Key Points
Question
Does the incidence of postoperative complications differ between conventional nipple-sparing mastectomy (C-NSM) and minimal access nipple-sparing mastectomy (M-NSM)?
Findings
In this case-control study of 1356 individuals who underwent C-NSM and 227 who underwent M-NSM. There was no significant difference between the 2 groups regarding short- and long-term postoperative complications.
Meaning
The incidence of complications following M-NSM was comparable to that following C-NSM, indicating its potential as a viable option for breast cancer treatment.
This case-control study evaluates outcomes following minimal access nipple-sparing mastectomy vs conventional nipple-sparing mastectomy.
Abstract
Importance
While nipple-sparing mastectomy (NSM) for breast cancer was only performed using the open method in the past, its frequency using endoscopic and robotic surgical instruments has been increasing rapidly. However, there are limited studies regarding postoperative complications and the benefits and drawbacks of minimal access NSM (M-NSM) compared with conventional NSM (C-NSM).
Objective
To examine the differences in postoperative complications between C-NSM and M-NSM.
Design, Setting, Participants
This was a retrospective multicenter cohort study enrolling 1583 female patients aged 19 years and older with breast cancer who underwent NSM at 21 university hospitals in Korea between January 2018 and December 2020. Those with mastectomy without preserving the nipple-areolar complex (NAC), clinical or pathological malignancy in the NAC, inflammatory breast cancer, breast cancer infiltrating the chest wall or skin, metastatic breast cancer, or insufficient medical records were excluded. Data were analyzed from November 2021 to March 2024.
Exposures
M-NSM or C-NSM.
Main Outcomes and Measures
Clinicopathological factors and postoperative complications within 3 months of surgery were assessed. Statistical analyses, including logistic regression, were used to identify the factors associated with complications.
Results
There were 1356 individuals (mean [SD] age, 45.47 [8.56] years) undergoing C-NSM and 227 (mean [SD] age, 45.41 [7.99] years) undergoing M-NSM (35 endoscopy assisted and 192 robot assisted). There was no significant difference between the 2 groups regarding short- and long-term postoperative complications (<30 days: C-NSM, 465 of 1356 [34.29%] vs M-NSM, 73 of 227 [32.16%]; P = .53; <90 days: C-NSM, 525 of 1356 [38.72%] vs M-NSM, 73 of 227 [32.16%]; P = .06). Nipple-areolar complex necrosis was more common in the long term after C-NSM than M-NSM (C-NSM, 91 of 1356 [6.71%] vs M-NSM, 5 of 227 [2.20%]; P = .04). Wound infection occurred more frequently after M-NSM (C-NSM, 58 of 1356 [4.28%] vs M-NSM, 18 of 227 [7.93%]; P = .03). Postoperative seroma occurred more frequently after C-NSM (C-NSM, 193 of 1356 [14.23%] vs M-NSM, 21 of 227 [9.25%]; P = .04). Mild or severe breast ptosis was a significant risk factor for nipple or areolar necrosis (odds ratio [OR], 4.75; 95% CI, 1.66-13.60; P = .004 and OR, 8.78; 95% CI, 1.88-41.02; P = .006, respectively). Conversely, use of a midaxillary, anterior axillary, or axillary incision was associated with a lower risk of necrosis (OR for other incisions, 32.72; 95% CI, 2.11-508.36; P = .01). Necrosis occurred significantly less often in direct-to-implant breast reconstruction compared to other breast reconstructions (OR, 2.85; 95% CI, 1.11-7.34; P = .03).
Conclusions and Relevance
The similar complication rates between C-NSM and M-NSM demonstrates that both methods were equally safe, allowing the choice to be guided by patient preferences and specific needs.
Introduction
Breast cancer is the most common type of cancer among women worldwide.1 The widespread uptake of breast cancer screening in many countries has led to a considerable increase in patients undergoing breast conserving surgery for early breast cancer. Even among patients diagnosed with advanced breast cancer, a substantial proportion of patients undergo breast conserving surgery after receiving neoadjuvant treatment; however, the rate of total mastectomy has remained greater than 30%.2 Furthermore, with an increased understanding of the BRCA1/2 variant, the frequency of prophylactic mastectomy has also increased.3
Nipple-sparing mastectomy (NSM) is increasingly performed owing to its superior esthetic outcomes compared with those of conventional mastectomy.4 However, conventional NSM (C-NSM) leaves a large visible scar on the breast and there is a high potential risk of necrosis of the nipple-areolar complex (NAC), depending on the approach used.5 Although the inframammary fold (IMF) approach eliminates visible scarring, it has the disadvantage of providing insufficient visual access; in addition, approaching the superior pole of the breast and the axillary area is challenging with this approach.6
Minimal access NSM (M-NSM) such as endoscopy-assisted or robot-assisted NSM refers to a surgical procedure for NSM that uses endoscopic or robotic devices.7,8 M-NSM allows the creation of relatively short incisions in less visible areas. M-NSM can be performed by inflating the breast with CO2 to create space or by using a gasless technique that retracts the skin via an incision.9 This method helps to overcome the disadvantages of C-NSM.10 The early experience of robotic surgery in Korea reported by the Korea Robot-Endoscopy Minimal Access Breast Surgery Study Group (KoREa-BSG) demonstrated the potential usefulness of M-NSM in patients with breast cancer,11 and several studies of robot-assisted NSM have been reported.12,13,14,15 However, some surgical oncologists have expressed apprehension regarding their inability to palpate breast tissue or lesions during M-NSM, especially in robot-assisted NSM.16 This limitation may increase the risk of complications, such as skin or NAC necrosis, which are critical for aesthetic outcomes. Moreover, extensive research on the advantages and disadvantages of M-NSM is lacking. This study aimed to compare postoperative complications between C-NSM and M-NSM and to identify factors that may influence such differences.
Methods
Patient Selection
This retrospective multicenter study included 1583 breast cancer patients who underwent C-NSM or M-NSM between January 2018 and December 2020 across 21 institutions in the Republic of Korea. This study was approved by the institutional review board of the Yongin Severance Hospital of Yonsei University. Due to the retrospective analysis, the requirement for patient consent was waived by the IRB.
The inclusion criterion for this study was female patients aged 19 years and older who underwent NSM for breast cancer irrespective of the location of the skin incision. The exclusion criteria were mastectomy without preserving the NAC, clinical or pathological malignancy in the NAC, inflammatory breast cancer, breast cancer infiltrating the chest wall or skin, metastatic breast cancer, and insufficient medical records. The patients were classified into 2 groups based on the surgical method: C-NSM (n = 1356) and M-NSM (n = 227). M-NSM was performed as endoscopy-assisted NSM in 35 individuals and robot-assisted NSM in 192 individuals. The analysis of complications was limited to complications that occurred within 3 months after surgery.
Clinicopathologic Variables
Clinicopathological variables, including age, body mass index, menopausal status, breast size, breast ptosis, history of smoking, medical history, germline variant status, adjuvant treatment, TNM stage, histological grade, histological type, estrogen receptor, progesterone receptor, human epidermal growth factor (HER) 2, and Ki-67, were collected. Surgical variables, including specimen weight, type of breast reconstruction, location of surgical incision, incision length, amount of intraoperative bleeding, and operation time, were analyzed. TNM staging was performed according to the anatomic stage of the American Joint Committee on Cancer, 8th edition.17 Estrogen receptor and progesterone receptor positivity were defined as 1% or greater nuclear staining in immunohistochemistry. HER2 positivity was defined as either 3+ staining in immunohistochemistry or 2+ staining in immunohistochemistry with confirmed amplification in fluorescence in situ hybridization or silver in situ hybridization, according to the guidelines of the American Society of Clinical Oncology and College of American Pathologists.18
C-NSM was performed through various skin incisions, including the upper outer radial, IMF, periareolar and extension, elliptical, periareolar only, horizontal, midaxillary or anterior axillary, inferior radial, and axillary incision. The M-NSM incision was made in the lateral chest, anterior axillary line, or midaxillary line. M-NSM involved the use of endoscopic devices, such as advanced energy devices, endoscopic forceps, endoscopic scissors, fiberoptic retractors, and self-retractors. For the gas-inflated technique, multiple single-access ports, such as Glove port (Nelis), Octo-port (Dalim SurgNet Corp), Uni-port (Dalim), Gelpoint Mini (Applied Medical), Oneport (Tebah), Lapsingle (Sejong Medical), and hand-made glove port were used for maintaining gas insufflation. For the gasless technique, Chung self-retractors or fiberoptic retractors were used to create and maintain working space. Previous researchers have described detailed M-NSM techniques.7,9,19,20,21
Postoperative Complications
Postoperative complications were categorized as short term (<30 days) and long term (<90 days). Postoperative complications were also classified according to the Clavien-Dindo classification.22 Complications of grade IIIb or higher based on the Clavien-Dindo classification include those requiring intervention under general anesthesia, life-threatening complications, and those leading to death. The records regarding postoperative complications, including NAC necrosis, skin necrosis, breast infection, wound dehiscence, bleeding or hematoma, postoperative seroma, and loss of breast implants, were collected. If more than 2 complications occurred in 1 patient, all complications were recorded and included in the analysis. NAC necrosis after surgery was classified into 6 stages (A-F) based on the extent and severity of necrosis within the nipple and areola23 (eFigure 1 in Supplement 1). The severity of skin necrosis was assessed by combining depth scores A-D and area scores 0-3.24 Depth of skin necrosis was defined as follows: A, no evidence of necrosis; B, only color change; C, partial-thickness necrosis; and D, full-thickness necrosis. The area of skin necrosis was scored according to the percentage of involved skin (score 0, <1%; score 1, 1%-10%; score 2, 11%-30%; and score 3, >30%) (eFigure 2 in Supplement 1).
Statistical Analysis
The Shapiro-Wilk test was used to assess the normality of distribution of continuous variables. Continuous variables were expressed as means with SDs and between-group differences were assessed for statistical significance using the t test. Categorical variables were expressed as frequencies with percentages and between-group differences were assessed using the χ2 or Fisher exact test.
For postoperative complications in the short term, statistical analysis could not be properly performed due to the low frequency of occurrence. However, for postoperative complications in the long term, statistical analysis was conducted differently for each factor. Logistic regression was used to determine the factors affecting NAC necrosis. Firth regression can overcome the problem of the lack of a finite confidence interval, which often occurs in regressions with a low number of events. Therefore, Firth regression was considered due to the low number of necrotic events. Univariate logistic regression analysis was performed to identify factors associated with necrosis. Subsequently, multivariate logistic regression was conducted to adjust for covariates in the model. The results are presented as odds ratios (ORs) and 95% CIs. Statistical analyses were performed using SAS version 9.4 (SAS Institute). Two-tailed P values <.05 were considered indicative of statistical significance.
Results
Among the 1583 patients included in the study, 1356 (mean [SD] age, 45.47 [8.56] years) were in the C-NSM group and 227 (mean [SD] age, 45.41 [7.99] years) in the M-NSM group (35 endoscopy assisted and 192 robot assisted). There were no significant differences between the 2 groups in terms of clinicopathological variables, except for menopausal status, grade of breast ptosis, BRCA1/2 variant, and neoadjuvant chemotherapy. The M-NSM group had a significantly higher proportion of premenopausal patients (C-NSM, 904 of 1356 [66.67%]; M-NSM, 167 of 227 [73.57%]; P = .02), as well as a higher proportion of nonptotic breasts (C-NSM, 424 of 1356 [31.27%]; M-NSM, 150 of 227 [66.08%]; P < .001). There were no significant differences between groups for T stage, N stage, estrogen receptor, progesterone receptor, HER2 gene status, or histologic grade (Table 1). The M-NSM group had significantly more cases of bilateral surgery (C-NSM, 128 of 1356 [9.44%]; M-NSM, 47 of 227 [20.70%]; P < .001), a higher rate of performing sentinel lymph node biopsy (C-NSM, 1267 of 1356 [93.44%]; M-NSM, 221 of 227 [97.36%]; P = .01), larger implant volume (mean [SD], C-NSM, 287.99 [189.36] mL3; M-16 NSM, 339.95 [114.75] mL3; P < .001), and smaller final incision length (mean [SD], C-NSM, 76.15 [17.55] mm; M-NSM, 48.61 [11.89] mm; P < .001). Regarding the patient’s position during surgery, while the 90° arm extension was most frequently used in the C-NSM group (n = 894 [65.93%]), the raising arm position was most frequently used in the M-NSM group (n = 113 [49.78%]) (P < .001). The most frequent incision method in C-NSM was upper outer radial incision (n = 468, 34.51%), whereas that in the M-NSM was midaxillary or anterior axillary incision (n = 177 [77.97%]) (P < .001). In the subcutaneous flap dissection for NSM, while electrocauterization alone was most preferred in C-NSM (n = 697 [51.40%]), a combination of hydrodissection and electrocauterization was most frequently used in M-NSM (n = 187 [82.38%]) (P < .001). The operative time was significantly longer in the M-NSM group than in the C-NSM group (mean [SD], C-NSM, 116.01 [47.21] minutes; M-NSM, 146.94 [47.09] minutes; P < .001); however, there was no significant between-group difference in specimen weight. There was no significant difference between 2 groups in the amount of intraoperative blood loss, but the volume of serous fluid drained after surgery was significantly greater in the M-NSM group (mean [SD], C-NSM, 959.33 [657.59] mL3; M-NSM, 1333.28 [859.29] mL3; P < .001), and the duration of placement of drainage tube was significantly longer in M-NSM than C-NSM (mean [SD], C-NSM, 12.61 [4.11] days; M-NSM, 16.88 [9.84] days; P < .001) (Table 2).
Table 1. Clinicopathologic Characteristics of Patients With Breast Cancer Who Underwent Conventional Nipple-Sparing Mastectomy (C-NSM) or Minimal Access Nipple-Sparing Mastectomy (M-NSM).
| Characteristic | No. (%) | P value | |
|---|---|---|---|
| C-NSM (n = 1356) | M-NSM (n = 227) | ||
| Age, mean (SD), y | 45.47 (8.56) | 45.41 (7.99) | .92 |
| BMI, mean (SD) | 22.71 (3.15) | 22.52 (3.04) | .39 |
| Menopausal status | |||
| Premenopausal | 904 (66.67) | 167 (73.57) | .02 |
| Postmenopausal | 291 (21.46) | 34 (14.98) | |
| Unknown | 161 (11.87) | 26 (11.45) | |
| Ptosis | |||
| Normal | 424 (31.27) | 150 (66.08) | <.001 |
| Mild | 172 (12.68) | 31 (13.66) | |
| Moderate | 145 (10.69) | 5 (2.2) | |
| Severe | 79 (5.83) | 2 (0.88) | |
| Pseudoptosis | 14 (1.03) | 2 (0.88) | |
| Unknown | 522 (38.5) | 37 (16.3) | |
| Smoking history | |||
| Nonsmoking | 866 (63.86) | 208 (91.63) | .24 |
| Smoking | 40 (2.95) | 14 (6.17) | |
| Unknown | 430 (31.71) | 5 (24.23) | |
| BRCA variant | |||
| No test | 1080 (79.65) | 143 (63) | <.001 |
| Negative | 174 (12.83) | 67 (29.52) | |
| Positive | 85 (6.27) | 11 (4.85) | |
| Variant of unknown significance | 26 (1.92) | 6 (2.64) | |
| pT stage | |||
| 0 or In situ | 257 (18.95) | 63 (27.75) | .15 |
| 1 | 516 (38.05) | 117 (51.54) | |
| 2 | 240 (17.7) | 36 (15.86) | |
| 3 | 26 (1.92) | 6 (2.64) | |
| Unknown | 317 (23.38) | 5 (2.2) | |
| pN stage | |||
| 0 or Micrometastasis | 825 (60.84) | 193 (85.02) | .06 |
| 1 | 169 (12.46) | 23 (10.13) | |
| 2 | 30 (2.21) | 3 (1.32) | |
| 3 | 9 (0.66) | 3 (1.32) | |
| Unknown | 323 (23.82) | 5 (2.2) | |
| Histologic grade | |||
| Well | 175 (12.91) | 33 (14.54) | .31 |
| Moderate | 680 (50.15) | 110 (48.46) | |
| Poor | 267 (19.69) | 34 (14.98) | |
| Unknown | 234 (17.26) | 50 (22.03) | |
| Estrogen receptor | |||
| Negative | 291 (21.46) | 41 (18.06) | .28 |
| Positive | 1058 (78.02) | 182 (80.18) | |
| Unknown | 7 (0.52) | 4 (1.76) | |
| Progesterone receptor | |||
| Negative | 398 (29.35) | 56 (24.67) | .18 |
| Positive | 950 (70.06) | 167 (73.57) | |
| Unknown | 8 (0.59) | 4 (1.76) | |
| HER2 gene | |||
| Negative | 959 (70.72) | 142 (62.56) | .12 |
| Equivocal | 144 (10.62) | 29 (12.78) | |
| Positive | 245 (18.07) | 50 (22.03) | |
| Unknown | 8 (0.59) | 6 (2.64) | |
| SISH or FISH | |||
| Negative | 230 (16.96) | 62 (27.31) | .16 |
| Positive | 47 (3.47) | 7 (3.08) | |
| Not applicable | 1079 (79.52) | 158 (69.6) | |
| Ki-67 index, mean (SD), % | 20.01 (19.71) | 19.7 (16.94) | .81 |
| Neoadjuvant chemotherapy | |||
| No | 1135 (83.7) | 204 (89.87) | .009 |
| Yes | 218 (16.08) | 21 (9.25) | |
| Unknown | 3 (0.22) | 2 (0.88) | |
| Adjuvant chemotherapy | |||
| No | 921 (67.92) | 159 (70.04) | .47 |
| Yes | 428 (31.56) | 66 (29.07) | |
| Unknown | 7 (0.52) | 2 (0.88) | |
| Adjuvant radiotherapy | |||
| No | 1096 (80.83) | 189 (83.26) | .33 |
| Yes | 252 (18.58) | 36 (15.86) | |
| Unknown | 8 (0.59) | 2 (0.88) | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HER2, human epidermal growth factor; FISH, fluorescence in situ hybridization; pN, pathological node; pT, pathological stage; SISH, silver in situ hybridization; VUS, variant of unknown significance.
Table 2. Comparative Analysis of Surgical Procedures and Outcomes in the Conventional Nipple-Sparing Mastectomy (C-NSM) and Minimal Access Nipple-Sparing Mastectomy (M-NSM) Groups.
| Variable | No. (%) | P value | |
|---|---|---|---|
| C-NSM (n = 1356) | M-NSM (n = 227) | ||
| Risk-reducing surgery | |||
| No | 1348 (99.41) | 226 (99.56) | >.99 |
| Yes | 8 (0.59) | 1 (0.44) | |
| Surgical extent | |||
| Unilateral | 1228 (90.56) | 180 (79.3) | <.001 |
| Bilateral | 128 (9.44) | 47 (20.7) | |
| Arm position | |||
| Raising arm | 17 (1.25) | 113 (49.78) | <.001 |
| Laying down | 129 (9.51) | 34 (14.98) | |
| 90° Extension | 894 (65.93) | 76 (33.48) | |
| Unknown | 316 (23.3) | 4 (1.76) | |
| Sentinel lymph node biopsy | |||
| No | 89 (6.56) | 6 (2.64) | .01 |
| Yes | 1267 (93.44) | 221 (97.36) | |
| Axillary lymph node dissection | |||
| No | 1135 (83.7) | 199 (87.67) | .07 |
| Yes | 221 (16.3) | 28 (12.33) | |
| Gas or gasless technique | |||
| Gasless | NA | 78 (34.36) | |
| Gas | NA | 149 (65.64) | |
| Incision location | |||
| Upper outer radial | 468 (34.51) | 6 (2.64) | <.001 |
| Inframammary | 332 (24.48) | 10 (4.41) | |
| Periareolar and extension | 153 (11.28) | 1 (0.44) | |
| Elliptical | 131 (9.66) | 0 | |
| Periareolar only | 16 (1.18) | 0 | |
| Horizontal | 28 (2.06) | 0 | |
| Mid or anterior axillary | 5 (0.37) | 177 (77.97) | |
| Inferior radial | 2 (0.15) | 0 | |
| Axillary | 0 | 12 (5.29) | |
| Other | 210 (15.49) | 18 (11.45) | |
| Initial incision size, mean (SD), cm | NA | 45.33 (11.49) | <.001 |
| Final incision size, mean (SD), cm | NA | 48.61 (11.89) | <.001 |
| Subcutaneous flap dissecting method | |||
| Hydrodissection | 21 (1.55) | 7 (3.08) | <.001 |
| Electrocauterization | 697 (51.4) | 33 (14.54) | |
| Both | 212 (15.63) | 187 (82.38) | |
| Unknown | 426 (31.42) | NA | |
| Time for mastectomy, mean (SD), min | 116.01 (47.21) | 146.94 (47.09) | <.001 |
| Specimen weight, mean (SD), g | 361.71 (197.53) | 347.88 (156.77) | .26 |
| Reconstruction method | |||
| Tissue expander | 338 (24.93) | 38 (16.74) | <.001 |
| Direct to implant | 682 (50.29) | 167 (73.57) | |
| TRAM/DIEP flap | 183 (13.5) | 14 (6.17) | |
| LD flap | 110 (8.11) | 4 (1.76) | |
| Other | 43 (3.17) | 4 (1.76) | |
| Using energy device | |||
| No | 441 (32.52) | 13 (5.73) | <.001 |
| Yes | 915 (67.48) | 214 (95.96) | |
| Using acellular dermal matrix | |||
| No | 382 (28.17) | 12 (5.29) | <.001 |
| Yes | 959 (70.72) | 215 (94.71) | |
| Unknown | 15 (11.06) | NA | |
| Implant volume, mean (SD), mL3 | 287.99 (189.36) | 339.95 (114.75) | <.001 |
| Amount of intraoperative bleeding, mean (SD), mL | 170.55 (195.72) | 136.24 (178.17) | .10 |
| Total amount of drainage, mean (SD), mL | 959.33 (657.59) | 1333.28 (859.29) | <.001 |
| Duration of drainage tube placement, mean (SD), d | 12.61 (4.11) | 16.88 (9.84) | <.001 |
Abbreviations: DIEP, deep inferior epigastric artery perforator; LD, latissimus dorsi myocutaneous; NA, not applicable; TRAM, transverse rectus abdominis musculocutaneous flap.
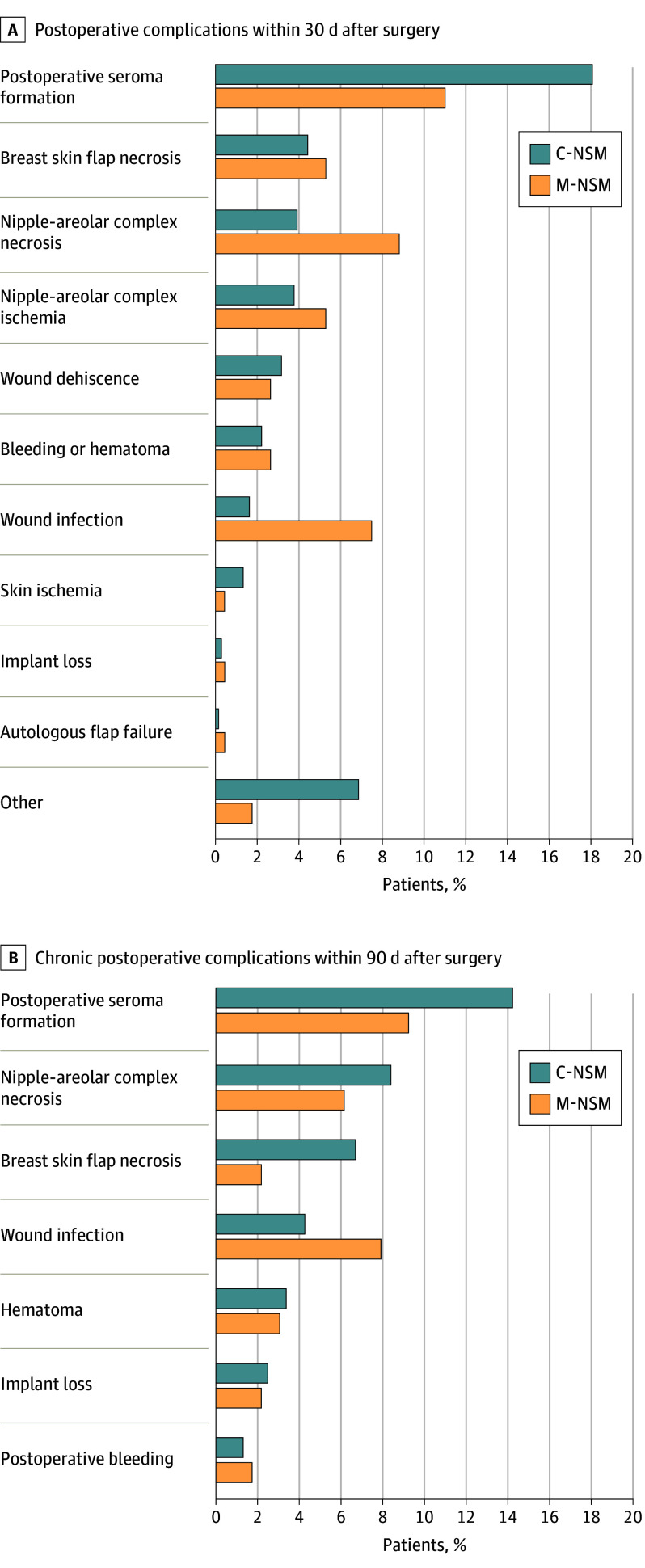
Based on Clavien-Dindo classification, 72 individuals (5.31%) in C-NSM and 7 (3.08%) in M-NSM developed grade IIIb or higher postoperative complications (P = .16). Although both short-term (<30 days) and long-term (<90 days) postoperative complications occurred more frequently in the C-NSM group, there was no statistical difference between the 2 groups (short term: C-NSM, 465 of 1356 [34.29%] vs M-NSM, 73 of 227 [32.16%]; P = .53; long term: C-NSM, 525 of 1356 [38.72%] vs M-NSM, 73 of 227 [32.16%], P = .06). Necrosis of the NAC occurred more frequently in the short term in the M-NSM group (C-NSM, 53 of 1356 [3.91%]; M-NSM, 20 of 227 [8.81%]); however, necrosis of the NAC in the long term occurred significantly more frequently in the C-NSM group (C-NSM, 91 of 1356 [6.71%]; M-NSM, 5 of 227 [2.20%]; P = .04). Wound infection occurred more frequently in the M-NSM group (short term: C-NSM, 22 of 1356 [1.62%] vs M-NSM, 17 of 227 [7.49%]; long term: C-NSM, 58 of 1356 [4.28%] vs M-NSM, 18 of 227 [7.93%]; P = .03). Postoperative seroma was significantly more frequently identified after C-NSM than M-NSM (C-NSM, 193 of 1356 [14.23%]; M-NSM, 21 of 227 [9.25%]; P = .04. In the C-NSM group, the amount of seroma was 3.68 times longer (P = .001), and the drainage period was 2.41 times greater (P < .001) than in the M-NSM group (Figure, Table 3).
Figure. Comparison of Postoperative Complications After Conventional Nipple-Sparing Mastectomy (C-NSM) or Minimal Access Mastectomy (M-NSM).
Table 3. Postoperative Complications and Outcomes in the Conventional Nipple-Sparing Mastectomy (C-NSM) and Minimal Access Nipple-Sparing Mastectomy (M-NSM) Groups.
| Variable | No. (%) | P value | |
|---|---|---|---|
| C-NSM (n = 1356) | M-NSM (n = 227) | ||
| Clavien-Dindo classification | |||
| 0-IIIa | 1241 (91.52) | 211 (92.95) | .16 |
| IIIb-V | 72 (5.31) | 7 (3.08) | |
| Unknown | 43 (3.17) | 9 (3.96) | |
| Less than postoperative 30 d | |||
| Occurrence of complications | |||
| No | 891 (65.71) | 154 (67.84) | .53 |
| Yes | 465 (34.29) | 73 (32.16) | |
| Postoperative seroma formation | 245 (18.07) | 25 (11.01) | NA |
| Breast skin flap necrosis | 60 (4.42) | 12 (5.29) | NA |
| Nipple-areolar complex necrosis | 53 (3.91) | 20 (8.81) | NA |
| Nipple-areolar complex ischemia | 51 (3.76) | 12 (5.29) | NA |
| Wound dehiscence | 43 (3.17) | 6 (2.64) | NA |
| Bleeding/hematoma | 30 (2.21) | 6 (2.64) | NA |
| Wound infection | 22 (1.62) | 17 (7.49) | NA |
| Skin ischemia | 18 (1.33) | 1 (0.44) | NA |
| Implant loss | 4 (0.29) | 1 (0.44) | NA |
| Autologous flap failure | 2 (0.15) | 1 (0.44) | NA |
| Other | 93 (6.86) | 4 (1.76) | NA |
| Less than postoperative 90 d | |||
| Occurrence of complications | |||
| No | 831 (61.28) | 154 (67.84) | .06 |
| Yes | 525 (38.72) | 73 (32.16) | |
| Breast skin flap necrosisa | |||
| A | 1236 (91.15) | 211 (92.95) | .62 |
| B0 | 13 (0.96) | 0 | |
| B1 | 34 (2.51) | 3 (1.32) | |
| B2 | 5 (0.37) | 1 (0.44) | |
| B3 | 0 | 0 | |
| C0 | 7 (0.52) | 0 | |
| C1 | 35 (2.58) | 9 (3.96) | |
| C2 | 8 (0.59) | 1 (0.44) | |
| C3 | 0 | 0 | |
| D0 | 5 (0.37) | 0 | |
| D1 | 3 (0.22) | 0 | |
| D2 | 2 (0.15) | 0 | |
| D3 | 2 (0.15) | 0 | |
| Unknown | 6 (0.44) | 2 (0.88) | |
| Nipple-areolar complex necrosisa | |||
| A | 1203 (88.72) | 200 (88.11) | .04 |
| B | 48 (3.54) | 0 | |
| C | 20 (1.47) | 2 (0.88) | |
| D | 8 (0.59) | 0 | |
| E | 5 (0.37) | 0 | |
| F | 10 (0.74) | 3 (1.32) | |
| Unknown | 62 (4.57) | 22 (9.69) | |
| Wound infection | |||
| No | 1293 (95.35) | 208 (91.63) | .03 |
| Minor infection | 35 (2.58) | 13 (5.73) | |
| Severe infection | 23 (1.7) | 5 (2.2) | |
| Unknown | 5 (0.37) | 1 (0.44) | |
| Postoperative bleeding | |||
| No | 1338 (98.67) | 223 (98.24) | .61 |
| Yes | 18 (1.33) | 4 (1.76) | |
| Hematoma | |||
| No | 1310 (96.61) | 220 (96.92) | .81 |
| Yes | 46 (3.39) | 7 (3.08) | |
| Postoperative seroma | |||
| No | 1163 (85.77) | 206 (90.75) | .04 |
| Yes | 193 (14.23) | 21 (9.25) | |
| Duration of seroma formation, mean (SD), d | 51.33 (108.51) | 13.93 (26.61) | .001 |
| Total volume of aspirated seroma, mean (SD), mL | 146.97 (270.1) | 60.8 (53) | <.001 |
| Implant loss | |||
| No | 1322 (97.49) | 222 (97.8) | .78 |
| Yes | 34 (2.51) | 5 (2.2) | |
Abbreviation: NA, not applicable.
Definitions are shown in the eFigures in Supplement 1.
Presence of breast ptosis, whether mild or severe, was associated with a significantly higher risk of nipple or areolar necrosis (mild ptosis: OR, 4.75; 95% CI, 1.66-13.60; P = .004; severe ptosis: OR, 8.78; 95% CI, 1.88-41.02; P = .006). Direct-to-implant breast reconstruction (OR, 2.85; 95% CI, 1.11-7.34; P = .03) and midaxillary, anterior axillary, or axillary incisions (OR, 32.72; 95% CI, 2.11-508.36; P = .01) were associated with a significantly lower incidence of NAC necrosis compared to alternative reconstruction methods (Table 4).
Table 4. Risk Factors Associated With Nipple or Areolar Necrosis After Nipple-Sparing Mastectomy.
| Variable | Univariate logistic regression | Multivariate logistic regression | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Breast ptosis | ||||
| Normal | 1 [Reference] | NA | 1 [Reference] | NA |
| Mild | 3.65 (1.816-7.336) | .26 | 4.75 (1.66-13.60) | .003 |
| Moderate | 4.232 (2.034-8.805) | .14 | 2.11 (0.60-7.48) | .25 |
| Severe | 5.252 (2.235-12.344) | .06 | 8.78 (1.88-41.02) | .01 |
| Pseudoptosis | 1.013 (0.053-19.246) | .47 | 5.59 (0.16-197.35) | .34 |
| History of smoking | ||||
| Never | 1 [Reference] | NA | 1 [Reference] | NA |
| Past | 4.416 (1.494-13.055) | .07 | 6.63 (0.73-60.61) | .09 |
| Current | 1.876 (0.485-7.252) | .88 | 0.71 (0.04-12.59) | .81 |
| Subcutaneous flap dissecting method | ||||
| Hydrodissection | 1 [Reference] | NA | 1 [Reference] | NA |
| Electrocauterization | 1.565 (0.403-6.076) | .32 | 2.88 (0.58-14.39) | .20 |
| Both | 0.616 (0.323-1.176) | .12 | 0.94 (0.36-2.47) | .91 |
| Reconstruction method | ||||
| Direct to implant | 1 [Reference] | NA | 1 [Reference] | NA |
| Other | 1.79 (1.177-2.721) | .006 | 2.85 (1.11-7.34) | .03 |
| Location of surgical incision | ||||
| Mid or anterior axillary or axillary | 1 [Reference] | NA | 1 [Reference] | NA |
| Other | 5.096 (1.431-18.147) | .01 | 32.72 (2.11-508.36) | .01 |
| Use of acellular dermal matrix | ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 0.502 (0.327-0.77) | .002 | 1.51 (0.51-4.49) | .46 |
| Neoadjuvant chemotherapy | ||||
| No | 1 [Reference] | NA | 1 [Reference] | NA |
| Yes | 1.025 (0.584-1.799) | .93 | 1.44 (0.47-4.46) | .52 |
Abbreviation: NA, not applicable.
No statistical difference in skin or NAC necrosis was observed between individuals who received C-NSM via IMF incision or M-NSM. However, the frequency of breast infection was significantly higher in the M-NSM group (C-NSM, 2 of 332 [0.60%]; M-NSM, 18 of 227 [7.93%]; P < .001), while the mean (SD) final length of incision was significantly longer in the C-NSM group using IMF incision than M-NSM (C-NSM, 83.62 [13.16] mm; M-NSM, 48.61 [11.89] mm; P < .001) (eTable in Supplement 1).
Discussion
In this case-control study, we compared the surgical outcomes of M-NSM and C-NSM in patients with breast cancer. Although there were no significant differences in overall frequency of postoperative complications, M-NSM showed advantages, such as significantly lower incidence of NAC necrosis and seroma. Also, the length of incision was significantly shorter in M-NSM group. Interestingly, despite the more frequent use of hydrodissection, advanced energy devices, and acellular dermal matrix, the surgical time was approximately 30 minutes longer in M-NSM, whereas breast reconstruction surgery was longer by approximately 44 minutes in C-NSM. More than 73% of reconstructions after M-NSM used direct implantation methods compared with flap surgery for C-NSM, contributing to the difference in duration. Total surgical time was similar between groups.
The rate of NAC necrosis after C-NSM varies from 0% to 48%.25,26,27,28,29,30,31 In a study of 12 358 C-NSM procedures, the NAC necrosis rate was reported as 5.9%.4 In a multicenter study of robot-assisted NSM and C-NSM, NAC necrosis rates were 2.1% and 7.8%, respectively.32 Similarly, in this study, NAC necrosis rates in the long term were 2.20% and 6.71% in the M-NSM and C-NSM groups, respectively. However, the NAC necrosis rate in the short term in the M-NSM group was 8.81% higher than that of C-NSM. Robot-assisted NSM in South Korea was first reported in 2018,20 and many institutions have started this procedure for NSM since then.11 Therefore, this study included early experiences of M-NSM and the grades of NAC necrosis may not be clearly reported. However, given that there was no difference in NAC necrosis rate in the long term, it is likely that most were low grade, manageable, and improved quickly.
Multivariate analysis in this study adjusted for factors like breast ptosis, smoking history, flap dissection method, reconstruction method, incision location, acellular dermal matrix use, and neoadjuvant chemotherapy to identify influences on NAC necrosis. Independent factors found were incision location, reconstruction method, and ptosis. Using an axillary incision in NSM helps preserve blood supply to the NAC and skin, potentially reducing necrosis rates. This preservation of the blood supply may have contributed to the reduced incidence of NAC necrosis. In addition, direct to implant breast reconstruction requires less manipulation compared to other surgical methods. These factors may explain the lower risk of NAC necrosis. Therefore, the use of endoscopy or robotics, enabling minimal access surgery, may facilitate smaller and strategically placed incisions away from the NAC, effectively reducing the rate of NAC necrosis.
A systematic review33 found that IMF incisions were not superior to other types of incisions in NSM. However, because the approach is similar to that of M-NSM, which does not cut the breast envelope, a subgroup analysis was performed on patients who underwent C-NSM with an IMF incision. No significant differences were seen in the incidence of skin flap necrosis, NAC necrosis, bleeding, hematoma, seroma, or implant loss between the 2 groups. Previous research on the association between NSM complications and incision type revealed that IMF incisions resulted in significantly lower rates of NAC necrosis than other approaches.5,30,34,35,36 Furthermore, shorter lengths of IMF incisions have been associated with a lower risk of ischemic complications.37 Compared to the C-NSM group, which used IMF incisions, the M-NSM group had a significantly higher incidence of breast infections. However, these infections were mostly minor and did not lead to serious complications, as there was no significant difference in implant loss between the 2 groups.
Endoscopy-assisted or robot-assisted NSM has the advantage of reducing the length of skin incision compared to C-NSM. Additionally, an instrument-based approach enables the hiding of skin incisions in the IMF or periareolar area. Shorter and more concealed incisions may improve aesthetic and psychological outcomes and potentially reduce pain levels experienced by patients. However, due to the retrospective nature of the study, pain severity was not evaluated. Nonetheless, Moon et al37 observed a reduction in immediate postoperative pain with robotic assistance, indicating that shorter incision lengths could contribute to this effect. Furthermore, the shorter incision line can lead to a shorter suturing time, which may shorten the duration of surgery and reduce the surgeon’s workload.38 The multicenter, retrospective design of this study and the variety of skin incision types used resulted in small sample sizes for each category, limiting the statistical power to draw firm conclusions about this variable. This limitation hinders more detailed analyses but reflects real-world data, allowing for a broader comparison of complication rates across different incisions with those of M-NSM.
In the M-NSM group, the incidence of infection was higher than that in C-NSM group, whereas the incidence of seroma was significantly lower. This can be attributed to the frequent use of implants, acellular dermal matrixes, and advanced energy devices. The relatively more frequent use of implants and acellular dermal matrixes in the M-NSM group is expected to contribute to a higher infection rate.39,40,41,42 Energy devices facilitate efficient tissue resection and hemostasis during surgery, which can help reduce the frequency of seroma.43,44 Therefore, in this study, the observed differences in infection and seroma rates between groups were likely due to the variations in surgical instruments used during the procedures.
Limitations
This study has several limitations that should be considered. First, the retrospective design may introduce selection bias. Nonetheless, the uniform application of selection criteria for NSM procedures across both C-NSM and M-NSM groups, which exclude patients with nipple or skin involvement, likely minimizes the impact of this bias on the results. Second, the retrospective nature of the study often results in missing critical data, as analyses are conducted postintervention. Efforts to collect data on patients’ bra cup sizes before surgery were hampered, with approximately 80% of these data missing and subsequently excluded. Additionally, data on breast ptosis were absent for about 36% of cases. It was also challenging to determine whether seromas originated in the breast or the axilla. Despite these limitations regarding data sufficiency, the study still managed to achieve significant findings. Another constraint was the nonrandom assignment of participants to the 2 study groups. However, the study involved more than 1500 patients from more than 20 centers in South Korea, and statistical adjustments were made for major factors that could influence the results. The findings of this study could serve as a measure to anticipate the results of ongoing clinical trials (eg, the Prospective Study of Mastectomy With Reconstruction Including Robot Endoscopic Surgery [MARRES] study and the Robot-assisted vs Open NSM With Immediate Breast Reconstruction [ROM] study).45 Third, this retrospective study, focusing on early data on endoscopic and robotic surgery, had a relatively small number of M-NSM cases compared to C-NSM, although the number aligns with those reported in other studies. The KoREa-BSG is collecting more data through the prospective MARRES study.45 Sentinel lymph node biopsy was also more common in M-NSM, reflecting the surgical consensus at the time that robotic NSM should be limited to clinically node-negative breast cancer.46 However, clinical indications for robotic surgery in breast cancer have expanded recently, including axillary lymph node dissection.47,48 Additionally, this retrospective study involved surgeons from 21 institutions and primarily early robotic surgery, possibly introducing bias and skewing results toward worse outcomes. However, no significant differences were found compared to studies from just 4 institutions, suggesting that these findings might offer a broader perspective by incorporating diverse early-stage experiences.32 Recent advancements in robotic systems have expanded the surgical options for M-NSM, including various flap surgeries, thus overcoming previous limitations in selection criteria.49,50,51,52
Conclusions
In conclusion, our study found no significant differences in the incidence and severity of postoperative complications between C-NSM and M-NSM. M-NSM, which uses endoscopic or robotic-assisted techniques, provides benefits such as less visible scarring, smaller incisions, and a reduced risk of NAC necrosis. The similar complication rates suggest that both C-NSM and M-NSM may be equally safe options. Therefore, the choice of surgical approach should be tailored to patient preferences and individual needs.
eFigure 1. Scoring system (A-F) for the degree of nipple or areolar necrosis after nipple-sparing mastectomy.
eFigure 2. Scoring system for degree of skin necrosis after nipple-sparing mastectomy based on the involved area and depth of skin necrosis.
eTable. Comparison of patients who underwent conventional nipple-sparing mastectomy (C-NSM) using inframammary fold (IMF) incision or minimal-access NSM (M-NSM)
The Korea Robot-endoscopy Minimal Access Breast Surgery Study Group members
Data sharing statement
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Kang SY, Lee SB, Kim YS, et al. ; Korean Breast Cancer Society . Breast cancer statistics in Korea, 2018. J Breast Cancer. 2021;24(2):123-137. doi: 10.4048/jbc.2021.24.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panchal H, Pilewskie ML, Sheckter CC, et al. National trends in contralateral prophylactic mastectomy in women with locally advanced breast cancer. J Surg Oncol. 2019;119(1):79-87. doi: 10.1002/jso.25315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Headon HL, Kasem A, Mokbel K. The oncological safety of nipple-sparing mastectomy: a systematic review of the literature with a pooled analysis of 12,358 procedures. Arch Plast Surg. 2016;43(4):328-338. doi: 10.5999/aps.2016.43.4.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donovan CA, Harit AP, Chung A, Bao J, Giuliano AE, Amersi F. Oncological and surgical outcomes after nipple-sparing mastectomy: do incisions matter? Ann Surg Oncol. 2016;23(10):3226-3231. doi: 10.1245/s10434-016-5323-z [DOI] [PubMed] [Google Scholar]
- 6.Roh TS, Kim JY, Jung BK, Jeong J, Ahn SG, Kim YS. Comparison of outcomes between direct-to-implant breast reconstruction following nipple-sparing mastectomy through inframammary fold incision versus noninframammary fold incision. J Breast Cancer. 2018;21(2):213-221. doi: 10.4048/jbc.2018.21.2.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park HS, Lee J, Lee DW, et al. Robot-assisted nipple-sparing mastectomy with immediate breast reconstruction: an initial experience. Sci Rep. 2019;9(1):15669. doi: 10.1038/s41598-019-51744-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang B, Keum H, Park HY, Jung JH, Kim WW, Lee J. Usefulness of cordless ultrasonic cutting energy devices in endoscopic nipple-sparing mastectomy: a retrospective study. Ann Surg Treat Res. 2024;106(3):147-154. doi: 10.4174/astr.2024.106.3.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H, Lee J, Lee K, Kim JY, Park HS. Comparison between gasless and gas-inflated robot-assisted nipple-sparing mastectomy. J Breast Cancer. 2021;24(2):183-195. doi: 10.4048/jbc.2021.24.e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai HW, Chen ST, Lin YJ, et al. Minimal access (endoscopic and robotic) breast surgery in the surgical treatment of early breast cancer-trend and clinical outcome from a single-surgeon experience over 10 years. Front Oncol. 2021;11:739144. doi: 10.3389/fonc.2021.739144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryu JM, Kim JY, Choi HJ, et al. Robot-assisted nipple-sparing mastectomy with immediate breast reconstruction: an initial experience of the Korea Robot-endoscopy Minimal Access Breast Surgery Study Group (KoREa-BSG). Ann Surg. 2022;275(5):985-991. doi: 10.1097/SLA.0000000000004492 [DOI] [PubMed] [Google Scholar]
- 12.Toesca A, Peradze N, Manconi A, et al. Robotic nipple-sparing mastectomy for the treatment of breast cancer: feasibility and safety study. Breast. 2017;31:51-56. doi: 10.1016/j.breast.2016.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai HW, Chen ST, Lin SL, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant: technique, preliminary results and patient-reported cosmetic outcome. Ann Surg Oncol. 2019;26(1):42-52. doi: 10.1245/s10434-018-6704-2 [DOI] [PubMed] [Google Scholar]
- 14.Filipe MD, de Bock E, Postma EL, et al. Robotic nipple-sparing mastectomy complication rate compared to traditional nipple-sparing mastectomy: a systematic review and meta-analysis. J Robot Surg. 2022;16(2):265-272. doi: 10.1007/s11701-021-01265-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houvenaeghel G, Bannier M, Rua S, et al. Breast cancer robotic nipple sparing mastectomy: evaluation of several surgical procedures and learning curve. World J Surg Oncol. 2019;17(1):27. doi: 10.1186/s12957-019-1567-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrow M. Robotic nipple-sparing mastectomy—ready for prime time? JAMA Surg. 2024;159(3):276. doi: 10.1001/jamasurg.2023.7007 [DOI] [PubMed] [Google Scholar]
- 17.Amin MB, Edge S, Greene F, et al. eds. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; 2017. [Google Scholar]
- 18.Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36(20):2105-2122. doi: 10.1200/JCO.2018.77.8738 [DOI] [PubMed] [Google Scholar]
- 19.Toesca A, Peradze N, Galimberti V, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with implant: first report of surgical technique. Ann Surg. 2017;266(2):e28-e30. doi: 10.1097/SLA.0000000000001397 [DOI] [PubMed] [Google Scholar]
- 20.Park HS, Kim JH, Lee DW, et al. Gasless robot-assisted nipple-sparing mastectomy: a case report. J Breast Cancer. 2018;21(3):334-338. doi: 10.4048/jbc.2018.21.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee HY, Chang YW, Yu DY, et al. Comparison of single incision endoscopic nipple-sparing mastectomy and conventional nipple-sparing mastectomy for breast cancer based on initial experience. J Breast Cancer. 2021;24(2):196-205. doi: 10.4048/jbc.2021.24.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187-196. doi: 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 23.Ahn SJ, Woo TY, Lee DW, Lew DH, Song SY. Nipple-areolar complex ischemia and necrosis in nipple-sparing mastectomy. Eur J Surg Oncol. 2018;44(8):1170-1176. doi: 10.1016/j.ejso.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 24.Lemaine V, Hoskin TL, Farley DR, et al. Introducing the SKIN score: a validated scoring system to assess severity of mastectomy skin flap necrosis. Ann Surg Oncol. 2015;22(9):2925-2932. doi: 10.1245/s10434-015-4409-3 [DOI] [PubMed] [Google Scholar]
- 25.Komorowski AL, Zanini V, Regolo L, Carolei A, Wysocki WM, Costa A. Necrotic complications after nipple- and areola-sparing mastectomy. World J Surg. 2006;30(8):1410-1413. doi: 10.1007/s00268-005-0650-4 [DOI] [PubMed] [Google Scholar]
- 26.Moyer HR, Ghazi B, Daniel JR, Gasgarth R, Carlson GW. Nipple-sparing mastectomy: technical aspects and aesthetic outcomes. Ann Plast Surg. 2012;68(5):446-450. doi: 10.1097/SAP.0b013e3182394bba [DOI] [PubMed] [Google Scholar]
- 27.Endara M, Chen D, Verma K, Nahabedian MY, Spear SL. Breast reconstruction following nipple-sparing mastectomy: a systematic review of the literature with pooled analysis. Plast Reconstr Surg. 2013;132(5):1043-1054. doi: 10.1097/PRS.0b013e3182a48b8a [DOI] [PubMed] [Google Scholar]
- 28.Gould DJ, Hunt KK, Liu J, et al. Impact of surgical techniques, biomaterials, and patient variables on rate of nipple necrosis after nipple-sparing mastectomy. Plast Reconstr Surg. 2013;132(3):330e-338e. doi: 10.1097/PRS.0b013e31829ace49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson GW, Chu CK, Moyer HR, Duggal C, Losken A. Predictors of nipple ischemia after nipple sparing mastectomy. Breast J. 2014;20(1):69-73. doi: 10.1111/tbj.12208 [DOI] [PubMed] [Google Scholar]
- 30.Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg. 2014;133(3):496-506. doi: 10.1097/01.prs.0000438056.67375.75 [DOI] [PubMed] [Google Scholar]
- 31.Dent BL, Small K, Swistel A, Talmor M. Nipple-areolar complex ischemia after nipple-sparing mastectomy with immediate implant-based reconstruction: risk factors and the success of conservative treatment. Aesthet Surg J. 2014;34(4):560-570. doi: 10.1177/1090820X14528352 [DOI] [PubMed] [Google Scholar]
- 32.Park HS, Lee J, Lai HW, et al. Surgical and oncologic outcomes of robotic and conventional nipple-sparing mastectomy with immediate reconstruction: international multicenter pooled data analysis. Ann Surg Oncol. 2022;29(11):6646-6657. doi: 10.1245/s10434-022-11865-x [DOI] [PubMed] [Google Scholar]
- 33.Daar DA, Abdou SA, Rosario L, et al. Is there a preferred incision location for nipple-sparing mastectomy? a systematic review and meta-analysis. Plast Reconstr Surg. 2019;143(5):906e-919e. doi: 10.1097/PRS.0000000000005502 [DOI] [PubMed] [Google Scholar]
- 34.Moo TA, Nelson JA, Sevilimedu V, et al. Strategies to avoid mastectomy skin-flap necrosis during nipple-sparing mastectomy. Br J Surg. 2023;110(7):831-838. doi: 10.1093/bjs/znad107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park S, Yoon C, Bae SJ, et al. Comparison of complications according to incision types in nipple-sparing mastectomy and immediate reconstruction. Breast. 2020;53:85-91. doi: 10.1016/j.breast.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willey SC, Fan KL, Luvisa K, et al. Predicting ischemic complications in the inframammary approach to nipple-sparing mastectomy: the midclavicular-to-inframammary fold measurement. Plast Reconstr Surg. 2020;145(2):251e-262e. doi: 10.1097/PRS.0000000000006439 [DOI] [PubMed] [Google Scholar]
- 37.Moon J, Lee J, Lee DW, et al. Postoperative pain assessment of robotic nipple-sparing mastectomy with immediate prepectoral prosthesis breast reconstruction: a comparison with conventional nipple-sparing mastectomy. Int J Med Sci. 2021;18(11):2409-2416. doi: 10.7150/ijms.56997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallbeck MS, Law KE, Lowndes BR, et al. Workload differentiates breast surgical procedures: NSM associated with higher workload demand than SSM. Ann Surg Oncol. 2020;27(5):1318-1326. doi: 10.1245/s10434-019-08159-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gubitosi A, Docimo G, Parmeggiani D, et al. Acellular bovine pericardium dermal matrix in immediate breast reconstruction after skin sparing mastectomy. Int J Surg. 2014;12(suppl 1):S205-S208. doi: 10.1016/j.ijsu.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 40.Lee KT, Mun GH. Updated evidence of acellular dermal matrix use for implant-based breast reconstruction: a meta-analysis. Ann Surg Oncol. 2016;23(2):600-610. doi: 10.1245/s10434-015-4873-9 [DOI] [PubMed] [Google Scholar]
- 41.Ooi ASh, Song DH. Reducing infection risk in implant-based breast-reconstruction surgery: challenges and solutions. Breast Cancer (Dove Med Press). 2016;8:161-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong TH, Chung KJ, Kim T, Lee JH. Effect of acellular dermal matrix thickness and surface area on direct-to-implant breast reconstruction. Gland Surg. 2022;11(8):1301-1308. doi: 10.21037/gs-22-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park HS, Lee J, Kim JY, Park JM, Kwon Y. A prospective randomized study to compare postoperative drainage after mastectomy using electrosurgical bipolar systems and conventional electro-cautery. J Breast Cancer. 2022;25(4):307-317. doi: 10.4048/jbc.2022.25.e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe J, Kataoka Y, Koike A, et al. Efficacy and safety of surgical energy devices for axillary node dissection: a systematic review and network meta-analysis. Breast Cancer. 2023;30(4):531-540. doi: 10.1007/s12282-023-01460-7 [DOI] [PubMed] [Google Scholar]
- 45.Ryu JM, Lee J, Lee J, et al. ; Korea Robot-endoscopy Minimal Access Breast Surgery Study Group (KoREa-BSG) . Mastectomy with Reconstruction Including Robotic Endoscopic Surgery (MARRES): a prospective cohort study of the Korea Robot-Endoscopy Minimal Access Breast Surgery Study Group (KoREa-BSG) and Korean Breast Cancer Study Group (KBCSG). BMC Cancer. 2023;23(1):571. doi: 10.1186/s12885-023-10978-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai HW, Toesca A, Sarfati B, et al. Consensus statement on robotic mastectomy-expert panel from International Endoscopic and Robotic Breast Surgery Symposium (IERBS) 2019. Ann Surg. 2020;271(6):1005-1012. doi: 10.1097/SLA.0000000000003789 [DOI] [PubMed] [Google Scholar]
- 47.Ahn JH, Park JM, Choi SB, et al. Early experience of robotic axillary lymph node dissection in patients with node-positive breast cancer. Breast Cancer Res Treat. 2023;198(3):405-412. doi: 10.1007/s10549-022-06760-8 [DOI] [PubMed] [Google Scholar]
- 48.Lee J, Park HS, Lee H, Lee K, Han DH, Lee DW. Axillary lymph node dissection using a robotic surgical system: initial experience. J Surg Oncol. 2020;122(6):1252-1256. doi: 10.1002/jso.26141 [DOI] [PubMed] [Google Scholar]
- 49.Selber JC. The robotic DIEP flap. Plast Reconstr Surg. 2020;145(2):340-343. doi: 10.1097/PRS.0000000000006529 [DOI] [PubMed] [Google Scholar]
- 50.Khan MTA, Won BW, Baumgardner K, et al. Literature review: robotic-assisted harvest of deep inferior epigastric flap for breast reconstruction. Ann Plast Surg. 2022;89(6):703-708. doi: 10.1097/SAP.0000000000003326 [DOI] [PubMed] [Google Scholar]
- 51.Vourtsis SA, Paspala A, Lykoudis PM, et al. Robotic-assisted harvest of latissimus dorsi muscle flap for breast reconstruction: review of the literature. J Robot Surg. 2022;16(1):15-19. doi: 10.1007/s11701-021-01232-5 [DOI] [PubMed] [Google Scholar]
- 52.Dermietzel A, Aitzetmüller M, Klietz ML, et al. Free flap breast reconstruction using a novel robotic microscope. J Plast Reconstr Aesthet Surg. 2022;75(7):2387-2440. doi: 10.1016/j.bjps.2022.04.086 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Scoring system (A-F) for the degree of nipple or areolar necrosis after nipple-sparing mastectomy.
eFigure 2. Scoring system for degree of skin necrosis after nipple-sparing mastectomy based on the involved area and depth of skin necrosis.
eTable. Comparison of patients who underwent conventional nipple-sparing mastectomy (C-NSM) using inframammary fold (IMF) incision or minimal-access NSM (M-NSM)
The Korea Robot-endoscopy Minimal Access Breast Surgery Study Group members
Data sharing statement