Abstract
Introduction
Neoadjuvant endocrine therapy (NET) is recommended for the treatment of invasive breast cancer (BC), particularly luminal subtypes, in locally advanced stages. Previous randomized studies have demonstrated the benefits of aromatase inhibitors in this context. However, NET is typically reserved for elderly or frail patients who may not tolerate neoadjuvant chemotherapy. Identifying non-responsive patients early and extending treatment for responsive ones would be ideal, yet optimal strategies are awaited.
Aims
This non-randomized phase 2 clinical trial aims to assess NET feasibility and efficacy in postmenopausal stage II and III luminal BC patients, identifying predictive therapeutic response biomarkers. Efficacy will be gauged by patients with Ki67 ≤ 10% after 4 weeks and Preoperative Endocrine Prognostic Index (PEPI) scores 0 post-surgery. Study feasibility will be determined by participation acceptance rate (recruitment rate ≥50%) and inclusion rate (>2 patients/month).
Methods
Postmenopausal women with luminal, HER2-tumors in stages II and III undergo neoadjuvant anastrozole treatment, evaluating continuing NET or receiving chemotherapy through early Ki67 analysis after 2 to 4 weeks. The study assesses NET extension for up to 10 months, using serial follow-ups with standardized breast ultrasound and clinical criteria-based NET suspension. Clinical and pathological responses will be measured overall and in the luminal tumor A subgroup. Toxicity, health-related quality of life, and circulating biomarkers predicting early NET response will also be evaluated.
Keywords: breast cancer, endocrine therapy, neoadjuvant, therapeutic response, anastrozole
Introduction
Breast cancer (BC) is a highly heterogeneous disease with considerable morphological diversity. Originally, cases with different clinical behaviors were classified into distinct molecular profiles based on cDNA microarray techniques, resulting in luminal A and B, basal-like, and HER2-enriched tumors.1-3 Subsequently, it was found that protein markers by immunohistochemistry could be a surrogate for intrinsic molecular subtypes with moderate concordance. 4 In practice, these subtypes are defined using immunohistochemistry.5,6
The treatment and prognosis of BC have been guided by TNM staging, histological grade, and the primary intrinsic molecular subtypes. Systemic treatment for early-stage BC can be administered before (neoadjuvant therapy) or after (adjuvant therapy) local curative treatment. Neoadjuvant treatment offers several advantages, such as tumor reduction, enabling conservative resection when mastectomy was initially planned, early treatment of potential micrometastases, and, most importantly, in vivo evaluation of tumor sensitivity to treatment. 7
Currently, there is increasing recognition that patients with hormone receptor-positive and HER2-negative (HR+/HER2-) BC derives limited benefit from neoadjuvant chemotherapy. An alternative approach is neoadjuvant endocrine therapy (NET); however, the classical way of assessing therapeutic response, by measuring pathological complete response, does not seem to be a good therapeutic endpoint in this context, since patients experience significant survival advantages through endocrine therapies, though achieving a pathological complete response is rare. 8 The most well accepted method for assessing response rates to endocrine therapy relies on the Prognostic Index for Preoperative Endocrine Therapy (PEPI) based on tissue evaluation. 9 PEPI incorporates tumor size, lymph node status, Ki67 levels, and ER staining score, categorizing patients into three risk groups (PEPI = 0 indicating low recurrence risk; PEPI = 1 to 3 indicating intermediate risk, and PEPI ≥4 indicating high risk). 10 It has been identified that Ki67 after 2 to 4 weeks of endocrine therapy correlates with Ki67 levels at the time of surgery. Additionally, studies have shown that patients with breast cancer and Ki67 > 10% after 2 to 4 weeks of hormone therapy have less than a 2% chance of achieving PEPI = 0 at surgery.11,12
Liquid biopsy refers to the analysis of body fluid samples that contain various tumor-derived materials, such as nucleic acids, circulating tumor cells (CTCs), or extracellular vesicles.13,14 Strategies based on biological fluids have gained significant attention in the last decade due to their potential as minimally invasive tools. Liquid biopsy-based approaches have shown promise in detecting biomarkers and providing valuable information about tumor dynamics, treatment response, and the emergence of resistance mechanisms. By analyzing circulating tumor DNA (ctDNA), CTCs, microRNAs or exosomes, liquid biopsy techniques offer a non-invasive and real-time assessment of tumor characteristics. In the context of BC, liquid biopsy has the potential to enhance personalized treatment strategies, enable early detection of treatment resistance, and provide valuable prognostic information. Integrating liquid biopsy into clinical trials and treatment protocols can contribute to improved patient management and outcomes. Currently, there is no liquid biopsy capable of selecting patients sensitive to NET.
This is a preliminary phase 2 clinical trial with a single-arm experimental design aiming to determine the efficacy of NET with anastrozole based on patients achieving Ki67 ≤ 10% after 4 weeks and PEPI score 0. It also seeks to evaluate the feasibility of incorporating tumor biopsies and serial breast ultrasounds into the NET approach through assessment of recruitment and acceptance rates. As secondary objectives, we will assess clinical, radiological, and pathological response rates in all included patients, as well as in the subgroup of patients with luminal A tumors. We will evaluate health-related quality of life (HRQOL), treatment toxicity, adherence to anastrozole, conversion rate to conservative surgeries, and explore new biomarkers for endocrine therapy response.
Methods and Analysis
Study Setting
Patients are recruited at the Barretos Cancer Hospital (BCH), a public tertiary oncology hospital that serves Brazilian patients from all regions of the country and is considered a reference in Latin America. The Attenuated Total Reflection-Fourier Transform Infrared (ATR-FTIR) analyses will be conducted at the Laboratory of Nanobiotechnology (Federal University of Uberlandia, Uberlandia, Minas Gerais, Brazil), and the miRNA analyses at the Molecular Oncology Research Center (BCH, Barretos, SP, Brazil). All biological samples are being stored at the Biobank of Barretos Cancer Hospital. 15
Study Population
Inclusion Criteria
• Age greater than or equal to 18 years.
• Patients with histologically confirmed unilateral primary invasive breast carcinoma are eligible. Patients with multicentric and/or multifocal tumors are also eligible if all histopathologically examined lesions meet the following pathological criteria:
Estrogen receptor-positive (Allred ≥6). If Allred is not available in the initial biopsy, any tumor with ≥66% ER expression meets this criterion; progesterone receptor-positive (any percentage); HER2-negative: negative in situ hybridization test or immunohistochemistry (IHC) status of 0 or 1+. If IHC is 2+, a negative in situ hybridization test (FISH, CISH, or SISH) is required to confirm HER2-negative status; histological grade according to Scarf-Bloom-Richardson 1 or 2; and Ki-67 antigen <50% on immunohistochemistry.
• TNM staging according to the eighth Edition cT2-4c cN0-3 M0.
• Clinically palpable tumor larger than 2 cm by palpation and/or imaging examination.
• Functional capacity assessed by Eastern Cooperative Oncology Group Performance Status (ECOG-PS) of 0-2.
• Adequate hematological, renal, and hepatic function.
• Absence of any psychological, familial, sociological, or geographical condition that could potentially hinder adherence to the study protocol and follow-up schedule.
• Tissue acquisition: The patient must agree to provide the necessary research biopsies at baseline, week 4 (rebiopsy), and surgery for biomarker research and biorepository.
Exclusion Criteria
• Inflammatory breast cancer.
• Excisional biopsy of the current BC.
• Hormone replacement therapy of any kind, megestrol acetate, or raloxifene within 1 week before inclusion.
• Axillary staging procedure before study entry. Note: Fine-needle aspiration or needle core biopsy of the axillary lymph node is allowed.
• Cutaneous breast implants that prevent necessary research biopsies or may interfere with palpation of the breast lesion.
• Any treatment for cancer before study entry.
• History of prior invasive BC.
• Patient with any other serious and/or uncontrolled concomitant medical condition that, in the Investigator’s opinion, may cause unacceptable safety risks, contraindicate the patient’s participation in the clinical study, or compromise adherence to the protocol or limit life expectancy to ≤5 years.
Patient Recruitment
Potentially eligible participants are identified during weekly multidisciplinary breast oncology meetings as well as through the active search for postmenopausal patients diagnosed with HR + BC. Subsequently, the cases are reviewed by the research coordinators and study investigators before approaching and inviting patients to participate in the study.
Interventions
Study Drug and Treatment Strategy
Upon signing the Informed Consent Form (ICF), participants have up to 14 days to undergo screening procedures, which include staging tests requested by the clinical oncologist, laboratory tests, breast US, mammography, breast MRI, and the placement of metallic clips on the tumor.
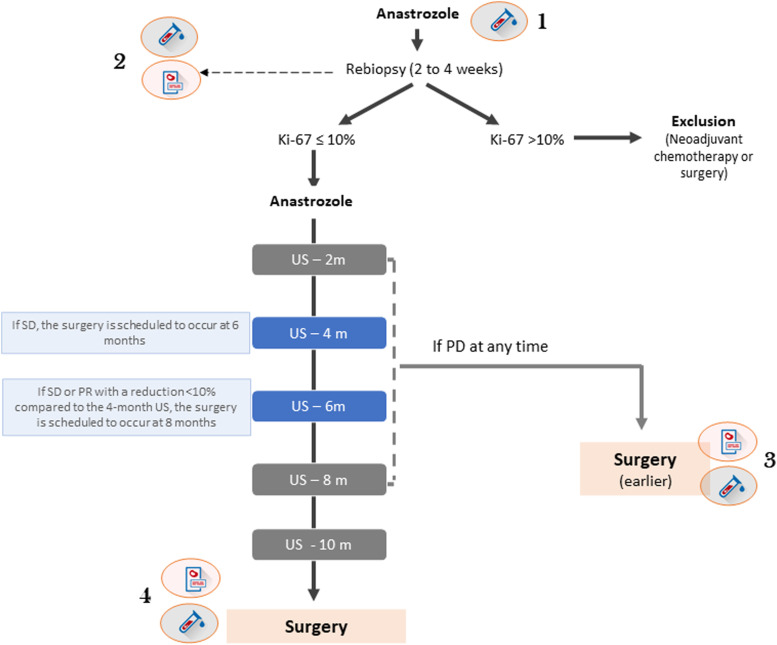
All study participants are receiving oral anastrozole (1 mg/day) continuously until the day prior to the surgical procedure or exclusion from the study. Within 2-4 weeks, a re-biopsy is conducted. If a patient exhibits a ki-67 > 10% in the rebiopsy, the NET is suspended. The patient is then excluded from the study and directed towards neoadjuvant chemotherapy (standard care) or immediate surgery, contingent upon the assessment of the attending clinical oncologist and surgeon. In such cases, all data collected at baseline, along with the therapeutic response assessed by Ki-67 to identify novel predictors, will be analyzed in the study. If a patient has ki-67 ≤ 10%, they continue participating in the study.
At 4 months, a breast US is performed; if there is stable disease (SD), the patient’s surgery is scheduled to take place at 6 months. If there is a partial response (PR), the patient continues to receive NET. At the 6-month breast US, if there is a reduction of ≥10% in the largest diameter compared to the 4-month breast US, the patient continues anastrozole until the next evaluation (8 months). If the reduction is less than 10% or an increase that does not qualify as progressive disease (PD), surgery is scheduled to take place at 8 months. If there is PD based on clinical examination at any time during the study, a breast US is performed for confirmation. If the breast US confirms PD, the patient is referred for surgery within 30 days (Figure 1).
Figure 1.
Schematic diagram of the conduct based on the breast ultrasound response evaluation. The rationale is to extend neoadjuvant endocrine therapy in patients with clinical response up to 10 months; however, surgery is anticipated in cases of little benefit. 1, Blood collection before starting anastrozole; 2, blood and tissue collection at the time of rebiopsy; 3, blood and tissue collection immediately before early surgery; 4, blood and tissue collection immediately before surgery.
Adjuvant Treatment
Adjuvant chemotherapy and radiotherapy are being recommended based on the institutional protocol and multidisciplinary discussions (departmental procedures). If the modified PEPI score is 0, it will be suggested by the protocol that the patient does not receive chemotherapy (although it is not mandatory) but instead receives adjuvant endocrine therapy. For adjuvant endocrine therapy, patients will receive a total of 5 years of adjuvant anastrozole as the standard. However, if there is disease progression during NET, the choice of medication will be at the discretion of the clinical oncologist.
Toxicity Assessment
The adverse events are being graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. 16 The following clinically significant adverse events will be monitored at all visits: Arthralgia; Musculoskeletal pain; Fatigue; Hot flashes; Vaginal discharge; Nausea; Headache; Insomnia; Anxiety; Depression; and Weight gain.
The Portuguese for Brazil Patient-Reported Outcomes version of the CTCAE (PRO-CTCAE Item Library Version 1.0) 17 is being completed alongside CTCAE to specifically assess the same events mentioned in CTCAE, except for weight gain, as it is not covered in PRO-CTCAE.
All patients complete the PRO-CTCAE at each monthly study visit. Additionally, adverse events are evaluated by the investigators using the CTCAE at the same time points.
Serious adverse events (SAEs) must be reported as a notification through the CEP/CONEP Brasil platform, in accordance with Circular Letter No. 13/2020 (National Commission for Ethics in Research, Ministry of Health, Brazil). While SAEs are reported within 48 hours, non-serious adverse event reports are submitted to the Ethics Committee biannually.
Assessment of Anastrozole Adherence
During each study visit, the study nurse counts the anastrozole tablets used by patients. The adherence rate is calculated using this formula: number of tablets used/number of tablets that should have been used in the period. Adherence rate for the entire study is determined by summing across all periods. The adherence criteria considered are as follows: adequate (>80%), reasonable (50%–80%), and poor (<50%).
Study Outcomes
Primary Outcomes
• To evaluate the efficacy of NET with anastrozole as a function of the rate of patients with ki67 ≤ 10% after 4 weeks and PEPI score 0 on the surgical specimen.
• Assess the feasibility of the study by assessing acceptance to participate in the study (recruitment rate ≥50%) and inclusion rate of >2 patients/month.
Secondary Outcomes
• Clinical response rate per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (partial response + complete response) by physical examination, US and MRI between baseline and final assessment (before surgery).
• Clinical response rate per RECIST 1.1 (partial response + complete response) by physical examination, US and MRI between baseline and final assessment (before surgery) in the group of patients classified as Luminal A.
• Evaluate the median time (in days) until the best response was obtained by physical examination and breast US, and longitudinally evaluate the medians of the largest diameters, tumor volume, and the percentages of tumor reduction.
• To assess the Time to Deterioration of Quality of Life (TDQOL) by considering the value of worsening HRQOL in the summed scores of the EORTC QLQ-C30 and BR-45 with a cut-off of 10 points.
• To evaluate the rate of conservative surgery among patients treated with anastrozole and the rate of mastectomy conversion or inoperability for conservative surgery after anastrozole.
• Evaluate the treatment adherence rate measured by the number of pills used/number of pills that should be used in the evaluated period, being considered adequate as >80%.
• To identify potential predictive biomarkers of response in Ki67 reduction on re-biopsy through the analysis of miRNA expression using NanoString technologies and isolated extracellular vesicles using the ATR-FTIR.
Sample Size
This study is based on the hypothesis that patients with TNM II and III HR + BC may be spared from receiving chemotherapy based on their response to NET. The minimum sample size for this phase II, non-comparative preliminary study was estimated through probability analysis using the formula N = z2 * p * (1-p)/e2; where z is the Z score for a specific confidence level, p is the assumed population proportion, and e is the desired margin of error. Given the results of the Alternate study, 18 where the response rate to NET (number of patients with PEPI 0 in the surgical specimen/total number of patients who initiated NET) was 18.6% (P = 0.186), with an alpha error of 5% (z = 1.96) and a maximum error of ±10% (e = 0.10), the minimum required sample size for the study is 59 patients.
Trial Status
The first patient was recruited on July 20, 2022, and currently 33 patients have been enrolled in the study, which is ongoing. It is anticipated that participant recruitment will be concluded by August 2024, and the final surgery will take place in July 2025.
Data Collection Methods and Measurements
Pathological Response Assessment
The evaluation of the pathological response will be primarily conducted using the PEPI score (tumor size, nodal status, ki-67 (%), and estrogen receptor status) 9 (Table 1). Additionally, study pathologists will categorize the tumors according to Residual Cancer Burden, 19 and CPS-EG.20,21
Table 1.
Preoperative Endocrine Prognostic Index (PEPI) Score.
| Variable | Category | PEPI Score - points | |
|---|---|---|---|
| RFS | BCSS | ||
| Tumor size | ypT1-2 | 0 | 0 |
| ypT3-4 | 3 | 3 | |
| Regional lymph nodes | Negative | 0 | 0 |
| Positive | 3 | 3 | |
| Ki-67 | 0%–2.7% | 0 | 0 |
| >2.7% a 7.3% | 1 | 1 | |
| >7.3% a 19.7% | 1 | 2 | |
| >19.7% a 53.1% | 2 | 3 | |
| >53.1% | 3 | 3 | |
| ER, allred score | 3-8 | 0 | 0 |
| 0-2 | 3 | 3 | |
Legend: RFS = Relapse free survival. BCSS = Breast cancer specific survival. ER = Estrogen receptor.
Clinical and Radiological Response Assessments
The assessment of response will follow the RECIST version 1.1, considering Complete Response (CR), Partial Response (PR), Stable Disease (SD), or Progressive Disease (PD).22,23 Each evaluation method will have an assessed response (clinical examination, breast US, and breast MRI). Target lesions will be selected based on their size (lesions with the largest diameter) and must serve as reproducible repeated measurements. Up to 2 lesions in the breast can be identified as target lesions. Pathological axillary lymph nodes should not be designated as target lesions, and their measurements should not be included in the sum of diameters.
Health-Related Quality of Life (HRQOL)
Evaluation of HRQOL will be conducted using the European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire (EORTC QLQ-C30) 24 and the Breast Cancer Specific Module (EORTC QLQ-BR45) at baseline and every 2 months until surgery.
The EORTC QLQ-C30 comprises 30 items, covering 5 functional scales, 3 symptom scales, a global health status scale, and individual items addressing common cancer-related symptoms. Responses are rated on a Likert scale ranging from 0 to 4 points, while items related to quality of life and overall health status use a 7-point Likert scale, with scores ranging from 0 to 100 (higher scores indicating better health-related quality of life and global health status, respectively).24,25
The EORTC QLQ-BR45 is an additional module specifically designed for assessing breast cancer patients undergoing chemotherapy, radiotherapy, and/or endocrine therapy. 26 It comprises 45 questions that assess body image, sexual functioning, sexual enjoyment, future perspective, side effects of systemic therapy, breast symptoms, arm symptoms, hair loss-related disruption, as well as hormonal therapy-related symptoms, skin, and sexual symptoms due to low hormone levels. The following domains will be used for the planned HRQOL analyses in the study:
• EORTC QLQ-C30: global health status, physical functioning, emotional functioning, cognitive functioning, role functioning, fatigue, nausea and vomiting, pain, and insomnia.
• EORTC QLQ-BR45: target therapy scale, particularly the sub-scale of endocrine therapy-related symptoms.
Ki-67 Immunohistochemical Protocol
Histological sections with a thickness of 3 micrometers from formalin-fixed paraffin-embedded tissue samples undergo a deparaffinization process and antigen retrieval at a temperature of 95°C for 60 minutes. The signal detection kit used is OptiView (Ventana Medical Systems, USA), integrated into the BenchMark Ultra platform (Ventana Medical Systems, USA). The antibody utilized is anti-Ki-67, clone 30-9 (Ventana Medical System, USA).
Digital Ki-67 Assessment Procedure in Tumor Re-Biopsy Samples
Immunohistochemical slides undergo a high-resolution scanning process using the Aperio CS2 platform (Leica Biosystems, Germany) at a magnification of ×400. The resulting scanning files will be imported into the QuPath v0.4.3 analytical software in DAB-brightfield representation. The employed analytical methodologies will include the positive_cell_detection and single_threshold algorithms, which quantitatively evaluate the extent of cell positivity. These algorithms will be applied to the region with the highest concentration of positive neoplastic cells (hotspot), manually selected by the responsible pathologist. The outcomes will be reported as a percentage.
Definition of Luminal A Cases
For the immunohistochemical definition of Luminal A subtype tumors, the following three items need to be present: (1) Positive Estrogen Receptor (ER); (2) Negative HER2; and (3) Ki67 < 14% or KI-67 between 14%–19% and Progesterone Receptor (PR) ≥20%. The molecular subtype (Luminal A, Luminal B, Basal-like, and HER2-enriched) will be determined, and the ROR (risk of recurrence) score will be calculated based on the PAM50.27,28
Molecular Analyses
The blood samples from baseline and rebiopsy will be used for liquid biopsy analyses (as described below). The other biological samples will be archived in the study’s biorepository (as approved by the Research Ethics Committee).
Sample Collection and Processing
Plasma total RNA isolation will be performed using the miRNAeasy Serum/Plasma kit (Qiagen). The extracted RNA was stored at −80°C until use. The RNA concentration and purity of each sample were assessed using the NanoDrop 2000 (Thermo Fisher Scientific, USA) and Qubit (Thermo Fisher Scientific, USA), respectively.
Evaluation of Cell-Free MicroRNAs (cfmiRNA) Profile by NanoString Technology
The miRNA expression can be assessed using the nCounter® miRNA Expression Assays panel (NanoString Technologies, Seattle, WA, USA). This panel consists of 800 targets for different cancer-associated miRNAs (https://www.nanostring.com/products/miRNA) and allows the expression analysis of different miRNAs in different types and subtypes of tumors. In summary, around 100 ng of total RNA will be subjected to tag binding and hybridization with the Reporter CodeSet and Capture ProbeSet of the assay, then processed using the NanoString PrepStation and immobilized on the nCounter cartridge according to manufacturer’s instructions (NanoString Technologies, Seattle, WA, USA). Finally, the assay will be placed in the nCounter® Digital Analyzer for image capture and data acquisition. Statistical analyzes of differential miRNA expression will be performed using the limma package of Bioconductor in the R environment, assuming a significance level of P < 0.05 between the evaluated groups.
Extracellular Vesicle (EV) Evaluation
EV will be precipitated using the ExoQuick™ Exosome Precipitation Solution (cat. no. EXOQ5A-1; System Biosciences). Briefly, plasma samples will be centrifuged at 3000 × g for 15 min at 4°C to remove clotted materials and cell debris. Thrombin will be used to pre-treat plasma samples to make them compatible with ExoQuick exosome precipitation. A volume of 4 μl Thrombin will be added to 0.5 mL plasma and incubated at room temperature for 5 min while mixing, then centrifuge at 10000 r/min for 5 min. The supernatant will be transferred to a new, clean tube. A volume of 126 μl ExoQuick Exosome Precipitation Solution will be added to 500 μl plasma pre-treated with Thrombin and the mixture will be refrigerated for 30 min. The mixture will be centrifuged at 1500 × g for 30 min at 4°C, and the supernatants will be discarded. The residual solution will be centrifuged at 1500 × g for 5 min at 4°C, and the supernatant will be removed. The exosome pellet will resuspend in 500 μl PBS. Finally, EV will be separated with qEV Size Exclusion Chromatography (cat. no. ICO-70; Izon Science). Briefly, 500 μl of samples will be centrifuged at 10000×g for 10 minutes prior to loading onto a qEV column, then allowed to run into the column and start collecting the samples fractions. For validation of EV separation, they will be analyzed by Western blotting (CD63, APOA1), NanoSight, and Transmission electron microscopy.
The isolated vesicles will be analyzed by ATR-FTIR using the Cary 630 equipment (Agilent Technologies) coupled to the diamond sensor, which functions as an internal reflection element for attenuated total reflectance. The MicroLab software (Agilent Technologies) will be used for data capture. The air spectrum will be used as background before analyzing each sample. The spectra will be analyzed in the wavenumber region from 4000 cm−1 to 650 cm−1, with 128 scans and a resolution of 4 cm−1. The obtained spectra will undergo preprocessing, including positive Rubberband baseline correction and normalization by minimum and maximum using Orange data mining software version 3.26. This software will also be used to apply derivatives when necessary, using the Savitzky-Golay filter.
Statistical Methods
Most of the study analyses are descriptive, with response rates calculated along with their respective 95% confidence intervals. Response rates will be compared based on molecular classification (intrinsic subtype) in Luminal A vs non-Luminal A using Chi-square or Fisher’s exact tests. Specifically, responses by clinical examination, breast ultrasound, and breast MRI will be categorized as CR/PR vs SD/PD. Regarding pathologic response, the categorizations will be PEPI score 0 vs PEPI score >1; Residual Cancer Burden (RCB) 0/I vs RCB II/III. Additionally, logistic regression analyses will be conducted with responses as outcomes and molecular subtype as a predictor variable, adjusted for T staging (cT2 vs cT3-4b) and N staging (cN0 vs cN1-3).
Concordance between clinical response evaluation methods and pathological evaluations will be measured using weighted Kappa test.
HRQOL indices will be assessed over time and presented graphically. TDQOL will be calculated using the cut-off point of 10, with baseline as the reference point, according to previous studies.29,30 Event-free survival times will be estimated using the Kaplan-Meier method, and the curves will be compared using the log-rank test.
Toxicities will be described in absolute values and percentages according to CTCAE categories, specifically (1) any category; (2) G2-4; (3) G3/4, separately. Besides an aggregated measure for the entire study duration, toxicities will be reported at specific time points (2, 4, 6, 8, and 10 months). To quantify the burden of chronic low-grade events, the area under the curve (AUC) for each adverse event, as measured by CTCAE, will be calculated and graphically illustrated. 31 To offer a descriptive view of the severity and progression of adverse events (measured by both CTCAE and PRO-CTCAE), toxicity “heatmaps” will be generated as outlined in previous studies.31,32 CTCAE grades zero to 4 will correspond to PRO-CTCAE responses of “not at all,” “a little bit,” “somewhat,” “quite a bit,” and “very much,” respectively. The longitudinal CTCAE and PRO-CTCAE heatmaps will be shown side by side, with patients arranged in the same sequence for easier visual comparison.
Biomarker analyses will be divided into (1) development (test) and (2) validation phases. The sample will be divided into 30 patients for the development cohort (potential biomarker [miRNA and EVs] search by NanoString and ATR-FTIR, respectively) and 29 patients for the validation of biomarkers by real-time PCR. For accuracy determination, samples classified with Ki67 ≤ 10% in the rebiopsy by immunohistochemistry will be considered as the “gold standard.” Therefore, the biomarkers identified in the development phase will be evaluated in terms of sensitivity, specificity, positive predictive value, negative predictive value, along with their respective 95% confidence intervals. Additionally, the continuous values of each biomarker variable will be assessed regarding the Area Under the Receiver Operating Characteristic (AUROC) curve.
A P-value <0.05 will be considered statistically significant. The statistical software SPSS v.21 will be used for the statistical analyses.
Ethics and Dissemination
The present study is conducted in accordance with the guidelines of Resolution CNS 466/12 (Brazilian National Health Council) and received approval from the Research Ethics Committee of the BCH (HCB number 2283/2021; approval number 5.213.699). This research protocol is registered with the Brazilian Registry of Clinical Trials (ReBEC, RBR-5pygzhj); UTN WHO International Clinical Trials Registry Plataform: U1111-1275-1903.
The responsible researchers are committed to ensuring the privacy of the participants, carefully preserving the confidentiality of their data and information. It is important to emphasize that the patients involved will receive necessary medical attention independent of their participation in the study, and their decision to withdraw or refuse participation will not result in any harm to their treatment. All eligible individuals will be invited to participate by the investigators in the study and, on a voluntary basis, will sign the ICF.
The findings of the trial will be disseminated through peer-reviewed scientific journals and conferences. Furthermore, the study findings will be shared with the Brazilian government, given that the study is funded by the Brazilian Ministry of Health. The study also holds potential for technological innovation through the identification of biomarker profiles linked to endocrine therapy sensitivity, which may lead to patent filings.
Data Management
All study data is entered into REDCap (Research Electronic Data Capture) 33 spreadsheets by a trained research coordinator, and consistency is verified by second researcher. The final trial dataset will be accessed by the principal investigator, the main study coordinator, and the biostatisticians who will carry out the statistical analyses.
Data Monitoring and Auditing
An independent monitoring committee comprising staff from the Center for Research Support (NAP) of BCH will oversee the collection and analysis of the study data at two moments. The committee is independent of the authors and the funder. Periodic and final reports, including study results, are submitted for approval to the study funding agency. No interim data analysis is planned for this study.
The reporting of this study conforms to SPIRIT guidelines. 34
Discussion
Given that many patients with HR+/HER2- BC receive neoadjuvant chemotherapy with little clinical benefit and potential negative impact on their HRQOL, strategies that select the most suitable patients for treatment with NET are awaited. Thus, the present preliminary study aims to evaluate the efficacy and feasibility of the neoadjuvant use of an aromatase inhibitor for postmenopausal women with luminal-HER2 negative BC in TNM stages II and III.
Although the role of neoadjuvant endocrine therapy (NET) is established in postmenopausal patients with HR+/HER2-breast tumors, particularly those with a low proliferative index, it is still more commonly used in practice for treating older patients or those with comorbidities. 35 Several previous clinical trials36-39 and a meta-analysis 40 have demonstrated that aromatase inhibitors are more effective than tamoxifen, yielding higher clinical and radiological response rates as well as higher rates of breast conservation surgeries. Additionally, phase 2 randomized clinical trials41,42 have shown that chemotherapy does not appear to be superior to NET in postmenopausal patients with luminal BCs. In recent years, the focus of research in NET has been on the combination of cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) and endocrine therapy. In the NEOPAL trial,43,44 the combination of letrozole and palbociclib was equivalent to standard chemotherapy in terms of response rates and survival times in a cohort of BC patients predominantly classified molecularly as luminal B. In the CORALLEEN trial, 45 the combination of neoadjuvant letrozole plus ribociclib resulted in similar molecular responses compared to standard chemotherapy in luminal B BC patients according to PAM50. However, we believe it is still necessary to define the profile of responders to aromatase inhibitors and the best way to assess responsiveness to endocrine therapy before adding a CDK4/6 inhibitor into routine practice, mainly due to the increased costs in health care systems with limited resources, such as the Brazilian system.
Most studies on NET have used an aromatase inhibitor for a duration of 3 to 6 months. However, two important studies have explored the concept of extending the duration of NET and its relationship with therapeutic response. Carpenter et al 46 evaluated patients with HR + BC who were ineligible for conservative surgery. These patients were treated with neoadjuvant letrozole and followed until they were considered eligible for conservative surgery. The median duration of NET was 7.5 months. Dixon et al 47 prospectively evaluated 182 patients undergoing NET. Of these, 63 received letrozole for more than 3 months. The median reduction in clinical volume was 52% in the first three months and 50% from three to 6 months. Further consistent reductions were observed between 6 and 12 months (37%) and between 12 and 24 months (33%). Therefore, we chose to extend the duration of NET for more than 6 months and defined a maximum period of 10 months due to logistical considerations in a clinical study. A novel protocol with standardized criteria to determine whether treatment should continue or be discontinued based on clinical and ultrasonography responses is being used.
Both the 21-gene Oncotype DX Breast Recurrence Score (RS) 48 and the 12-gene EndoPredict molecular score 49 have been identified as useful molecular predictor tools for identifying the best candidates for NET. For Oncotype DX, 55% of patients with a recurrence score (RS) of less than 18 achieved partial or complete response, compared with 22% of those with an RS above 31 (P < 0.001). Additionally, in multivariable analyses, continuous RS results were among the variables associated with clinical response. Regarding EndoPredict, patients classified as low and high risk had a 27.3% and 7.7% chance, respectively, of achieving residual cancer burden (RCB) 0/1 (P < 0.001). In the ALTERNATE trial, 50 the percentage change in Ki67 at week 4 from pretreatment Ki67 levels was −84.8% and −76.7% in Luminal A and Luminal B, respectively. Furthermore, among patients with pretreatment Ki67 levels >10%, the rate of Ki67 > 10% at week 4 was 13.5% in Luminal A and 43.7% in Luminal B. Although these findings were not statistically compared, they strongly suggest that PAM50 classification of Luminal A or B is a useful tool for predicting good NET responders. In the ANNE trial, PAM50 analyses will be performed on baseline tumor samples to investigate biological subtype and risk of relapse (ROR). These results will be correlated with ongoing Ki67 response, as well as clinical, radiological, and pathologic response.
Among the distinctive aspects of this study, the researchers intend to standardize tumor rebiopsy within 2-4 weeks and employ digital immunohistochemistry analysis of Ki67. Most importantly, the authors aim to identify potential biomarkers of endocrine sensitivity at the time of rebiopsy, thus avoiding the need for future tumor resampling.
Anticipated outcomes of this study are expected to significantly influence the design of a subsequent, larger randomized clinical trial. Essential aspects encompass the standardization of rebiopsy procedures, the utilization of digital Ki67 analysis, and the carefully delineated clinical and ultrasonography follow-up process to determine the optimal timing for surgery. The results regarding efficacy and feasibility will subsequently guide the formulation of the ensuing study.
The present research protocol has its limitations. The primary limitation is the small sample size and limited potential for generalizability. However, this should not necessarily be seen as a shortcoming of the study, but rather as an inherent characteristic of this type of research, as it is a preliminary phase 2 study. We strongly believe that conducting a preliminary study is essential for the design and planning of a larger, subsequent study, allowing for better management of costs and human resources. A specific limitation is the lack of assessment of EndoPredict or Oncotype DX in the baseline samples to compare with the PAM50 results (molecular subtypes and ROR score), as well as microRNA and ATR-FTIR findings as potential novel NET predictors of response. Nonetheless, given the availability of sufficient biological material, it is possible that additional funding could be secured for these future analyses, contingent upon ethical approval. Another limitation of the study is that the adjuvant treatment is not predefined by the protocol and is left to the discretion of the treating oncologist. Although there is a suggestion to forgo chemotherapy for patients with a PEPI score of 0, the research therapeutic protocol itself concludes with surgical management.
In conclusion, the standardized framework to be established within this ongoing study may serve as a foundation for the future integration of cyclin inhibitors, mTOR or PI3K inhibitors, and other innovative drugs into upcoming trials. This framework could be implemented either initially (as a first-line therapy) or after the failure of initial endocrine therapy (based on ki-67 rebiopsy or dependent on a novel liquid biopsy biomarker). This approach would streamline treatment strategies and enhance patient care.
Acknowledgments
We would like to express our gratitude to the biostatistician Marcos de Lima for his valuable contributions to the study design. We also extend our heartfelt thanks to Dr Cristiano de Pádua Souza, Dr Vinicius Duval da Silva, Dr Chrissie Casella Amirati, Dr Rui Manuel Vieira Reis, and Dr Antonio Bailão Júnior for their insightful comments and suggestions during the study’s development.
Appendix.
Abbreviations
- NET
Neoadjuvant endocrine therapy
- BC
Breast cancer
- PEPI
Preoperative Endocrine Prognostic Index
- CTCs
Circulating tumor cells
- ctDNA
Circulating tumor DNA
- BCH
Barretos Cancer Hospital
- ATR-FTIR
Attenuated Total Reflection-Fourier Transform Infrared
- IHQ
Immunohistochemistry
- FISH
Fluorescence In Situ Hybridization
- CISH
Chromogenic In Situ Hybridization
- SISH
Silver In Situ Hybridization
- ECOG-PS
Eastern Cooperative Oncology Group Performance Status
- CR
Complete Response
- SD
Stable disease
- PR
Partial response
- PD
Progressive disease
- CTCAE
Common Terminology Criteria for Adverse Events
- PRO-CTCAE
Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events
- TDQOL:
Time to Deterioration of Quality of Life
- US:
Ultrasound
- MRI
Magnetic resonance image
- HRQOL:
Health-related quality of life
- EORTC QLQ-C30
European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire
- ROR
Risk of recurrence
- cfmiRNA
Cell-Free MicroRNAs
- EV
Extracellular Vesicle
- AUROC:
Area Under the Receiver Operating Characteristic
- miRNAs
microRNAs
- mRNAs
messenger RNAs.
Author Contributions: CEP and ATFS drafted the manuscript. All other authors critically revised and approved the final version in its current form.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is funded by the Ministry of Health, Department of Science and Technology (DECIT), under the CNPq/MS-SCTIE-DECIT Call No. 50/2022, Process No. 406775/2022-2. CEP is a recipient of CNPq’s Productivity Scholarship, Level 2 (Call No. 09/2020, Process No. 313414/2020-3) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) Grant numbers: APQ-01339-21 and APQ-01961-23. The study funders have no role in the design, collection, management, analysis, and interpretation of research data, the writing of the report, or the decision to submit the report for publication.
Trial Registration: This research protocol is registered with the Brazilian Registry of Clinical Trials (ReBEC, RBR-5pygzhj). UTN WHO International Clinical Trials Registry Plataform: U1111-1275-1903.
Protocol Version: The current version of the project is dated May 16, 2023. Significant changes to the research protocol require prior approval from the Ethics Committee of the Barretos Cancer Hospital.
Roles and Responsibilities: CEP serves as the Principal Investigator (PI) and oversees study conduct, as well as financial resources. YCPM handles ATR-FTIR analyses, while MMCM is responsible for microRNA analyses. GRT conducts Ki67 analysis and pathology review. AHUW and NO perform the study evaluation ultrasounds. BSRP plays a role in designing and analyzing quality of life and toxicity scores. IOJ and DCL contribute to study design from the mastology and clinical oncology departments. Additionally, ISSO, VSG, and ATFS are master’s, Ph.D., and postdoctoral students involved in data collection and molecular analysis.
Ethical Statement
Ethical Approval and Consent to Participate
The present study is conducted in accordance with the guidelines of Resolution CNS 466/12 (Brazilian National Health Council) and received approval from the Research Ethics Committee of the Barretos Cancer Hospital (HCB number 2283/2021; approval number 5.213.699). All patients included in the study signed an Informed Consent Form in person (written), which was duly approved by the Ethics Committee.
ORCID iD
Carlos Eduardo Paiva https://orcid.org/0000-0002-7934-1451
References
- 1.Van De Vijver MJ, He YD, Van ’T Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999-2009. [DOI] [PubMed] [Google Scholar]
- 2.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747-752. [DOI] [PubMed] [Google Scholar]
- 3.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869-10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maisonneuve P, Disalvatore D, Rotmensz N, et al. Proposed new clinicopathological surrogate definitions of luminal A and luminal B (HER2-negative) intrinsic breast cancer subtypes. Breast Cancer Res. 2014;16:R65. doi: 10.1186/BCR3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the st gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gnant M, Harbeck N, Thomssen C. St. Gallen 2011: summary of the consensus discussion. Breast Care. 2011;6:136-141. doi: 10.1159/000328054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39:1485-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burstein HJ. Systemic therapy for estrogen receptor-positive, HER2-negative breast cancer. N Engl J Med. 2020;383:2557-2570. [DOI] [PubMed] [Google Scholar]
- 9.Ellis MJ, Tao Y, Luo J, et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst. 2008;100:1380-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis MJ, Suman VJ, Hoog J, et al. Ki67 proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: results from the American college of surgeons oncology group Z1031 trial (alliance). J Clin Oncol. 2017;35:1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson JA, Budd GT, Carey LA, et al. Improved surgical outcomes for breast cancer patients receiving neoadjuvant aromatase inhibitor therapy: results from a multicenter phase II trial. J Am Coll Surg. 2009;208:906-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99:167-170. [DOI] [PubMed] [Google Scholar]
- 13.Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-548. [DOI] [PubMed] [Google Scholar]
- 14.Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4:650-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuber AC, Tostes CH, Ribeiro AG, et al. The biobank of barretos cancer hospital: 14 years of experience in cancer research. Cell Tissue Bank. 2022;23:271-284. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) Common Terminology Criteria for Adverse Events v4.0 (CTCAE). Bethesda, MA: National Cancer Institute; 2009. [Google Scholar]
- 17.Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106:dju244. doi: 10.1093/JNCI/DJU244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma CX, Suman VJ, Leitch AM, et al. ALTERNATE: neoadjuvant endocrine treatment (NET) approaches for clinical stage II or III estrogen receptor-positive HER2-negative breast cancer (ER+ HER2- BC) in postmenopausal (PM) women: alliance A011106. J Clin Oncol. 2020;38:504. [Google Scholar]
- 19.Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414-4422. [DOI] [PubMed] [Google Scholar]
- 20.Marmé F, Lederer B, Blohmer JU, et al. Utility of the CPS + EG staging system in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer treated with neoadjuvant chemotherapy. Eur J Cancer. 2016;53:65-74. [DOI] [PubMed] [Google Scholar]
- 21.Mittendorf EA, Jeruss JS, Tucker SL, et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol. 2011;29:1956-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [DOI] [PubMed] [Google Scholar]
- 24.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365-376. [DOI] [PubMed] [Google Scholar]
- 25.Paiva CE, Carneseca EC, Barroso EM, et al. Further evaluation of the EORTC QLQ-C30 psychometric properties in a large Brazilian cancer patient cohort as a function of their educational status. Support Care Cancer. 2014;22:2151-2160. doi: 10.1007/s00520-014-2206-3 [DOI] [PubMed] [Google Scholar]
- 26.Bjelic-Radisic V, Cardoso F, Cameron D, et al. An international update of the EORTC questionnaire for assessing quality of life in breast cancer patients: EORTC QLQ-BR45. Ann Oncol. 2020;31:283-288. [DOI] [PubMed] [Google Scholar]
- 27.Wallden B, Storhoff J, Nielsen T, et al. Development and verification of the PAM50-based Prosigna breast cancer gene signature assay. BMC Med Genom. 2015;8:54. doi: 10.1186/s12920-015-0129-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker JS, Mullins M, Cheang MCU, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27:1160-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anota A, Hamidou Z, Paget-Bailly S, et al. Time to health-related quality of life score deterioration as a modality of longitudinal analysis for health-related quality of life studies in oncology: do we need RECIST for quality of life to achieve standardization? Qual Life Res. 2015;24:5-18. doi: 10.1007/s11136-013-0583-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139-144. [DOI] [PubMed] [Google Scholar]
- 31.Thanarajasingam G, Leonard JP, Witzig TE, et al. Longitudinal Toxicity over Time (ToxT) analysis to evaluate tolerability: a case study of lenalidomide in the CALGB 50401 (Alliance) trial. Lancet Haematol. 2020;7:e490-e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filho OM, Giobbie-Hurder A, Lin NU, et al. A dynamic portrait of adverse events for breast cancer patients: results from a phase II clinical trial of eribulin in advanced HER2-negative breast cancer. Breast Cancer Res Treat. 2021;185:135-144. [DOI] [PubMed] [Google Scholar]
- 33.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiba A, Hoskin TL, Heins CN, Hunt KK, Habermann EB, Boughey JC. Trends in neoadjuvant endocrine therapy use and impact on rates of breast conservation in hormone receptor-positive breast cancer: a national cancer data base study. Ann Surg Oncol. 2017;24:418-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cataliotti L, Buzdar AU, Noguchi S, et al. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative ‘Arimidex’ Compared to Tamoxifen (PROACT) trial. Cancer. 2006;106:2095-2103. [DOI] [PubMed] [Google Scholar]
- 37.Eiermann W, Paepke S, Appfelstaedt J, et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: a randomized double-blind multicenter study. Ann Oncol. 2001;12:1527-1532. [DOI] [PubMed] [Google Scholar]
- 38.Masuda N, Sagara Y, Kinoshita T, et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): a double-blind, randomised phase 3 trial. Lancet Oncol. 2012;13:345-352. [DOI] [PubMed] [Google Scholar]
- 39.Ellis MJ, Coop A, Singh B, et al. Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol. 2001;19:3808-3816. [DOI] [PubMed] [Google Scholar]
- 40.Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2:1477-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alba E, Calvo L, Albanell J, et al. Chemotherapy (CT) and hormonotherapy (HT) as neoadjuvant treatment in luminal breast cancer patients: results from the GEICAM/2006-03, a multicenter, randomized, phase-II study. Ann Oncol. 2012;23:3069-3074. [DOI] [PubMed] [Google Scholar]
- 42.Semiglazov VF, Semiglazov VV, Dashyan GA, et al. Phase 2 randomized trial of primary endocrine therapy versus chemotherapy in postmenopausal patients with estrogen receptor-positive breast cancer. Cancer. 2007;110:244-254. [DOI] [PubMed] [Google Scholar]
- 43.Cottu P, D’Hondt V, Dureau S, et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann Oncol. 2018;29:2334-2340. [DOI] [PubMed] [Google Scholar]
- 44.Delaloge S, Dureau S, D’Hondt V, et al. Survival outcomes after neoadjuvant letrozole and palbociclib versus third generation chemotherapy for patients with high-risk oestrogen receptor-positive HER2-negative breast cancer. Eur J Cancer. 2022;166:300-308. [DOI] [PubMed] [Google Scholar]
- 45.Prat A, Saura C, Pascual T, et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2020;21:33-43. [DOI] [PubMed] [Google Scholar]
- 46.Carpenter R, Doughty JC, Cordiner C, et al. Optimum duration of neoadjuvant letrozole to permit breast conserving surgery. Breast Cancer Res Treat. 2014;144:569-576. [DOI] [PubMed] [Google Scholar]
- 47.Dixon JM, Renshaw L, MacAskill EJ, et al. Increase in response rate by prolonged treatment with neoadjuvant letrozole. Breast Cancer Res Treat. 2009;113:145-151. [DOI] [PubMed] [Google Scholar]
- 48.Iwata H, Masuda N, Yamamoto Y, et al. Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, HER2-negative breast cancer: the TransNEOS study. Breast Cancer Res Treat. 2019;173:123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubsky PC, Singer CF, Egle D, et al. The EndoPredict score predicts response to neoadjuvant chemotherapy and neoendocrine therapy in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer patients from the ABCSG-34 trial. Eur J Cancer. 2020;134:99-106. [DOI] [PubMed] [Google Scholar]
- 50.Ma CX, Suman VJ, Sanati S, et al. Endocrine-sensitive disease rate in postmenopausal patients with estrogen receptor-rich/ERBB2-negative breast cancer receiving neoadjuvant anastrozole, fulvestrant, or their combination: a phase 3 randomized clinical trial. JAMA Oncol. 2024;10:362-371. [DOI] [PMC free article] [PubMed] [Google Scholar]