Abstract
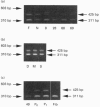
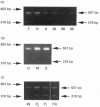
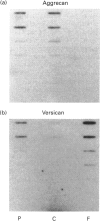
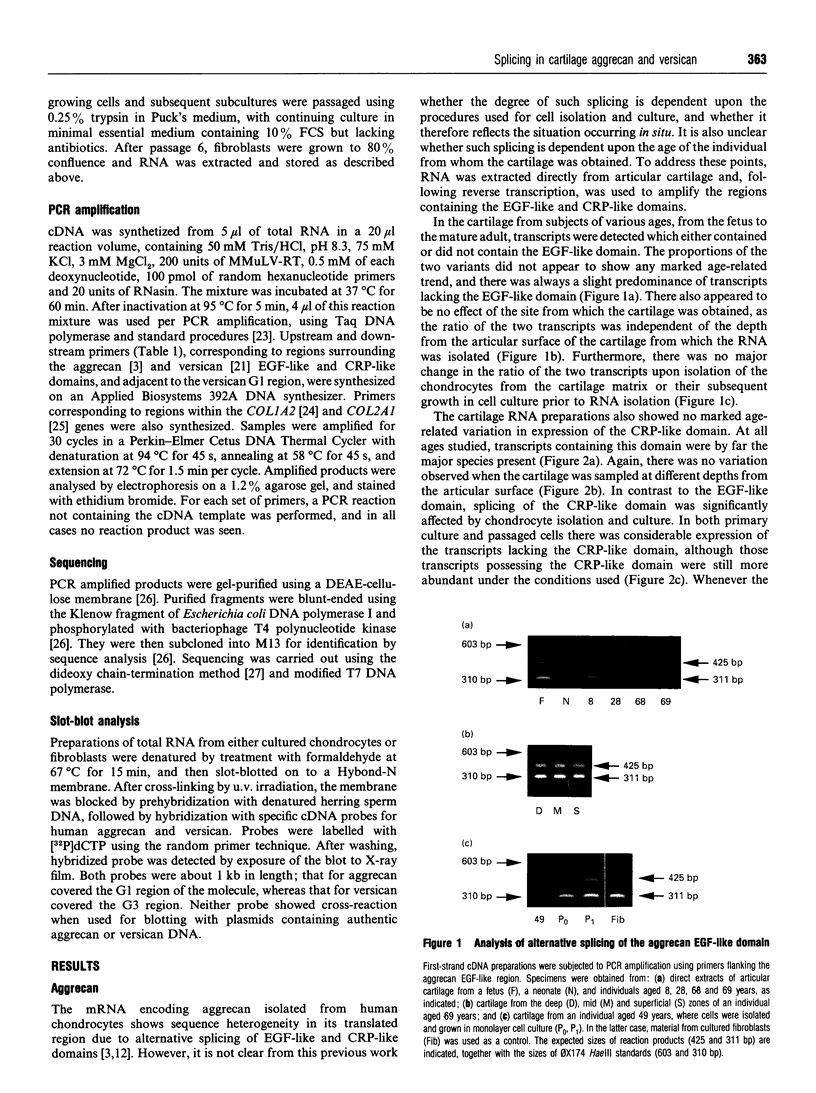
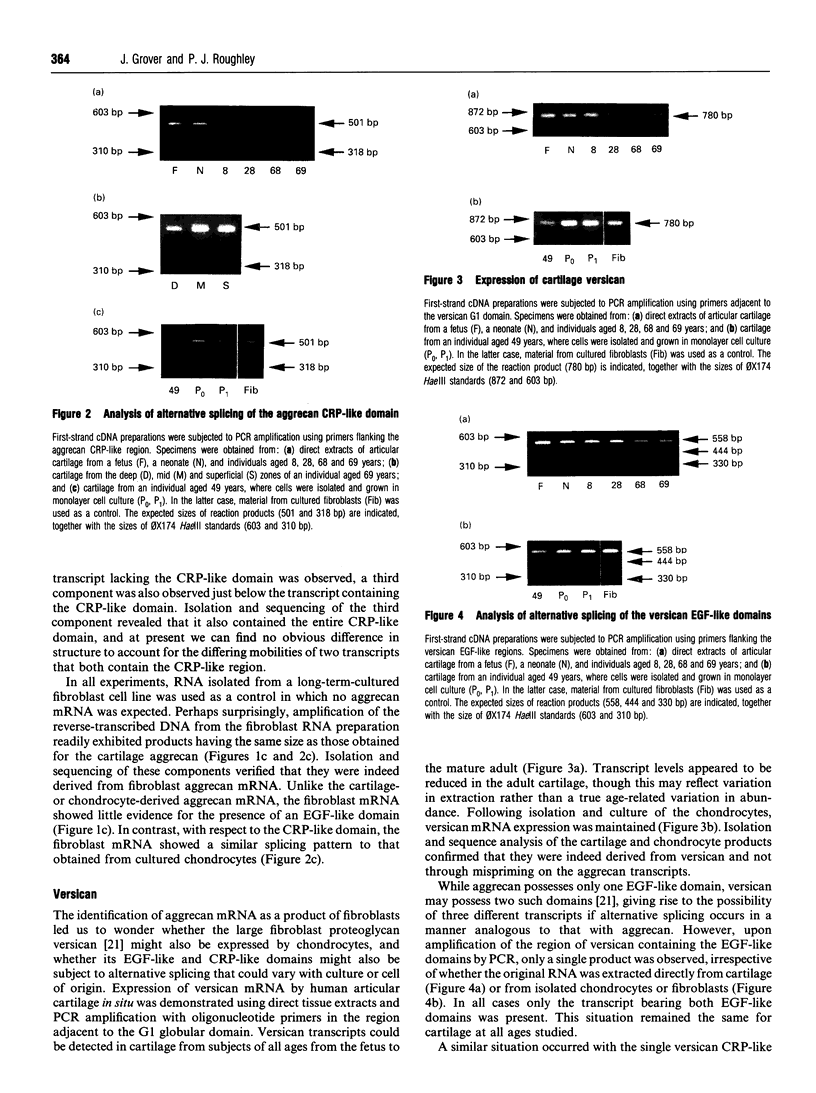
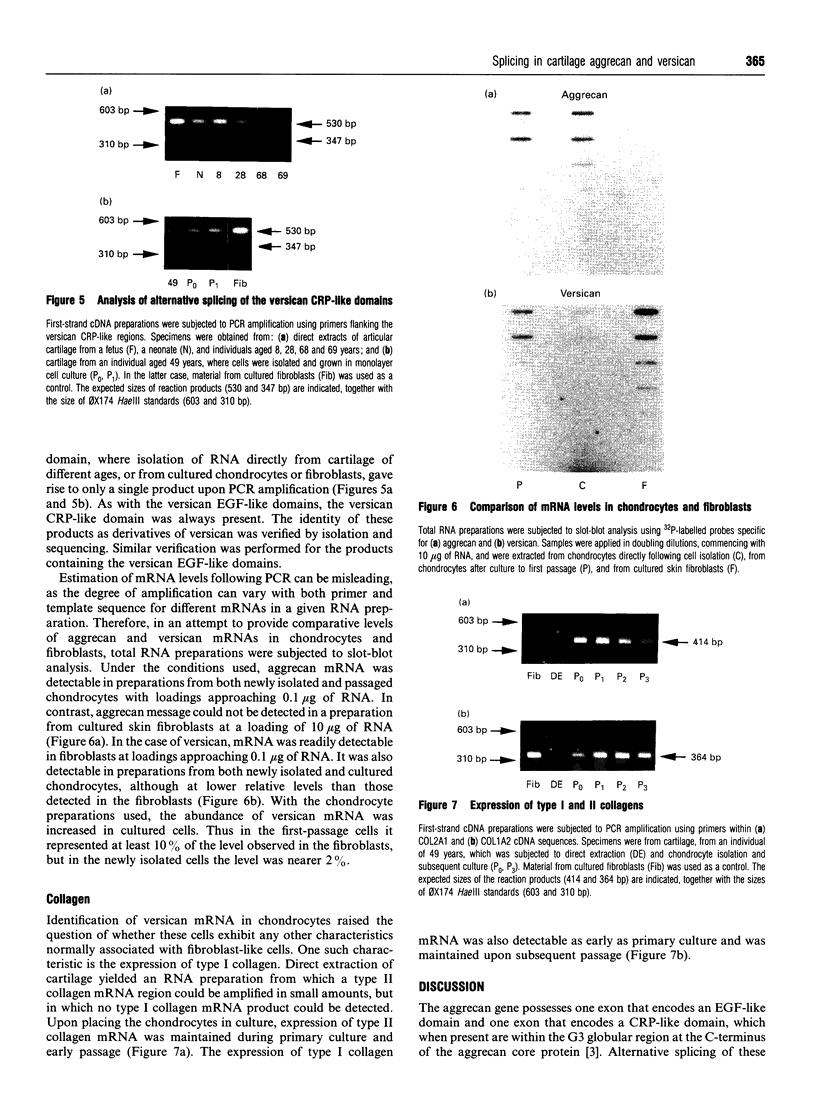
The chondrocytes in human articular cartilage from subjects of all ages express mRNAs for both of the aggregating proteoglycans aggrecan and versican, although the level of expression of versican mRNA is much lower than that of aggrecan mRNA. Aggrecan shows alternative splicing of the epidermal growth factor (EGF)-like domain within its C-terminal globular region, but there is no evidence for a major difference in situ in the relative expression of this domain with age. At all ages studied from birth to the mature adult, a greater proportion of transcripts lacked the EGF domain. The relative proportions of the two transcripts did not change upon culture and passage of isolated chondrocytes. In contrast, the neighbouring complement regulatory protein (CRP)-like domain was predominantly expressed irrespective of age, but cell culture did result in variation of the splicing of this domain. Versican possesses two EGF-like domains and one CRP-like domain, but at all ages the three domains were predominantly present in all transcripts. This situation persisted upon culture and passage of the chondrocytes. Thus, unlike aggrecan, the versican expressed by human articular cartilage does not appear to undergo alternative splicing of its C-terminal globular region, either in cartilage in situ or in chondrocytes in culture.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonsson P., Heinegård D., Oldberg A. The keratan sulfate-enriched region of bovine cartilage proteoglycan consists of a consecutively repeated hexapeptide motif. J Biol Chem. 1989 Sep 25;264(27):16170–16173. [PubMed] [Google Scholar]
- Baldwin C. T., Reginato A. M., Prockop D. J. A new epidermal growth factor-like domain in the human core protein for the large cartilage-specific proteoglycan. Evidence for alternative splicing of the domain. J Biol Chem. 1989 Sep 25;264(27):15747–15750. [PubMed] [Google Scholar]
- Chelly J., Concordet J. P., Kaplan J. C., Kahn A. Illegitimate transcription: transcription of any gene in any cell type. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2617–2621. doi: 10.1073/pnas.86.8.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Doege K. J., Sasaki M., Kimura T., Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991 Jan 15;266(2):894–902. [PubMed] [Google Scholar]
- Doege K., Sasaki M., Horigan E., Hassell J. R., Yamada Y. Complete primary structure of the rat cartilage proteoglycan core protein deduced from cDNA clones. J Biol Chem. 1987 Dec 25;262(36):17757–17767. [PubMed] [Google Scholar]
- Doege K., Sasaki M., Yamada Y. Rat and human cartilage proteoglycan (aggrecan) gene structure. Biochem Soc Trans. 1990 Apr;18(2):200–202. doi: 10.1042/bst0180200. [DOI] [PubMed] [Google Scholar]
- Elima K., Vuorio T., Vuorio E. Determination of the single polyadenylation site of the human pro alpha 1(II) collagen gene. Nucleic Acids Res. 1987 Nov 25;15(22):9499–9504. doi: 10.1093/nar/15.22.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang A. J., Hardingham T. E. Isolation of the N-terminal globular protein domains from cartilage proteoglycans. Identification of G2 domain and its lack of interaction with hyaluronate and link protein. Biochem J. 1989 Aug 1;261(3):801–809. doi: 10.1042/bj2610801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetinck P. F., Stirpe N. S., Tsonis P. A., Carlone D. The tandemly repeated sequences of cartilage link protein contain the sites for interaction with hyaluronic acid. J Cell Biol. 1987 Nov;105(5):2403–2408. doi: 10.1083/jcb.105.5.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Björne-Persson A., Cöster L., Franzén A., Gardell S., Malmström A., Paulsson M., Sandfalk R., Vogel K. The core proteins of large and small interstitial proteoglycans from various connective tissues form distinct subgroups. Biochem J. 1985 Aug 15;230(1):181–194. doi: 10.1042/bj2300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss I., Deák F., Mestrić S., Delius H., Soos J., Dékány K., Argraves W. S., Sparks K. J., Goetinck P. F. Structure of the chicken link protein gene: exons correlate with the protein domains. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6399–6403. doi: 10.1073/pnas.84.18.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knebelmann B., Deschenes G., Gros F., Hors M. C., Grünfeld J. P., Zhou J., Tryggvason K., Gubler M. C., Antignac C. Substitution of arginine for glycine 325 in the collagen alpha 5 (IV) chain associated with X-linked Alport syndrome: characterization of the mutation by direct sequencing of PCR-amplified lymphoblast cDNA fragments. Am J Hum Genet. 1992 Jul;51(1):135–142. [PMC free article] [PubMed] [Google Scholar]
- Kähäri V. M., Larjava H., Uitto J. Differential regulation of extracellular matrix proteoglycan (PG) gene expression. Transforming growth factor-beta 1 up-regulates biglycan (PGI), and versican (large fibroblast PG) but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J Biol Chem. 1991 Jun 5;266(16):10608–10615. [PubMed] [Google Scholar]
- McQuillan D. J., Findlay D. M., Hocking A. M., Yanagishita M., Midura R. J., Hascall V. C. Proteoglycans synthesized by an osteoblast-like cell line (UMR 106-01). Biochem J. 1991 Jul 1;277(Pt 1):199–206. doi: 10.1042/bj2770199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörgelin M., Paulsson M., Malmström A., Heinegård D. Shared and distinct structural features of interstitial proteoglycans from different bovine tissues revealed by electron microscopy. J Biol Chem. 1989 Jul 15;264(20):12080–12090. [PubMed] [Google Scholar]
- Neame P. J., Christner J. E., Baker J. R. Cartilage proteoglycan aggregates. The link protein and proteoglycan amino-terminal globular domains have similar structures. J Biol Chem. 1987 Dec 25;262(36):17768–17778. [PubMed] [Google Scholar]
- Neame P. J., Christner J. E., Baker J. R. The primary structure of link protein from rat chondrosarcoma proteoglycan aggregate. J Biol Chem. 1986 Mar 15;261(8):3519–3535. [PubMed] [Google Scholar]
- Oldberg A., Antonsson P., Hedbom E., Heinegård D. Structure and function of extracellular matrix proteoglycans. Biochem Soc Trans. 1990 Oct;18(5):789–792. doi: 10.1042/bst0180789. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Antonsson P., Heinegård D. The partial amino acid sequence of bovine cartilage proteoglycan, deduced from a cDNA clone, contains numerous Ser-Gly sequences arranged in homologous repeats. Biochem J. 1987 Apr 1;243(1):255–259. doi: 10.1042/bj2430255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsson M., Mörgelin M., Wiedemann H., Beardmore-Gray M., Dunham D., Hardingham T., Heinegård D., Timpl R., Engel J. Extended and globular protein domains in cartilage proteoglycans. Biochem J. 1987 Aug 1;245(3):763–772. doi: 10.1042/bj2450763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada J. A., Thoft R. A., Hassell J. R. Increased aggrecan (cartilage proteoglycan) production in the sclera of myopic chicks. Dev Biol. 1991 Oct;147(2):303–312. doi: 10.1016/0012-1606(91)90288-e. [DOI] [PubMed] [Google Scholar]
- Roughley P. J., McNicol D., Santer V., Buckwalter J. The presence of a cartilage-like proteoglycan in the adult human meniscus. Biochem J. 1981 Jul 1;197(1):77–83. doi: 10.1042/bj1970077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., Mort J. S. Ageing and the aggregating proteoglycans of human articular cartilage. Clin Sci (Lond) 1986 Oct;71(4):337–344. doi: 10.1042/cs0710337. [DOI] [PubMed] [Google Scholar]
- Sai S., Tanaka T., Kosher R. A., Tanzer M. L. Cloning and sequence analysis of a partial cDNA for chicken cartilage proteoglycan core protein. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5081–5085. doi: 10.1073/pnas.83.14.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Boynton R. E., Flannery C. R. Analysis of the catabolism of aggrecan in cartilage explants by quantitation of peptides from the three globular domains. J Biol Chem. 1991 May 5;266(13):8198–8205. [PubMed] [Google Scholar]
- Sandy J. D., Flannery C. R., Boynton R. E., Neame P. J. Isolation and characterization of disulfide-bonded peptides from the three globular domains of aggregating cartilage proteoglycan. J Biol Chem. 1990 Dec 5;265(34):21108–21113. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanescu V., Chaminade F., Pham T. D. Immunological detection of the EGF-like domain of the core proteins of large proteoglycans from human and baboon cartilage. Connect Tissue Res. 1991;26(4):283–293. doi: 10.3109/03008209109152445. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Heinegård D. Characterization of proteoglycans from adult bovine tendon. J Biol Chem. 1985 Aug 5;260(16):9298–9306. [PubMed] [Google Scholar]
- Zimmermann D. R., Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989 Oct;8(10):2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet W., Bernard M., Benson-Chanda V., Chu M. L., Dickson L., Weil D., Ramirez F. Organization of the human pro-alpha 2(I) collagen gene. J Biol Chem. 1987 Nov 25;262(33):16032–16036. [PubMed] [Google Scholar]