Abstract
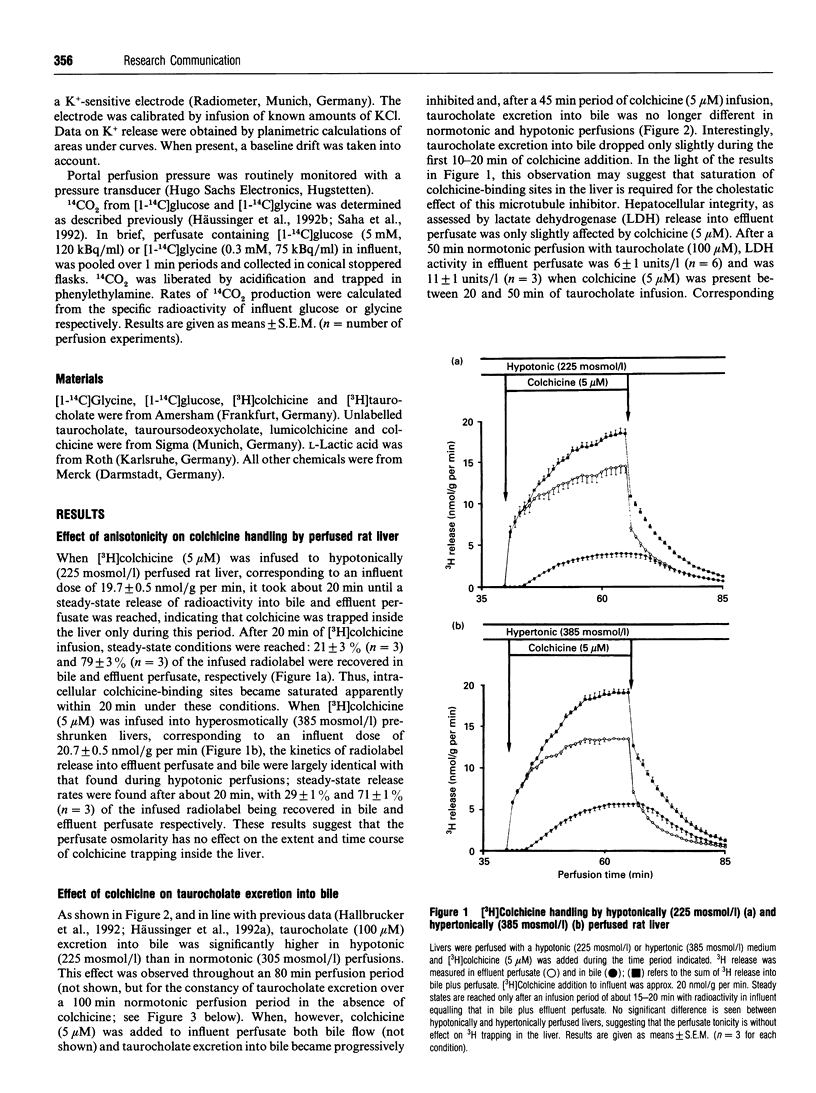
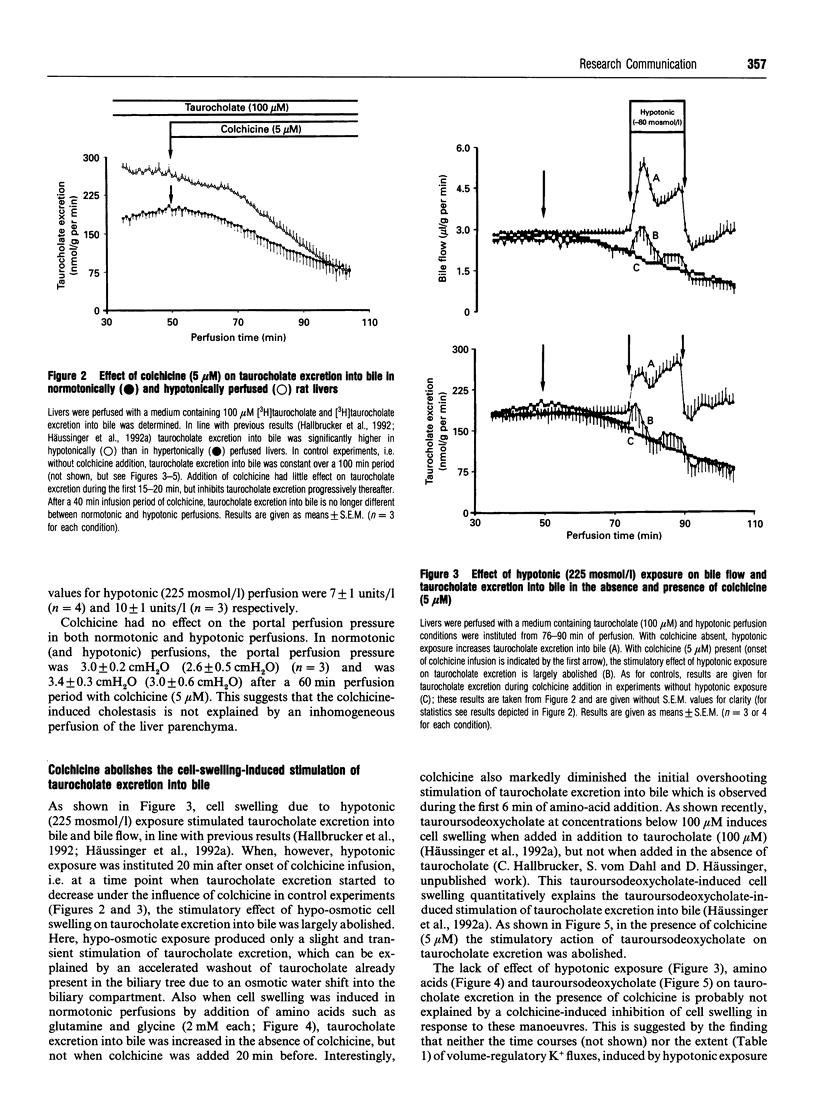
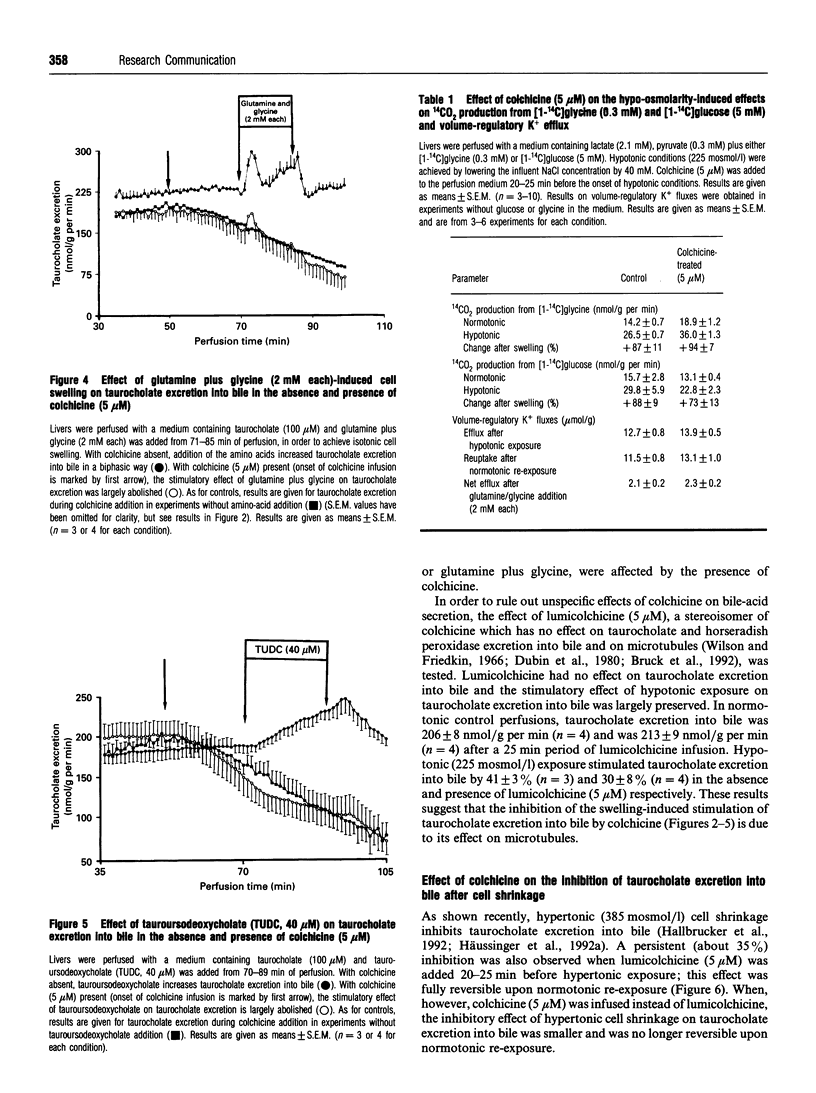
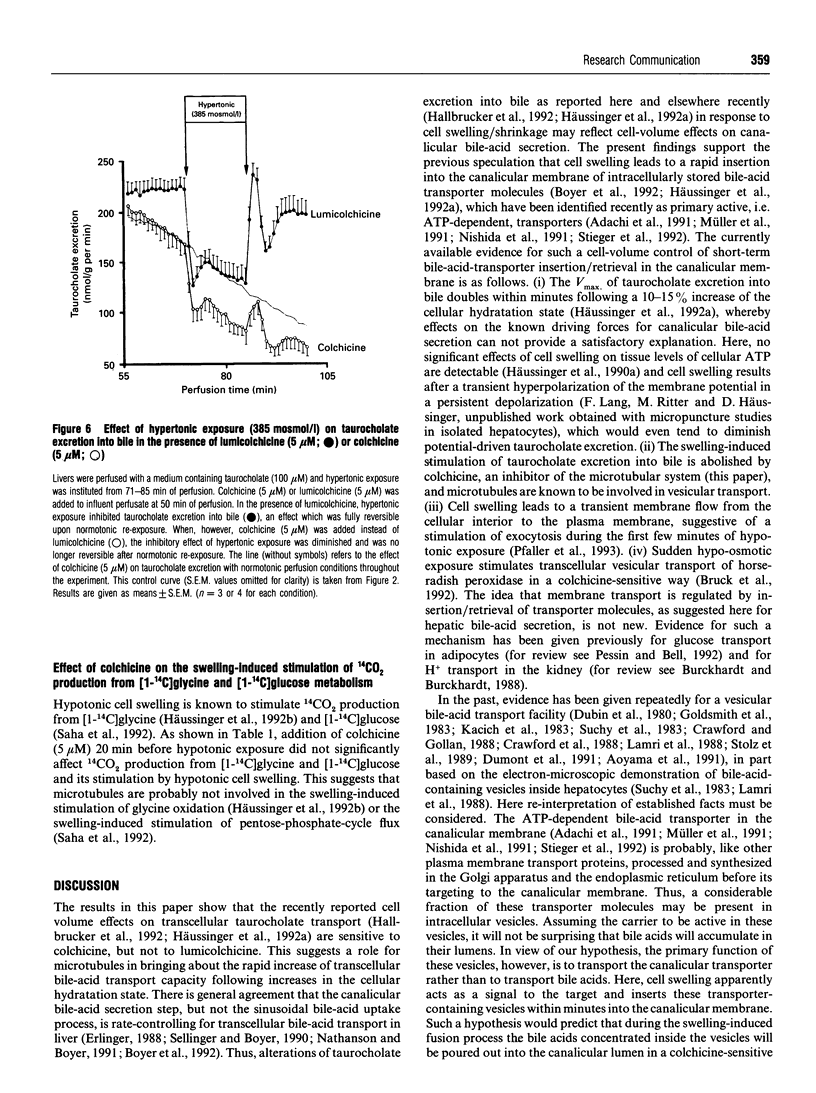
An increase of the hepatocellular hydratation state, induced by hypotonic exposure, amino acids or tauroursodeoxycholate, was shown to increase within minutes the Vmax of transcellular taurocholate transport and excretion into bile [Häussinger, Hallbrucker, Saha, Lang and Gerok (1992) Biochem. J. 288, 681-689]. This stimulatory effect of cell swelling on taurocholate excretion into bile is abolished in the presence of colchicine (5 microM). On the other hand, colchicine did not affect the stimulatory action of hypotonic cell swelling on 14CO2 production from [1-14C]glycine or [1-14C]glucose. Likewise, volume regulatory K+ fluxes following anisotonic exposure were not influenced in the presence of colchicine. Lumicolchicine (5 microM), a stereoisomer of colchicine without an inhibitory effect on microtubules, did not abolish the stimulation of taurocholate excretion into bile following hypo-osmotic exposure. Hypertonic cell shrinkage decreased taurocholate excretion into bile by about 35%; this effect was fully reversible upon normotonic re-exposure. With colchicine pretreatment, however, the hypertonicity-induced inhibition of taurocholate excretion was blunted and was no longer reversible upon normotonic re-exposure. The results suggest that stimulation of taurocholate excretion into bile in response to cell swelling involves a colchicine-sensitive, probably microtubule-dependent, mechanism, but not the stimulation of other cell-volume-sensitive pathways such as glycine oxidation or the pentose-phosphate shunt. It is hypothesized that the swelling-induced stimulation of taurocholate excretion into bile is due to a microtubule-dependent insertion of bile acid transporter molecules into the canalicular membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Kobayashi H., Kurumi Y., Shouji M., Kitano M., Yamamoto T. ATP-dependent taurocholate transport by rat liver canalicular membrane vesicles. Hepatology. 1991 Oct;14(4 Pt 1):655–659. doi: 10.1016/0270-9139(91)90053-x. [DOI] [PubMed] [Google Scholar]
- Aoyama N., Ohya T., Chandler K., Gresky S., Holzbach R. T. Transcellular transport of organic anions in the isolated perfused rat liver: the differential effects of monensin and colchicine. Hepatology. 1991 Jul;14(1):1–9. doi: 10.1002/hep.1840140102. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Graf J., Meier P. J. Hepatic transport systems regulating pHi, cell volume, and bile secretion. Annu Rev Physiol. 1992;54:415–438. doi: 10.1146/annurev.ph.54.030192.002215. [DOI] [PubMed] [Google Scholar]
- Bruck R., Haddad P., Graf J., Boyer J. L. Regulatory volume decrease stimulates bile flow, bile acid excretion, and exocytosis in isolated perfused rat liver. Am J Physiol. 1992 May;262(5 Pt 1):G806–G812. doi: 10.1152/ajpgi.1992.262.5.G806. [DOI] [PubMed] [Google Scholar]
- Cornet M., Delpire E., Gilles R. Study of microfilaments network during volume regulation process of cultured PC 12 cells. Pflugers Arch. 1987 Sep;410(1-2):223–225. doi: 10.1007/BF00581921. [DOI] [PubMed] [Google Scholar]
- Crawford J. M., Berken C. A., Gollan J. L. Role of the hepatocyte microtubular system in the excretion of bile salts and biliary lipid: implications for intracellular vesicular transport. J Lipid Res. 1988 Feb;29(2):144–156. [PubMed] [Google Scholar]
- Crawford J. M., Gollan J. L. Hepatocyte cotransport of taurocholate and bilirubin glucuronides: role of microtubules. Am J Physiol. 1988 Jul;255(1 Pt 1):G121–G131. doi: 10.1152/ajpgi.1988.255.1.G121. [DOI] [PubMed] [Google Scholar]
- Dubin M., Maurice M., Feldmann G., Erlinger S. Influence of colchicine and phalloidin on bile secretion and hepatic ultrastructure in the rat. Possible interaction between microtubules and microfilaments. Gastroenterology. 1980 Oct;79(4):646–654. [PubMed] [Google Scholar]
- Dumont M., D'Hont C., Durand-Schneider A. M., Legrand-Defretin V. L., Feldmann G., Erlinger S. Inhibition by colchicine of biliary secretion of diethylmaleate in the rat: evidence for microtubule-dependent vesicular transport. Hepatology. 1991 Jul;14(1):10–15. doi: 10.1002/hep.1840140103. [DOI] [PubMed] [Google Scholar]
- Flessner M. F., Wall S. M., Knepper M. A. Ammonium and bicarbonate transport in rat outer medullary collecting ducts. Am J Physiol. 1992 Jan;262(1 Pt 2):F1–F7. doi: 10.1152/ajprenal.1992.262.1.F1. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. A., Huling S., Jones A. L. Hepatic handling of bile salts and protein in the rat during intrahepatic cholestasis. Gastroenterology. 1983 May;84(5 Pt 1):978–986. [PubMed] [Google Scholar]
- Hallbrucker C., Lang F., Gerok W., Häussinger D. Cell swelling increases bile flow and taurocholate excretion into bile in isolated perfused rat liver. Biochem J. 1992 Feb 1;281(Pt 3):593–595. doi: 10.1042/bj2810593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh J. E., Pryme I. F. Interaction between mRNA, ribosomes and the cytoskeleton. Biochem J. 1991 Jul 1;277(Pt 1):1–10. doi: 10.1042/bj2770001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D., Hallbrucker C., Saha N., Lang F., Gerok W. Cell volume and bile acid excretion. Biochem J. 1992 Dec 1;288(Pt 2):681–689. doi: 10.1042/bj2880681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häussinger D., Lang F., Bauers K., Gerok W. Interactions between glutamine metabolism and cell-volume regulation in perfused rat liver. Eur J Biochem. 1990 Mar 30;188(3):689–695. doi: 10.1111/j.1432-1033.1990.tb15451.x. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F. Cell volume in the regulation of hepatic function: a mechanism for metabolic control. Biochim Biophys Acta. 1991 Dec 12;1071(4):331–350. doi: 10.1016/0304-4157(91)90001-d. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Stoll B., Morimoto Y., Lang F., Gerok W. Anisoosmostic liver perfusion: redox shifts and modulation of alpha-ketoisocaproate and glycine metabolism. Biol Chem Hoppe Seyler. 1992 Aug;373(8):723–734. doi: 10.1515/bchm3.1992.373.2.723. [DOI] [PubMed] [Google Scholar]
- Hüssinger D., Lang F., Bauers K., Gerok W. Control of hepatic nitrogen metabolism and glutathione release by cell volume regulatory mechanisms. Eur J Biochem. 1990 Nov 13;193(3):891–898. doi: 10.1111/j.1432-1033.1990.tb19414.x. [DOI] [PubMed] [Google Scholar]
- Kacich R. L., Renston R. H., Jones A. L. Effects of cytochalasin D and colchicine on the uptake, translocation, and biliary secretion of horseradish peroxidase and [14C]sodium taurocholate in the rat. Gastroenterology. 1983 Aug;85(2):385–394. [PubMed] [Google Scholar]
- Lamri Y., Roda A., Dumont M., Feldmann G., Erlinger S. Immunoperoxidase localization of bile salts in rat liver cells. Evidence for a role of the Golgi apparatus in bile salt transport. J Clin Invest. 1988 Oct;82(4):1173–1182. doi: 10.1172/JCI113714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Ishikawa T., Berger U., Klünemann C., Lucka L., Schreyer A., Kannicht C., Reutter W., Kurz G., Keppler D. ATP-dependent transport of taurocholate across the hepatocyte canalicular membrane mediated by a 110-kDa glycoprotein binding ATP and bile salt. J Biol Chem. 1991 Oct 5;266(28):18920–18926. [PubMed] [Google Scholar]
- Nathanson M. H., Boyer J. L. Mechanisms and regulation of bile secretion. Hepatology. 1991 Sep;14(3):551–566. [PubMed] [Google Scholar]
- Nishida T., Gatmaitan Z., Che M., Arias I. M. Rat liver canalicular membrane vesicles contain an ATP-dependent bile acid transport system. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6590–6594. doi: 10.1073/pnas.88.15.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessin J. E., Bell G. I. Mammalian facilitative glucose transporter family: structure and molecular regulation. Annu Rev Physiol. 1992;54:911–930. doi: 10.1146/annurev.ph.54.030192.004403. [DOI] [PubMed] [Google Scholar]
- Pfaller W., Willinger C., Stoll B., Hallbrucker C., Lang F., Häussinger D. Structural reaction pattern of hepatocytes following exposure to hypotonicity. J Cell Physiol. 1993 Feb;154(2):248–253. doi: 10.1002/jcp.1041540206. [DOI] [PubMed] [Google Scholar]
- Saha N., Stoll B., Lang F., Häussinger D. Effect of anisotonic cell-volume modulation on glutathione-S-conjugate release, t-butylhydroperoxide metabolism and the pentose-phosphate shunt in perfused rat liver. Eur J Biochem. 1992 Oct 1;209(1):437–444. doi: 10.1111/j.1432-1033.1992.tb17307.x. [DOI] [PubMed] [Google Scholar]
- Schulz W. A., Eickelmann P., Hallbrucker C., Sies H., Häussinger D. Increase of beta-actin mRNA upon hypotonic perfusion of perfused rat liver. FEBS Lett. 1991 Nov 4;292(1-2):264–266. doi: 10.1016/0014-5793(91)80880-c. [DOI] [PubMed] [Google Scholar]
- Sellinger M., Boyer J. L. Physiology of bile secretion and cholestasis. Prog Liver Dis. 1990;9:237–259. [PubMed] [Google Scholar]
- Sies H. The use of perfusion of liver and other organs for the study of microsomal electron-transport and cytochrome P-450 systems. Methods Enzymol. 1978;52:48–59. doi: 10.1016/s0076-6879(78)52005-3. [DOI] [PubMed] [Google Scholar]
- Stieger B., O'Neill B., Meier P. J. ATP-dependent bile-salt transport in canalicular rat liver plasma-membrane vesicles. Biochem J. 1992 May 15;284(Pt 1):67–74. doi: 10.1042/bj2840067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll B., Gerok W., Lang F., Häussinger D. Liver cell volume and protein synthesis. Biochem J. 1992 Oct 1;287(Pt 1):217–222. doi: 10.1042/bj2870217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz A., Takikawa H., Ookhtens M., Kaplowitz N. The role of cytoplasmic proteins in hepatic bile acid transport. Annu Rev Physiol. 1989;51:161–176. doi: 10.1146/annurev.ph.51.030189.001113. [DOI] [PubMed] [Google Scholar]
- Suchy F. J., Balistreri W. F., Hung J., Miller P., Garfield S. A. Intracellular bile acid transport in rat liver as visualized by electron microscope autoradiography using a bile acid analogue. Am J Physiol. 1983 Nov;245(5 Pt 1):G681–G689. doi: 10.1152/ajpgi.1983.245.5.G681. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos P. A., Stournaras C., Stoll B., Markogiannakis E., Lang F., Gravanis A., Häussinger D. Hepatocyte swelling leads to rapid decrease of the G-/total actin ratio and increases actin mRNA levels. FEBS Lett. 1992 Oct 26;311(3):241–245. doi: 10.1016/0014-5793(92)81111-x. [DOI] [PubMed] [Google Scholar]
- Wilson L., Friedkin M. The biochemical events of mitosis. I. Synthesis and properties of colchicine labeled with tritium in its acetyl moiety. Biochemistry. 1966 Jul;5(7):2463–2468. doi: 10.1021/bi00871a042. [DOI] [PubMed] [Google Scholar]


