Abstract
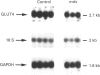
Glucose transporter protein levels have been investigated in mdx and control (C57Bl/10) mice. Crude membrane fractions (microsomes plus plasma membranes) were prepared from skeletal muscle, heart, diaphragm and brain of 5-6-week-old and 6-7-month-old control and mdx mice. Using Western blot analysis with C-terminal-specific anti-peptide antibodies, we investigated the glucose transporters GLUT4 in the different muscle tissues and GLUT1 in brain. In skeletal tissue from the hindlegs, GLUT4 was increased by approximately 55% in mdx mice compared with control mice at both ages studied. In the diaphragm, the amount of GLUT4 protein was unchanged in young mdx mice, and was decreased by 37.4 +/- 4.7% in older mice compared with age-matched control mice. No difference was observed between mdx and control mice in the amounts of GLUT4 and GLUT1 in heart and brain preparations respectively. To determine whether the change in GLUT4 protein observed in the diaphragm and skeletal muscle of mdx mice was regulated through changes at the level of glucose transporter mRNA, Northern blot analyses were performed. In skeletal muscle, GLUT4 mRNA level per tissue was not different between the two groups of mice at both ages studied. In contrast, the decrease in the amount of GLUT4 protein observed in the diaphragm of 6-7-month-old mdx mice was accompanied by a decrease in the GLUT4 mRNA level. In conclusion, the levels of GLUT4 protein were modified in muscle tissues from mdx compared with control mice, and these modifications were different depending on the muscle involved and the age of the mice. An increase in the amount of GLUT4 protein in the skeletal muscle of mdx mice was not due to changes at the mRNA level. The diaphragms of 6-7-month-old mdx mice exhibited decreases in GLUT4 protein and mRNA levels that were not detected in young animals (5-6 weeks old).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Eguchi C., Ishihara T., Nonaka I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988 Jun 30;333(6176):861–863. doi: 10.1038/333861a0. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Bonilla E., Samitt C. E., Miranda A. F., Hays A. P., Salviati G., DiMauro S., Kunkel L. M., Hoffman E. P., Rowland L. P. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988 Aug 12;54(4):447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- Bridges L. R. The association of cardiac muscle necrosis and inflammation with the degenerative and persistent myopathy of MDX mice. J Neurol Sci. 1986 Feb;72(2-3):147–157. doi: 10.1016/0022-510x(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Bulfield G., Siller W. G., Wight P. A., Moore K. J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnwath J. W., Shotton D. M. Muscular dystrophy in the mdx mouse: histopathology of the soleus and extensor digitorum longus muscles. J Neurol Sci. 1987 Aug;80(1):39–54. doi: 10.1016/0022-510x(87)90219-x. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. S., Pearlman J. A., Muzny D. M., Gibbs R. A., Ranier J. E., Caskey C. T., Reeves A. A. Expression of the murine Duchenne muscular dystrophy gene in muscle and brain. Science. 1988 Mar 18;239(4846):1416–1418. doi: 10.1126/science.3347839. [DOI] [PubMed] [Google Scholar]
- Charron M. J., Brosius F. C., 3rd, Alper S. L., Lodish H. F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christie K. N. Chymostatin has no apparent beneficial effect on muscular dystrophy in the mdx mouse. J Neurol Sci. 1988 Apr;84(2-3):341–346. doi: 10.1016/0022-510x(88)90138-4. [DOI] [PubMed] [Google Scholar]
- Cullen M. J., Jaros E. Ultrastructure of the skeletal muscle in the X chromosome-linked dystrophic (mdx) mouse. Comparison with Duchenne muscular dystrophy. Acta Neuropathol. 1988;77(1):69–81. doi: 10.1007/BF00688245. [DOI] [PubMed] [Google Scholar]
- Dangain J., Vrbova G. Muscle development in mdx mutant mice. Muscle Nerve. 1984 Nov-Dec;7(9):700–704. doi: 10.1002/mus.880070903. [DOI] [PubMed] [Google Scholar]
- Douen A. G., Ramlal T., Rastogi S., Bilan P. J., Cartee G. D., Vranic M., Holloszy J. O., Klip A. Exercise induces recruitment of the "insulin-responsive glucose transporter". Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem. 1990 Aug 15;265(23):13427–13430. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Glesby M. J., Rosenmann E., Nylen E. G., Wrogemann K. Serum CK, calcium, magnesium, and oxidative phosphorylation in mdx mouse muscular dystrophy. Muscle Nerve. 1988 Aug;11(8):852–856. doi: 10.1002/mus.880110809. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Bell G. I. Facilitative glucose transporters: an expanding family. Trends Biochem Sci. 1990 Jan;15(1):18–23. doi: 10.1016/0968-0004(90)90125-u. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Jockusch H., Friedrich G., Zippel M. Serum parvalbumin, an indicator of muscle disease in murine dystrophy and myotonia. Muscle Nerve. 1990 Jun;13(6):551–555. doi: 10.1002/mus.880130613. [DOI] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Young D. A., Holloszy J. O. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987 Nov 16;224(1):224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Jeanrenaud B., Freychet P. Insulin binding and effects in isolated soleus muscle of lean and obese mice. Am J Physiol. 1978 Apr;234(4):E348–E358. doi: 10.1152/ajpendo.1978.234.4.E348. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y., Olichon-Berthe C., Grémeaux T., Tanti J. F., Rochet N., Van Obberghen E. Glucose transporter in insulin sensitive tissues of lean and obese mice. Effect of the thermogenic agent BRL 26830A. Endocrinology. 1990 Dec;127(6):2687–2695. doi: 10.1210/endo-127-6-2687. [DOI] [PubMed] [Google Scholar]
- MacLennan P. A., Edwards R. H. Protein turnover is elevated in muscle of mdx mice in vivo. Biochem J. 1990 Jun 15;268(3):795–797. doi: 10.1042/bj2680795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan P. A., McArdle A., Edwards R. H. Acute effects of phorbol esters on the protein-synthetic rate and carbohydrate metabolism of normal and mdx mouse muscles. Biochem J. 1991 Apr 15;275(Pt 2):477–483. doi: 10.1042/bj2750477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco A. P. Dystrophin, the protein product of the Duchenne/Becker muscular dystrophy gene. Trends Biochem Sci. 1989 Oct;14(10):412–415. doi: 10.1016/0968-0004(89)90290-9. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K., Ervasti J. M., Snook J. B., Campbell K. P. Dystrophin-glycoprotein complex is highly enriched in isolated skeletal muscle sarcolemma. J Cell Biol. 1991 Jan;112(1):135–148. doi: 10.1083/jcb.112.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons F., Augier N., Heilig R., Léger J., Mornet D., Léger J. J. Isolated dystrophin molecules as seen by electron microscopy. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7851–7855. doi: 10.1073/pnas.87.20.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. Modification of body composition by clenbuterol in normal and dystrophic (mdx) mice. Biosci Rep. 1985 Sep;5(9):755–760. doi: 10.1007/BF01119873. [DOI] [PubMed] [Google Scholar]
- Sicinski P., Geng Y., Ryder-Cook A. S., Barnard E. A., Darlison M. G., Barnard P. J. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989 Jun 30;244(4912):1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Stedman H. H., Sweeney H. L., Shrager J. B., Maguire H. C., Panettieri R. A., Petrof B., Narusawa M., Leferovich J. M., Sladky J. T., Kelly A. M. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991 Aug 8;352(6335):536–539. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- Torres L. F., Duchen L. W. The mutant mdx: inherited myopathy in the mouse. Morphological studies of nerves, muscles and end-plates. Brain. 1987 Apr;110(Pt 2):269–299. doi: 10.1093/brain/110.2.269. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Potential mechanism of insulin action on glucose transport in the isolated rat diaphragm. Apparent translocation of intracellular transport units to the plasma membrane. J Biol Chem. 1981 Jul 25;256(14):7090–7093. [PubMed] [Google Scholar]
- Watkins S. C., Hoffman E. P., Slayter H. S., Kunkel L. M. Immunoelectron microscopic localization of dystrophin in myofibres. Nature. 1988 Jun 30;333(6176):863–866. doi: 10.1038/333863a0. [DOI] [PubMed] [Google Scholar]