Abstract
Background
Opt2Move is a theory-guided moderate and vigorous physical activity (MVPA) promotion trial that uses multiphase optimization strategy (MOST) methodology to evaluate the individual and combined effects of four intervention components in a full factorial experiment among young adult cancer survivors (YACS; N = 304). All participants will receive the core mHealth MVPA intervention, which includes a Fitbit and standard self-monitoring Opt2Move smartphone application. YACS will be randomized to one of 16 conditions to receive between zero and four additional components each with two levels (yes v. no): E-Coach, buddy, general mindfulness, and MVPA-specific mindfulness.
Objective
The primary aim is to determine the individual and combined effects of the components on MVPA post-intervention (12-weeks) and at 24-week follow-up. The secondary aim is to examine how changes in MVPA are associated with patient-reported outcomes, light-intensity activity, sedentary time, and sleep duration and quality. Potential mediators and moderators of component effects will also be examined.
Results
Results will support the selection of a package of intervention components optimized to maximize MVPA to be tested in a randomized controlled trial.
Conclusion
Opt2Move represents the first systematic effort to use MOST to design an optimized, scalable mHealth MVPA intervention for YACS and will lead to an improved understanding of how to effectively change YACS’ MVPA and ultimately, improve health and disease outcomes.
Keywords: cancer, health coaching, health promotion, mhealth, mindfulness, young adults
Background/Rationale
Young adult cancer survivors (YACS; aged 18-39 at diagnosis) are a growing population with an estimated 90 000 new cases annually in the United States. 1 Adolescence and young adulthood is a unique developmental period comprised of major life transitions (i.e., school, work, dating, marriage, child-rearing).2,3 Facets of this developmental period coupled with disruptions from a cancer diagnosis can result in anxiety, depression, sleep disturbance, fatigue, loneliness, and reduced quality of life (QOL).4–7 YACS experience a number of treatment late-effects and an increased risk of early morbidity and mortality compared to healthy age-matched controls.8,9
Increased moderate-to-vigorous physical activity (MVPA) mitigates treatment late-effects and improves QOL among cancer survivors.10–12 However, nearly 60% of YACS do not meet recommended 150 minutes/week of MVPA.13,14 Most YACS demonstrate sharp declines of MVPA following diagnosis 15 and poorer fitness levels than healthy controls. 16 However, a review of physical activity in YACS 17 revealed few existing interventions targeting physical activity in YACS. Those that do exist were limited to short-term follow-up and did not include a control group or randomization.18–20 All studies used bundled multicomponent treatment packages, making it impossible to determine what intervention components contributed to effects. No studies measured objective MVPA, included post-intervention follow-up assessments, or used smartphones or wearables, and sample sizes were small. Recent findings from a 6-month social cognitive theory-guided mHealth physical activity intervention, adapted specifically for YACS (N = 280), found modest increases in physical activity (digital tools [Fitbit, digital scale] plus Facebook = +24.7 minutes/week; digital tools = +11.4 minutes/week). 21 There is need for rigorous testing of different mHealth MVPA intervention components addressing YACS’ unique needs to optimize MVPA interventions and health outcomes in YACS.
Social support and emotion regulation-promoting practices, such as mindfulness training, may be particularly useful for promoting MVPA in YACS. Social support is considered a key theoretical construct of health behavior change.22,23 In the context of exercise and cancer, social support is leveraged to aid an individual’s effort to change and improve behavior through positive reinforcement and accountability. 24 For cancer survivors, higher levels of social support are associated with increased MVPA.25–27 A review of 12 health behavior change interventions for YACS found five out of six efficacious interventions included some form of social support. 28 Mindfulness-based interventions may also increase MVPA in YACS by way of emotion regulation and reappraisal.19,29,30 Meta-analytic findings demonstrated a small to moderate effect of mindfulness-based intervention on increased MVPA among adults with overweight/obesity. 31 However, little is known about the unique roles of social support and mindfulness in MVPA promotion. Granular information about discrete effects of these components is needed to optimize intervention configuration to maximize MVPA in YACS.
Intervention development typically involves testing a bundled treatment package consisting of multiple components to determine whether the intervention, as a whole, improves the outcome. 32 What remains unknown, is which components are essential to produce a positive outcome vs which could be eliminated to reserve resources without loss of benefit. Using traditional study designs (i.e., randomized controlled trial [RCT]) is costly and inefficient. Multiphase Optimization Strategy (MOST) is an innovative multi-phase framework using highly efficient experiments to systematically evaluate intervention components, or component levels, and individual and combined effects.33,34 Based on engineering principles of resource management and continuous optimization, the overall goal of MOST is to maximize public health impact (efficacy x intervention reach) using available resources. The present study will apply MOST methodology to examine the effect of 4 intervention components on MVPA. Our optimization objective is to develop an intervention optimized to produce the largest expected increase in daily minutes of MVPA most efficiently (i.e., with no inactive treatment components). To the best of our knowledge, no studies have applied MOST to MVPA intervention development specifically for YACS.
The primary aim of Opt2Move is to identify which intervention components contribute to a statistically significant (P < 0.05) increase in daily MVPA at 12- and 24-weeks (Aim 1). The secondary aim is to examine how changes in MVPA at 12- and 24-weeks influence symptom burden, time spent in light-intensity activity, sedentary behaviors, and sleep duration and quality (Aim 2). We will also examine mediators and moderators (of the four intervention components) on MVPA at 12- and 24-weeks in YACS (Aim 3).
Detailed Methods
Study Design
All participants receive the “core” intervention. Participants are randomly assigned to one of 16 combinations (24 = 16) of four intervention components (E-coaching, buddy general mindfulness, MVPA-specific mindfulness), each with two possible levels (yes vs no) in a full factorial experiment (see Table 1). The 16 experimental conditions reflect all possible combinations of the four intervention components. We selected a factorial experimental design because it: a) separates component effects enabling the estimation of the main effect of each candidate component and interactions between components; and b) has greater economical use in terms of adequate power to examine the main effects of multiple intervention components simultaneously with essentially the same sample size needed for a single intervention component. 33 This factorial design should not be considered a 16-arm RCT. All estimated main effects and interactions use all experimental conditions (e.g., main effect of Buddy is estimated by comparing outcomes of participants randomized to conditions 1-8 in Table 1 to participants randomized to conditions 9-16).
Table 1.
Experimental Conditions of Four Possible Component Assignments.
| Condition | Core Intervention | E-Coach | Buddy | General Mindfulness Training | MVPA Specific Mindfulness Training |
|---|---|---|---|---|---|
| 1 | On | On | On | On | On |
| 2 | On | On | Off | On | On |
| 3 | On | On | On | Off | Off |
| 4 | On | On | Off | Off | Off |
| 5 | On | On | On | On | Off |
| 6 | On | On | Off | On | Off |
| 7 | On | On | On | Off | On |
| 8 | On | On | Off | Off | On |
| 9 | On | Off | On | On | Off |
| 10 | On | Off | Off | On | Off |
| 11 | On | Off | On | Off | On |
| 12 | On | Off | Off | Off | On |
| 13 | On | Off | On | On | On |
| 14 | On | Off | Off | On | On |
| 15 | On | Off | On | Off | Off |
| 16 | On | Off | Off | Off | Off |
Study Population
Individuals meeting the following criteria are eligible to participate: 1) 18-39 years old during study screening; 2) diagnosed with cancer between 18-39 years old and <5 years since diagnosis; 3) >3 months post-completion of primary treatment (i.e., surgery, chemotherapy, and/or radiation); may still be undergoing endocrine or hormone therapies; 4) currently engage in <60 minutes per week of MVPA (via self-report); 5) own a smartphone (either an iPhone or an Android model 5.0 or greater); 6) have access to internet; 7) fluent in spoken and written English; and 8) willing to identify a buddy to participate with them, if randomly assigned to this component. Exclusion criteria include: 1) metastatic cancer diagnosis (cancer spread beyond initial site or lymph nodes, to another part of the body); 2) any absolute contraindications to exercise (e.g., acute myocardial infarction, severe orthopedic conditions); 3) planned elective surgery in next 12 months; 4) plans to become pregnant in next 18 months; 5) plans to move out of United States in next 18 months; and 6) current enrollment in another dietary or physical activity trial. We chose to restrict our sample to young adults and exclude adolescents (ages 15-18) because main intervention is not developmentally appropriate for those <18 years of age. There will be no restrictions on geographic location within the United States. All participants will be required to pass the American College of Sports Medicine Exercise Preparticipation Health Screening Questionnaire adapted for self-administration or obtain medical clearance to participate in the trial. 35 This study is registered on Clinicaltrials.gov (NCT05375162). All trial procedures are approved by Northwestern University’s Institutional Review Board (#STU00210628).
Recruitment, Screening, and Consent
Participants are recruited through electronic health records and clinician referrals at affiliated oncology clinics at two large U.S. cancer centers (1 in Midwest and 1 in Southeast) and via social media sites, online forums, and fliers at community organizations and events. Participants complete screening online or via phone to determine initial eligibility for study participation. Eligible participants receive a copy of study informed consent and a study overview document via email and complete a phone call to confirm eligibility and review study in more detail. All participants are required to read and sign a university Institutional Review Board approved informed consent prior to participation in study activities and are emailed a secure, individualized link to complete online forms via REDCap (Research Electronic Data Capture).36,37
Randomization
After completing baseline assessments and receiving physician consent (if needed), eligible participants are randomly assigned to one of the 16 intervention conditions using computer-generated randomly permuted blocks. To prevent bias, allocation is concealed as follows: 1) all 16 conditions are assigned a code to correspond to condition and key for this coding scheme will be kept separate from randomization allocation; 2) a concealed allocation sequence in REDCap is used, which is inaccessible to anyone until randomization occurs. The individual conducting randomization is blinded until allocation has been completed. The nature of intervention precludes blinding of staff and participants, and the statistician is also blinded. Participants are notified of their assigned intervention components via e-mail after their intervention materials are mailed. Participants receive a personalized QR code to download the Opt2Move app with modules enabled for components to which they have been assigned to “Yes” level. Participants assigned to “No” level for components do not receive access to the corresponding module.
Intervention Packets and Orientation Call
All participants are mailed a Fitbit device and an intervention packet including study overview, basic safety information, instructions for using the Fitbit and downloading and using the Opt2Move and Fitbit apps, and component-specific instructions. Participants complete a 15-20-minute orientation call, where study staff reiterate expectations for overall intervention, describe each assigned intervention component, answer any questions, and troubleshoot any technical issues they may be having.
Core Intervention
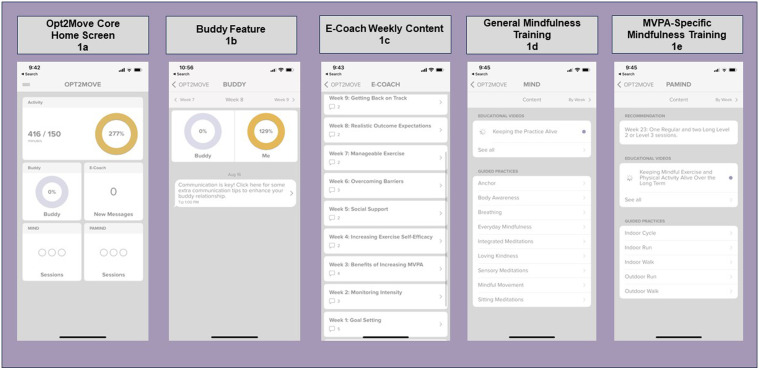
All participants receive the core intervention to build essential competencies necessary for progressively increasing MVPA safely. The core includes the Fitbit Inspire 3 (or similar) and the custom-built Opt2Move MVPA self-monitoring core app module adapted from our previous study in breast cancer survivors.38,39 Participants are asked to download the Fitbit app and wear the Fitbit continuously throughout the 24-week study period. Fitbit data automatically syncs wirelessly with the Fitbit app and the Opt2Move app to provide real-time Fitbit data to the study team. The core Opt2Move app includes educational information on MVPA, and effective behavior change strategies for building self-efficacy, overcoming barriers, enhancing facilitators, setting realistic outcome expectations, and self-regulatory skill-building (i.e., reviewing progress, goal setting, action-planning, and coping with disruptions). Figure 1 displays the Opt2Move core app module home screen (Figure 1A) and additional intervention component app modules (Figure 1B–E). Participants are provided with an exercise prescription to gradually increase MVPA, build efficacy, and ensure safe progression towards an overall goal of 150 minutes per week. All participants begin with a MVPA goal of 60 minutes per week and gradually increase the goal by 15 minutes of MVPA each week until 150 minutes of MVPA is reached. Participants receive a hard copy of the exercise prescription, which is also included in educational information in the app. The weekly goal is displayed on the Opt2Move app home screen. Participants are instructed to self-monitor progress towards these goals via feedback on Fitbit Inspire 3 and progress information in the Opt2Move app. Participants receive app notifications when they reach 50%, 75%, and 100% of their current week’s MVPA goal and a weekly notification with a summary of the previous week’s MVPA progress.
Figure 1.
Opt2Move intervention mobile app home screen and component features.
Rationale for Selection of the Four Candidate Components
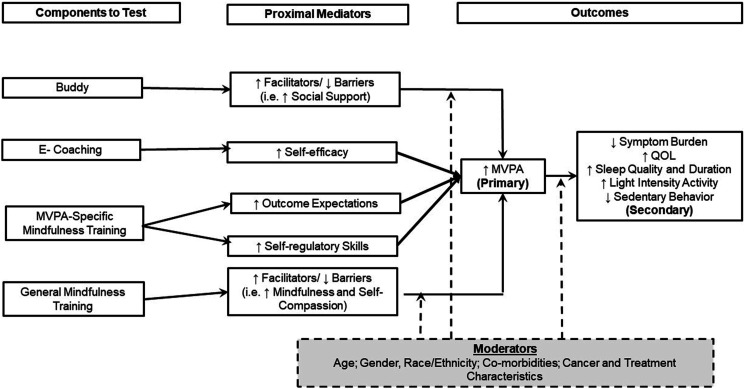
The four components to be tested were chosen for examination based on evidence from prior studies in cancer survivors and social cognitive theory (SCT). 22 See Figure 2 for the Opt2Move conceptual model. SCT is recognized as a useful framework for designing MVPA interventions in cancer survivors. 40 SCT specifies a core set of determinants (self-efficacy, goal-setting, facilitators and barriers, outcome expectations) and mechanisms by which behavior change occurs. 41 SCT posits self-efficacy is both directly and indirectly related to MVPA via facilitators and barriers (i.e., lack of access to facilities, social support, self-compassion), goal-setting/self-regulation (i.e., monitoring MVPA, using feedback and body awareness to measure progress), and outcome expectations (i.e., belief MVPA will result in a specific outcome).42,43 Each component is remotely delivered. While each of the four components might impact multiple SCT constructs, each was developed and hypothesized to target one to two unique primary SCT construct(s). In turn, we hypothesize that increased MVPA will decrease symptom burden and sedentary time while increasing sleep duration, sleep quality, and light-intensity activity time.
Figure 2.
Opt2Move conceptual model.
Intervention Components
Buddy
Personal and peer support from survivors’ friends and family has been associated with increased MVPA among cancer survivors.44,45 Personal support may increase MVPA through encouragement, accountability, and exercising together. 45 We hypothesize that the buddy component will primarily enhance facilitators/reduce barriers by increasing social support. Participants assigned to “Yes” level for buddy select a buddy (i.e., friend, co-worker, caregiver, or family member) to support them in increasing their MVPA and have access to the Buddy module in the Opt2Move app. Buddies must be ≥ 18 years old and willing to share their Fitbit data with the study team. Buddies need to pass the Exercise Preparticipation Health Screening Questionnaire or receive medical clearance to participate. 35 The buddy is mailed a Fitbit and provided access to, and instructions for, all Opt2Move app modules the participant has access to for their assigned condition, with the exception of E-coach. The buddy participates in a 15-30-minute orientation led by a research team member over Zoom or phone. In the Opt2Move buddy module (see Figure 1B), the buddy and participant can view each other’s weekly MVPA progress toward their goal. A message is posted in the buddy module when the buddy or participant reaches 50%, 75%, and 100% of their weekly goal. They can comment on progress using encouraging messages pre-populated by the study team (i.e., “You inspire me to keep going!” or “Wow! I am so proud of you for reaching your goals!”). The study team also posts a “Buddy Breakdown” strategy for increasing support (i.e., going for a walk together, sharing an exercise selfie) one time per week. Table 2 displays weekly content by intervention component. Four 10-15-minute buddy training podcasts are posted to the Opt2Move Buddy module at Weeks 3, 6, 9, and 12. These podcasts are primarily designed to help the buddy effectively support YACS, but YACS can access them if interested. Buddies receive the same standardized exercise prescription as participants. Buddies are informed that they are not required to increase or change their MVPA, but they may use the exercise prescription as a guide if they opt to do so. On the Opt2Move app home screen, the buddy tile displays their buddy’s relative progress towards their weekly MVPA goal.
Table 2.
Weekly Intervention Content by Component.
| Week | E-Coaching | Buddy Education | General Mindfulness Training | MVPA-Specific Training |
|---|---|---|---|---|
| 1 | Goal setting | Benefits of having a buddy | Defining mindfulness | Defining mindfulness in relation to MVPA |
| 2 | Monitoring intensity | Buddy communication | Body awareness | Body awareness in relation to MVPA |
| 3 | Benefits of increasing MVPA | Goal setting with a buddy | Introduction to mindful movement | Mindful movement and MVPA |
| Benefits of social support for MVPA a | ||||
| 4 | Increasing exercise self-efficacy | Increasing motivation with a buddy | Qualities of mindfulness | Qualities of mindfulness in relation to MVPA |
| 5 | Social support | Effective social support | Working with the wandering mind | Working with the wandering mind during MVPA |
| 6 | Overcoming barriers | Overcoming barriers together | Noticing pleasant events | Noticing pleasant events during MVPA |
| Types of social support a | ||||
| 7 | Manageable exercise | Holding each accountable | Pain vs suffering | Pain vs suffering in the context of MVPA |
| 8 | Realistic outcome expectations | Refreshing communication tips (check-in) | Responding vs reacting | Responding vs reacting in relation to MVPA |
| 9 | Getting back on track | Helping your buddy get back on track | Awareness and compassion | Awareness and compassion during MVPA |
| Active listening a | ||||
| 10 | Healthy rewards | Healthy rewards | Loving- kindness | Loving-kindness during MVPA |
| 11 | Reflecting on your progress | Reflections on progress with buddy | Observing thoughts | Observing thoughts during MVPA |
| 12 | Relapse prevention | Relapse prevention | Keeping practice alive | Keeping MVPA mindfulness practice alive |
| Relationship building a |
aBuddy Podcast.
E-Coach
Professional support from trained coaches may increase MVPA in cancer survivors and the general population. 46 Increases in MVPA have been observed with individualized physical activity coaching delivered via various modes, including web-based, 47 interactive voice response (IVR), 48 and text message, 49 suggesting asynchronous coaching may be effective. We hypothesize that E-coaching will primarily enhance self-efficacy through SCT constructs (addressing barriers to physical activity, developing and managing outcome expectations, facilitating social support, and promoting mastery experiences). Participants assigned to the “Yes” level of this component receive the Opt2Move E-coaching module, which consists of real-time messaging with a trained bachelor’s or master’s level coach. Two text messages are scheduled to automatically be sent from the coach to the participant each week during the 12-week intervention period. In the first weekly E-coach message, the coach will provide a brief overview of the weekly behavior change topic (See Table 2 for weekly e-coach topics) and ask participants a question about the weekly topic. The second weekly E-coach message provides feedback on participants’ recent progress and goals and helps troubleshoot and problem-solve. Each message ends with an open-ended question to facilitate conversation. Participants receive an app notification when a new message is generated from their coach. The number of “new messages” from the coach is displayed on the Coach tile of the Opt2Move app home screen. Participants who have not responded to their coach within 48 hours receive a reminder notification to check their coach messages. In addition to scheduled messaging, participants are instructed to reach out to their coach with questions, or for additional assistance. Coaches respond to participants within 24 hours on weekdays and 48 hours on weekends and check for new messages twice daily. Coaches use a semi-structured response guide tailored to each week to guide coach responses. Coaches also meet with other coaches once a week to ensure fidelity and consistency across coaches.
General Mindfulness Training (MIND)
Increased mindfulness is associated with a higher likelihood to follow through on MVPA intentions and maintain an exercise program.50,51 It is hypothesized mindfulness may enhance MVPA via improved emotion regulation and cognitive flexibility,52–54 resulting in better recognition and regulation of negative experiences during MVPA (e.g., uncomfortable bodily sensations, emotions) and enhanced intensity of positive aspects of MVPA. 55 General mindfulness training is hypothesized to increase facilitators/reduce barriers to MVPA by increasing mindfulness and self-compassion. Participants in the “Yes” level for this component receive the General Mindfulness module in the Opt2Move app. Participants with this component receive 1 new brief weekly mindfulness education video each week (1-2 minutes), which lays the foundation for what mindfulness is and how it can be an important health behavior (see Table 2 for weekly MIND topics). Each week participants are also asked to listen to general guided mindfulness audio files ≥3 days/week and have a progress bar on their app to show how many they have completed each week. Each week during the 12-week intervention, a new mindfulness practice audio file becomes available within the General Mindfulness app module. Participants continue to have access to all guided practices released in previous weeks. Participants are recommended to listen to newly released audio files each given week but are permitted to listen to any of the mindfulness audio files. The length of recordings gradually increases each week from 3-5 to 25-30 minutes. If not completed, participants are sent reminders. All videos and recordings are available via the Opt2Move app. Participants receive an app notification when new MIND videos and audios are available and a reminder notification if they have not watched the videos and/or completed their weekly mindfulness sessions. Their weekly progress towards 3 sessions of general mindfulness training is also tracked on the MIND tile on the Opt2Move app home screen.
MVPA-Specific Mindfulness Training (PAMIND)
Combining mindfulness into performance of physical activity has demonstrated preliminary feasibility and acceptability and decreased ratings of perceived exertion, compared to physical activity alone despite equivalent physiological responses.56–58 Thus, we hypothesize that MVPA-specific mindfulness will increase realistic outcome expectations, which may make MVPA more tolerable and enhance self-regulatory/goal setting-skills. Participants in the “Yes” level for this component receive the MVPA-Specific Mindfulness Training Opt2Move app module. They receive access to 1 new brief mindfulness education video (1-2 minutes) each week, which lays the foundation for what mindfulness is and the importance of linking mindful awareness with MVPA. Participants have access to a library of 45 MVPA-specific guided mindfulness audio files varying in intensity and duration (e.g., 10-minute treadmill walking meditation, 20-minute bicycling meditation) and are released to participants on a schedule corresponding with their exercise prescription to ensure safe progression. See Table 2 for weekly PAMIND topics. Participants in this condition are asked to listen to ≥3 MVPA sessions/week and are given a recommendation for the length and intensity of these audio files each week corresponding to their progression in the program. Participants receive an app notification when new audio or videos are released and a reminder notification if they have not watched weekly videos and/or completed their weekly mindfulness sessions. On the PAMIND tile on the Opt2Move homescreen, participants see their progress in completing their weekly PAMIND sessions.
Outcome Measures
Measures are administered at baseline, 12-week (post-intervention), and 24-week (maintenance). The primary outcome is accelerometer-assessed MVPA. Secondary outcomes include symptoms, light-intensity activity, sedentary time, sleep, and social cognitive theory constructs. Demographic and disease characteristics are collected as potential moderators of MVPA changes.
An accelerometer is mailed to all participants at each time point. Participants are instructed to wear the activity monitor for 7 consecutive days (see below for further details) and mail it back to study investigators using self-addressed stamped envelope provided. Fitbit data are collected throughout the full study duration. Demographic and disease characteristics, social cognitive constructs, patient-reported outcomes, and behavior will be assessed via a personalized link to a battery of questionnaires e-mailed to participants at each time point to complete online via REDCap. Participants receive reminders via phone or email to complete assessments. Participants are incentivized to complete assessments (up to $50/time point) and permitted to keep the Fitbit.
Primary Outcome
The primary outcome for the study is daily minutes of MVPA measured using an ActiGraph (model wGT3X – BT, ActiGraph, Pensacola, FL) that is worn continuously for 7 consecutive days at each timepoint (non-dominant hip during waking hours and non-dominant wrist during sleep) excluding bathing or swimming. Participants complete a daily log to indicate when the monitor is worn at each of the body locations and sleep and wake times. Activity data are collected at 40 Hz in 10-second intervals (epochs) and are reintegrated into 60 s epochs for processing. Upon receipt of the accelerometer, data are screened for valid wear time using ActiLife version 6.13.5. If there is not at least 5 days of valid measurement, 59 participants are asked to re-wear the monitor. Data processed for wear periods of ≥3 valid days at 12- and 24-weeks are included in analyses.60–62 Valid minutes of waking wear time are categorized according to intensity (counts/min) using established cut-points: sedentary (<100), light activity (100-2019), and MVPA (>2020).63,64
Secondary Outcomes
Self-Reported Physical Activity
Participants complete the Godin Leisure Time Exercise Questionnaire, a valid and reliable measure of physical activity participation. 65 They are asked to report the frequency of participation in strenuous (e.g., jogging), moderate (e.g., fast walking), and mild (e.g., easy walking) exercise over the past 7 days and the average time spent in each intensity. Activity frequencies are multiplied by metabolic equivalents and summed to create a total leisure time activity score.
Symptom Burden
Participants complete the PROMIS 29 Profile for pain intensity, and seven health domains (physical function, fatigue, pain interference, depressive symptoms, anxiety, ability to participate in social roles and activities, and sleep disturbance) using four items for each domain over the past 7 days. 66
Time Spent in Other Activity Intensities
Daily time spent in sedentary behavior (<100 counts) and light-intensity physical activity (100-2019 counts) is examined as described above.
Sleep
The ActiGraph has been well-validated for sleep. 67 We use the validated Cole-Kripke Sleep Scoring Algorithm built into ActiLife software to process device-based sleep measures, including sleep duration, sleep efficiency, sleep onset latency, and wakefulness after sleep onset. We also measure perceptions or sleep-related impairment using well-validated PROMIS sleep disturbance short form 4a. 68
Moderators/Mediators
Potential moderators include demographic and disease characteristics and intervention fidelity, feasibility, and acceptability. Potential mediators include SCT constructs (i.e., goal setting, self-efficacy, facilitators/barriers, and outcome expectations). Table 3 includes a more detailed description of each measure.
Table 3.
Mediators and Moderators.
| Name | Description | Time Point | ||
|---|---|---|---|---|
| Baseline | Week 12 | Week 24 | ||
| Demographics and health history | ||||
| Demographics | Participants will report on the following: Age, gender, race, ethnicity, marital status, education, employment, occupation, income, technology use, number of children | ✓ | ||
| Health history questionnaire | Participants will report on the following: Height; most recent weight; tobacco/alcohol use, fruit and vegetable consumption, caffeine consumption, date of diagnosis; age at diagnosis; cancer diagnosis; clinical stage; previous cancer diagnosis; current and past cancer treatment and start/completion dates; current medications; menses and menopausal status; co-morbid medical conditions; and clinical events (e.g., receipt of conventional cancer treatment or procedure, cardiac events, diagnosis of comorbid condition, medical problem requiring emergency room visit or hospitalization); access to and use of exercise equipment and resources | ✓ | ✓ | ✓ |
| Social cognitive theory constructs | ||||
| Barriers self-efficacy scale 66 | Assesses the beliefs in ability to be regularly active despite common barriers over the next 12 weeks | ✓ | ✓ | ✓ |
| E-coaching component a | ||||
| Exercise self-efficacy scale 66 | Assesses the beliefs in ability to be regularly active over the next 12 weeks | ✓ | ✓ | ✓ |
| E-coaching component a | ||||
| Multidimensional outcome expectations for exercise scale 67 | Assesses social, self-evaluative, and physical outcome expectations for physical activity | ✓ | ✓ | ✓ |
| MVPA-specific mindfulness training component a | ||||
| Social support for exercise scale 68 | Assesses support for physical activity received from friends, family, and other cancer patients/survivors | ✓ | ✓ | ✓ |
| Buddy component a | ||||
| Mindfulness | ||||
| Southampton mindfulness questionnaire 69 | Assesses mindfulness-based emotion regulation during distressing thoughts and images | ✓ | ✓ | ✓ |
| PROMIS b mindful presence short form 4a 70 | Assesses qualities of attention, awareness, and curiosity | ✓ | ✓ | ✓ |
| PROMIS mindful awareness short form 4a 70 | Assesses moment-to-moment conscious attunement with sensory, mental, and emotional experience | ✓ | ✓ | ✓ |
| PROMIS mindful curiosity short form 4a 70 | Assesses the quality of connecting with one’s present moment experience with open, unbiased inquiry | ✓ | ✓ | ✓ |
| PROMIS mindful acceptance short form 4a 70 | Assesses being open to one’s experiences as they are without trying to change or control them | ✓ | ✓ | ✓ |
| PROMIS mindful self-kindness short form 4a 70 | Assesses being compassionate and caring towards oneself | ✓ | ✓ | ✓ |
| PROMIS mindful insight – decentering short form 4a 70 | Assesses the observation of thoughts and emotions as temporary experiences or mental events that are different from one another | ✓ | ✓ | ✓ |
| Fidelity and adherence to intervention components | ||||
| Core intervention c | Proportion of days fitbit is worn; proportion of number of days Opt2Move app is opened; proportion of intervention weeks MVPA goal is met |

|
||
| E-coach c | Average number of e-coach exchanges; proportion of e-coach messages responded to |

|
||
| Buddy c | Proportion of buddy podcasts listened to by buddy; average number of buddy breakdowns read; average number of buddy interactions and proportion of weeks buddies interact within the Opt2Move app |

|
||
| MVPA-specific mindfulness c | Average number of educational videos opened and watched; average number and length recordings listened to; proportion of weeks do ≥ 3 audio practices each week |

|
||
| General mindfulness c | Average number of educational videos opened and watched; average number and length recordings listened to; proportion of weeks do ≥ 3 audio practices each week |

|
||
| Feasibility | ||||
| Participant retention | Ratio of participants who drop out to participants retained in each component | ✓ | ✓ | |
| Safety | The number and severity of adverse events reported spontaneously and during non-spontaneous adverse event assessments. Acceptability is measured via a process evaluation to assess |

|
||
| Acceptability | ||||
| Post-program evaluation | Assesses: a) Intervention components’ perceived effectiveness, b) plans to continue physical activity and intervention tool use; c) intervention elements liked/disliked; and d) overall satisfaction with study experience | ✓ | ||
aComponent targeting specific construct.
bPatient-Reported Outcomes Measurement Information System.
cData are continuously measured throughout the duration of the study.
Safety Monitoring
This study has a data and safety monitoring board. Participants are instructed to report injuries to study staff within 24 hours of occurrence. Additionally, they are emailed a questionnaire via REDCap every 8 weeks to report any potential adverse events that were not previously reported. We reach out to emergency contacts for participants who do not have any data for ≥14 days and have not responded to contact attempts during that period to ensure safety and re-engage participation.
Privacy and Confidentiality
To protect participant privacy, no personal information is stored or associated with their Opt2Move app account. All Fitbit data transmitted via the Opt2Move app are collected using the Northwestern University server cluster, which has limited physical access, is firewalled, and is regularly monitored for security issues. All phone-encrypted data transmissions use a secure sockets layer protocol with a unique token for each participant. All data are backed up regularly. Any personal health information is stored on separate data clusters with unique keys and limited firewalled access. The study consent form details potential privacy and confidentiality risks and practices implemented to ensure protection.
Sample Size and Power
We will enroll 304 YACS (19 per intervention condition). A sample size calculation was conducted based on an ability to detect an effect size of 0.3, which would correspond to a mean increase of 5.13 minutes/day of MVPA from baseline between participants with a component “on” vs those with it “off.” This equates to approximately 35 minutes per week or about 2.0 MET/hours per week, which has been associated with a ∼4% reduction in cancer mortality in cancer survivors. 69 We assumed a type 1 error rate of 0.05, an ICC of 0.36, and a standard deviation of 17 minutes at 12- and 24-weeks based on existing data 38 and using existing formulas for group differences in longitudinal settings. 70 Attrition was assumed to be 11% at 12-weeks and 20% at 24-weeks.
Data Analysis
We will use intent-to-treat principles and aim to collect all outcome measures even if participants do not engage with their assigned treatments. Analyses will be conducted in SAS 9.4. Descriptive statistics will be summarized by participant characteristics and baseline variables.
Aim 1 will test the difference in daily minutes of MVPA by intervention components across the time points (baseline, 12- and 24-weeks) using mixed-effects regression modeling. Covariates will include time—modeled using two indicator variables for 12- and 24-weeks (with baseline as the reference group), and the interaction of time and indicator variables for each of the 4 components. Exploratory analyses involving interactions between two components will be tested by fitting additional models that include 3-way time by component-by-component interactions.
Aim 2 will investigate the time-varying effects of MVPA as a covariate on health behaviors and health outcomes over the course of the intervention and will be modeled using generalized linear mixed-effects models with the outcomes of symptom burden, time spent in other activity intensities, or sleep duration and quality. We will use the following decomposition of MVPA to estimate the effect of a participant’s baseline level of MVPA and their change from baseline on Aim 2 outcomes at 12- and 24-weeks
These models will control for age, gender, time since diagnosis, treatment, and cancer type.
Aim 3 will expand upon the model in Aim 1 to include a moderation analyses. We will augment the Aim 1 model to examine the effect of moderators of the intervention components by including a moderator main effect, its interaction with time, and a 3-way moderator by time by component interactions. These three-way interactions will estimate whether the effect of a component differs as a function of a moderator. We will examine moderators one at a time using this framework. Models to assess the effect of potential mediators of the intervention components will follow Krull and Mackinnon’s approach for estimating mediation in multilevel models wherein mediation is assessed by fitting two models. 72 Model 1 will include the mediating variable as a time-dependent covariate, and Model 2 will estimate the effect of the components on the mediator itself over time (using mixed-effects models with the same covariates as in Aim 1). We will estimate a separate model for each mediation variable and multiply the mediator’s coefficients from Model 1 by the coefficient for the time-by-component interaction of interest from Model 2 for the mediation effect.
Component Selection
Our optimization criterion for component selection is the combination of components corresponding to the largest increase in MVPA at 12- or 24-weeks. We will identify an effective combination of components by using the posterior expected value approach developed by Strayhorn and colleagues. 71 Our primary analysis will use informative priors for the regression coefficients that place more prior weight on the grand mean and main effects. Sensitivity to this prior specification will be assessed by using non-informative priors for all the regression coefficients. The combination of components with the largest effects at weeks 12 or 24 will be retained and considered for inclusion in the optimized intervention as some components may be more or less important for MVPA initiation (i.e., 12-weeks) or maintenance (i.e., 24-weeks). If none of the components are effective at either time point, we will proceed with pilot testing additional or revised components in a future optimization study.
Discussion
Considering the increasing rates of cancer among YACS, there is a need for resource- and cost-efficient health interventions to meet the unique preferences and developmental needs of YACS. 73 mHealth physical activity interventions, including social support and/or mindfulness, may be a viable approach. However, traditional methods of testing social support and mindfulness in physical activity interventions do not delineate which specific components have the greatest effect on increasing physical activity and improving quality of life, particularly among YACS.
To the best of our knowledge, Opt2Move is the first study to apply MOST methodology to determine individual and combined effects of social support (i.e., buddy, E-coaching) and mindfulness (i.e., general mindfulness training, MVPA-specific mindfulness training) components on physical activity. MOST is a rigorous, resource-efficient approach with the ability to streamline efficacious behavioral health interventions. Thus, this study has the potential to identify a fully remote, scalable, and optimized mHealth physical activity intervention that is efficacious at improving physical activity and quality of life in YACS. Following study completion, we will revise intervention components as necessary based on participant feedback. Then, we will test the identified optimal set of intervention components in a treatment package in a fully powered RCT. If an RCT to test an optimized treatment package is not warranted based on our findings, we will use information from other data collected and our mediators and moderators to refine the most promising components and/or identify new potential components to be tested in an additional optimization study consistent with the continuous optimization principle of MOST. 74
This study is not without limitations. While MOST factorial study designs can be complex with multiple component testing, previous work suggests this approach is feasible.57,75 As with most technology- or mobile-based interventions, generalizability poses a threat to external validity. In that, our findings could be limited to individuals who have access to technology. On the other hand, we extend our reach and generalizability by recruiting nationwide and including individuals who might experience barriers to participation, including transportation, time, and access. As in any factorial design, many permutations of intervention components could be tested. The components chosen to be tested are based on data for the efficacy, scalability, and cost of these components. This study will lay the groundwork for future RCTs evaluating the newly optimized intervention. Furthermore, while this investigation concentrates on one aspect of exercise recommendations for cancer survivors, subsequent research could broaden the scope to encompass other forms of exercise recommendations, such as strength training.
Conclusion
Opt2Move is the first mHealth physical activity intervention for YACS tested in a MOST factorial trial. This trial will add to the growing body of research applying MOST to intervention development, particularly for physical activity and mindfulness-based interventions. Distinguishing efficacious and cost- and resource-efficient intervention components for promoting physical activity is an important next step for disseminating scalable and optimized physical activity interventions. Ultimately, this work will significantly contribute to understanding how to effectively increase and maintain physical activity to improve health and disease outcomes in cancer survivors.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health [R01CA262357; T32CA193193], and National Cancer Institute (R01CA262357, T32CA193193).
ORCID iDs
Jean Miki Reading https://orcid.org/0000-0003-3603-6040
David Victorson https://orcid.org/0000-0002-3530-8633
References
- 1.American Cancer Society. Cancer Facts & Figures 2020. Atlanta, GA: 2020. American Cancer Society. Accessed December 13, 2023, https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/special-section-cancer-in-adolescents-and-young-adults-2020.pdf [Google Scholar]
- 2.Arnett JJ, Žukauskienė R, Sugimura K. The new life stage of emerging adulthood at ages 18-29 years: implications for mental health. Lancet Psychiatr. 2014;1(7):569-576. doi: 10.1016/S2215-0366(14)00080-7 [DOI] [PubMed] [Google Scholar]
- 3.Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol. 2000;55(5):469-480. [PubMed] [Google Scholar]
- 4.Bleyer A. Young adult oncology: the patients and their survival challenges. CA Cancer J Clin. 2007;57(4):242-255. doi: 10.3322/canjclin.57.4.242 [DOI] [PubMed] [Google Scholar]
- 5.Mattson MR, Demshar RK, Daly BJ. Quality of life of young adult survivors of hematologic malignancies. Cancer Nurs. 2013;36(2):E1-E7. doi: 10.1097/NCC.0b013e31824242dd [DOI] [PubMed] [Google Scholar]
- 6.Parsons HM, Harlan LC, Lynch CF, et al. Impact of cancer on work and education among adolescent and young adult cancer survivors. J Clin Oncol. 2012;30(19):2393-2400. doi: 10.1200/JCO.2011.39.6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salsman JM, Garcia SF, Yanez B, Sanford SD, Snyder MA, Victorson D. Physical, emotional, and social health differences between posttreatment young adults with cancer and matched healthy controls. Cancer. 2014;120(15):2247-2254. doi: 10.1002/cncr.28739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smitherman AB, Anderson C, Lund JL, Bensen JT, Rosenstein DL, Nichols HB. Frailty and comorbidities among survivors of adolescent and young adult cancer: a cross-sectional examination of a hospital-based survivorship cohort. J Adolesc Young Adult Oncol. 2018;7(3):374-383. doi: 10.1089/jayao.2017.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rugbjerg K, Mellemkjaer L, Boice JD, Kober L, Ewertz M, Olsen JH. Cardiovascular disease in survivors of adolescent and young adult cancer: a Danish cohort study, 1943-2009. J Natl Cancer Inst. 2014;106(6):dju110. doi: 10.1093/jnci/dju110 [DOI] [PubMed] [Google Scholar]
- 10.Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104(11):815-840. doi: 10.1093/jnci/djs207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rock CL, Thomson CA, Sullivan KR, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022;72(3):230-262. doi: 10.3322/caac.21719 [DOI] [PubMed] [Google Scholar]
- 13.Rock CL, Thomson C, Gansler T, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70(4):245-271. doi: 10.3322/caac.21591 [DOI] [PubMed] [Google Scholar]
- 14.Liguori G. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2021. [Google Scholar]
- 15.Jarvela LS, Niinikoski H, Lahteenmaki PM, et al. Physical activity and fitness in adolescent and young adult long-term survivors of childhood acute lymphoblastic leukaemia. J Cancer Surviv. 2010;4(4):339-345. doi: 10.1007/s11764-010-0131-0 [DOI] [PubMed] [Google Scholar]
- 16.Love B, Moskowitz MC, Crook B, et al. Defining adolescent and young adult (AYA) exercise and nutrition needs: concerns communicated in an online cancer support community. Patient Educ Counsel. 2013;92(1):130-133. doi: 10.1016/j.pec.2013.02.011 [DOI] [PubMed] [Google Scholar]
- 17.Brunet J, Wurz A, Shallwani SM. A scoping review of studies exploring physical activity among adolescents and young adults diagnosed with cancer. Psycho Oncol. 2018;27(8):1875-1888. doi: 10.1002/pon.4743 [DOI] [PubMed] [Google Scholar]
- 18.Belanger LJ, Plotnikoff RC, Clark A, Courneya KS. Physical activity and health-related quality of life in young adult cancer survivors: a Canadian provincial survey. J Cancer Surviv. 2011;5(1):44-53. doi: 10.1007/s11764-010-0146-6 [DOI] [PubMed] [Google Scholar]
- 19.Rabin C, Pinto B, Fava J. Randomized trial of a physical activity and meditation intervention for young adult cancer survivors. J Adolesc Young Adult Oncol. 2016;5(1):41-47. doi: 10.1089/jayao.2015.0033 [DOI] [PubMed] [Google Scholar]
- 20.Valle CG, Tate DF, Mayer DK, Allicock M, Cai J. A randomized trial of a Facebook-based physical activity intervention for young adult cancer survivors. J Cancer Surviv. 2013;7(3):355-368. doi: 10.1007/s11764-013-0279-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valle CG, Diamond MA, Heiling HM, et al. Effect of an mHealth intervention on physical activity outcomes among young adult cancer survivors: the IMPACT randomized controlled trial. Cancer. 2023;129(3):461-472. doi: 10.1002/cncr.34556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandura A. Health promotion from the perspective of social cognitive theory. Psychol Health. 1998;13:623-649. [Google Scholar]
- 23.Glanz K, Rimer BK. Health Behavior Theory, Research, and Practice. 5th ed. San Francisco, CA: Jossey-Bass; 2015. [Google Scholar]
- 24.Ungar N, Wiskemann J, Weißmann M, Knoll A, Steindorf K, Sieverding M. Social support and social control in the context of cancer patients’ exercise: a pilot study. Health Psychol Open. 2016;3(2):2055102916680991. doi: 10.1177/2055102916680991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilliam MB, Madan-Swain A, Whelan K, Tucker DC, Demark-Wahnefried W, Schwebel DC. Cognitive influences as mediators of family and peer support for pediatric cancer survivors’ physical activity. Psycho Oncol. 2013;22(6):1361-1368. doi: 10.1002/pon.3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su JA, Yeh DC, Chang CC, et al. Depression and family support in breast cancer patients. Neuropsychiatric Dis Treat. 2017;13:2389-2396. doi: 10.2147/NDT.S135624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barber FD. Social support and physical activity engagement by cancer survivors. Clin J Oncol Nurs. 2012;16(3):E84-E98. doi: 10.1188/12.CJON.E84-E98 [DOI] [PubMed] [Google Scholar]
- 28.Pugh G, Gravestock HL, Hough RE, King WM, Wardle J, Fisher A. Health behavior change interventions for teenage and young adult cancer survivors: a systematic review. J Adolesc Young Adult Oncol. 2016;5(2):91-105. doi: 10.1089/jayao.2015.0042 [DOI] [PubMed] [Google Scholar]
- 29.Meyer JD, Torres ER, Grabow ML, et al. Benefits of 8-wk mindfulness-based stress reduction or aerobic training on seasonal declines in physical activity. Med Sci Sports Exerc. 2018;50(9):1850-1858. doi: 10.1249/MSS.0000000000001636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas AR, Focht BC, Cohn DE, Buckworth J, Klatt MD. A mindfulness-based lifestyle intervention for obese, inactive endometrial cancer survivors: a feasibility study. Integr Cancer Ther. 2017;16(3):263-275. doi: 10.1177/1534735416668257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruffault A, Czernichow S, Hagger MS, et al. The effects of mindfulness training on weight-loss and health-related behaviours in adults with overweight and obesity: a systematic review and meta-analysis. Obes Res Clin Pract. 2017;11(5 Suppl 1):90-111. doi: 10.1016/j.orcp.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 32.Collins L. Optimization of Behavioral, Biobehavioral, and Biomedical Interventions. Berlin: Springer; 2018. [Google Scholar]
- 33.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32(5):S112-S118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins LM, Kugler KC, Gwadz MV. Optimization of multicomponent behavioral and biobehavioral interventions for the prevention and treatment of HIV/AIDS. AIDS Behav. 2016;20 Suppl 1(1):197-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magal M, Riebe D. New preparticipation health screening recommendations: what exercise professionals need to know. ACSM's Health & Fit J. 2016;20(20):22-27. [Google Scholar]
- 36.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inf. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phillips SM, Penedo FJ, Collins LM, et al. Optimization of a technology-supported physical activity promotion intervention for breast cancer survivors: results from Fit2Thrive. Cancer. 2022;128(5):1122-1132. doi: 10.1002/cncr.34012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welch WA, Solk P, Auster-Gussman L, et al. User-centered development of a smartphone application (Fit2Thrive) to promote physical activity in breast cancer survivors. Transl Behav Med. 2022;12(2):203-213. doi: 10.1093/tbm/ibab112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stacey FG, James EL, Chapman K, Courneya KS, Lubans DR. A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. J Cancer Surviv. 2015;9(2):305-338. doi: 10.1007/s11764-014-0413-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bandura A. The primacy of self-regulation in health promotion. Appl Psychol: Int Rev. 2005;54(2):245-254. [Google Scholar]
- 42.Phillips SM, Conroy DE, Keadle SK, et al. Breast cancer survivors' preferences for technology-supported exercise interventions. Support Care Cancer. 2017;25(10):3243-3252. doi: 10.1007/s00520-017-3735-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coughlin SS, Whitehead M, Sheats JQ, Mastromonico J, Smith S. A review of smartphone applications for promoting physical activity. Jacobs J Community Med. 2016;2(1):021. [PMC free article] [PubMed] [Google Scholar]
- 44.Demark-Wahnefried W, Jones LW, Snyder DC, et al. Daughters and Mothers against Breast Cancer (DAMES): main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer. 2014;120(16):2522-2534. doi: 10.1002/cncr.28761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee MK, Park SY, Choi GS. Association of support from family and friends with self-leadership for making long-term lifestyle changes in patients with colorectal cancer. Eur J Cancer Care. 2018;27(3):e12846. doi: 10.1111/ecc.12846 [DOI] [PubMed] [Google Scholar]
- 46.Bus K, Peyer KL, Bai Y, Ellingson LD, Welk GJ. Comparison of in-person and online motivational interviewing-based health coaching. Health Promot Pract. 2018;19(4):513-521. doi: 10.1177/1524839917746634 [DOI] [PubMed] [Google Scholar]
- 47.Short CE, Rebar A, James EL, et al. How do different delivery schedules of tailored web-based physical activity advice for breast cancer survivors influence intervention use and efficacy? J Cancer Surviv. 2017;11(1):80-91. doi: 10.1007/s11764-016-0565-0 [DOI] [PubMed] [Google Scholar]
- 48.Pekmezi D, Ainsworth C, Holly T, et al. Physical activity and related psychosocial outcomes from a pilot randomized trial of an interactive voice response system-supported intervention in the deep south. Health Educ Behav. 2018;45(6):957-966. doi: 10.1177/1090198118775492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gell NM, Grover KW, Humble M, Sexton M, Dittus K. Efficacy, feasibility, and acceptability of a novel technology-based intervention to support physical activity in cancer survivors. Support Care Cancer. 2017;25(4):1291-1300. doi: 10.1007/s00520-016-3523-5 [DOI] [PubMed] [Google Scholar]
- 50.Ulmer CS, Stetson BA, Salmon PG. Mindfulness and acceptance are associated with exercise maintenance in YMCA exercisers. Behav Res Ther. 2010;48(8):805-809. doi: 10.1016/j.brat.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 51.Chatzisarantis NL, Hagger MS. Mindfulness and the intention-behavior relationship within the theory of planned behavior. Pers Soc Psychol Bull. 2007;33(5):663-676. doi: 10.1177/0146167206297401 [DOI] [PubMed] [Google Scholar]
- 52.Arch JJ, Craske MG. Mechanisms of mindfulness: emotion regulation following a focused breathing induction. Behav Res Ther. 2006;44(12):1849-1858. doi: 10.1016/j.brat.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 53.Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatr. 1982;4(1):33-47. doi: 10.1016/0163-8343(82)90026-3 [DOI] [PubMed] [Google Scholar]
- 54.Chambers R, Gullone E, Allen NB. Mindful emotion regulation: an integrative review. Clin Psychol Rev. 2009;29(6):560-572. doi: 10.1016/j.cpr.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 55.Tsafou KE, Lacroix JP, van Ee R, Vinkers CD, De Ridder DT. The relation of trait and state mindfulness with satisfaction and physical activity: a cross-sectional study in 305 Dutch participants. J Health Psychol. 2017;22(10):1221-1232. doi: 10.1177/1359105315624748 [DOI] [PubMed] [Google Scholar]
- 56.Cox AE, Roberts MA, Cates HL, McMahon AK. Mindfulness and affective responses to treadmill walking in individuals with low intrinsic motivation to exercise. Int J Exerc Sci. 2018;11(5):609-624. [PMC free article] [PubMed] [Google Scholar]
- 57.Sala M, Geary B, Baldwin AS. A mindfulness-based physical activity intervention: a randomized pilot study. Psychosom Med. 2020;83(6):615-623. doi: 10.1097/PSY.0000000000000885 [DOI] [PubMed] [Google Scholar]
- 58.Starikovsky J, Victorson D, Welch W, et al. Evaluating the acceptability of mindfulness training during physical activity. In: Presented at: Society of Behavioral Medicine, Phoenix, AZ, 2023. [Google Scholar]
- 59.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188. [DOI] [PubMed] [Google Scholar]
- 60.Western B, Demmelmaier I, Vistad I, et al. How many days of continuous physical activity monitoring reliably represent time in different intensities in cancer survivors. PLoS One. 2023;18(4):e0284881. doi: 10.1371/journal.pone.0284881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37(11 Suppl):S531-S543. doi: 10.1249/01.mss.0000185657.86065.98 [DOI] [PubMed] [Google Scholar]
- 62.Keadle SK, Shiroma EJ, Freedson PS, Lee IM. Impact of accelerometer data processing decisions on the sample size, wear time and physical activity level of a large cohort study. BMC Publ Health. 2014;14:1210. doi: 10.1186/1471-2458-14-1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol. 2008;167(7):875-881. doi: 10.1093/aje/kwm390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crane TE, Skiba MB, Miller A, Garcia DO, Thomson CA. Development and evaluation of an accelerometer-based protocol for measuring physical activity levels in cancer survivors: development and usability study. JMIR Mhealth Uhealth. 2020;8(9):e18491. doi: 10.2196/18491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Godin G, Jobin J, Bouillon J. Assessment of leisure time exercise behavior by self-report: a concurrent validity study. Can J Public Health. 1986;77(5):359-362. [PubMed] [Google Scholar]
- 66.Hays RD, Spritzer KL, Schalet BD, Cella D. PROMIS®-29 v2.0 profile physical and mental health summary scores. Qual Life Res. 2018;27(7):1885-1891. doi: 10.1007/s11136-018-1842-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Full KM, Kerr J, Grandner MA, et al. Validation of a physical activity accelerometer device worn on the hip and wrist against polysomnography. Sleep Health. 2018;4(2):209-216. doi: 10.1016/j.sleh.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33(6):781-792. doi: 10.1093/sleep/33.6.781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li T, Wei S, Shi Y, et al. The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med. 2016;50(6):339-345. doi: 10.1136/bjsports-2015-094927 [DOI] [PubMed] [Google Scholar]
- 70.Hedeker D, Gibbons R, Waternaux C. Sample size estimation for longitudinal designs with attrition: comparing time-related constrasts between two groups. J Educ Behav Stat. 1999;41(1):70-93. [Google Scholar]
- 71.Strayhorn JC, Collins LM, Vanness DJ. A posterior expected value approach to decision-making in the multiphase optimization strategy for intervention science. Psychol Methods 2023;Advanced online publication. doi: 10.1037/met0000569 [DOI] [PubMed] [Google Scholar]
- 72.Krull JL, MacKinnon DP. Multilevel modeling of individual and group level mediated effects. Multivariate Behav Res. 2001;36(2):249-277. doi: 10.1207/S15327906MBR3602_06 [DOI] [PubMed] [Google Scholar]
- 73.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289. doi: 10.3322/caac.21349 [DOI] [PubMed] [Google Scholar]
- 74.Collins LM, Baker TB, Mermelstein RJ, et al. The multiphase optimization strategy for engineering effective tobacco use interventions. Ann Behav Med. 2011;41(2):208-226. doi: 10.1007/s12160-010-9253-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spring B, Pfammatter AF, Marchese SH, et al. A factorial experiment to optimize remotely delivered behavioral treatment for obesity: results of the opt-IN study. Obesity. 2020;28(9):1652-1662. doi: 10.1002/oby.22915 [DOI] [PMC free article] [PubMed] [Google Scholar]