagia. Diabetologia. 1969 Oct;5(5):300–303. doi: 10.1007/BF00452902. [DOI] [PubMed] [Google Scholar]
- Wiley J. H., Leveille G. A. Significance of insulin in the metabolic adaptation of rats to meal ingestion. J Nutr. 1970 Sep;100(9):1073–1080. doi: 10.1093/jn/100.9.1073. [DOI] [PubMed] [Google Scholar]
- Wilson M. D., Blake W. L., Salati L. M., Clarke S. D. Potency of polyunsaturated and saturated fats as short-term inhibitors of hepatic lipogenesis in rats. J Nutr. 1990 Jun;120(6):544–552. doi: 10.1093/jn/120.6.544. [DOI] [PubMed] [Google Scholar]


