Abstract
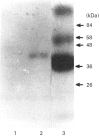
G-protein subunits were characterized from Medicago sativa (alfalfa) seedlings. Crude membranes and GTP-Sepharose-purified fractions were electrophoresed on SDS/polyacrylamide gels and analysed by Western blotting with 9193 (anti-alpha common) and AS/7 (anti-alpha t, anti-alpha i1 and anti-alpha i2) polyclonal antibodies. These procedures led to the identification of a specific polypeptide band of about 43 kDa. Another polypeptide reacting with the SW/1 (anti-beta) antibody, of about 37 kDa, was also detected. The 43 kDa polypeptide bound specifically [alpha-32P]GTP by a photoaffinity reaction and was ADP-ribosylated by activated cholera toxin, but not by pertussis toxin. Irradiation of etiolated Medicago sativa protoplast preparations at 660 nm for 1 min produced a maximal increase in the guanosine 5'-[gamma-thio]triphosphate (GTP[35S])-binding rate. After this period of irradiation, the binding rate tended to decrease. The effect of a red-light (660 nm) pulse on the binding rate was reversed when it was immediately followed by a period of far-red (> 730 nm) illumination. These results may suggest that activation of GTP[S]-binding rate was a consequence of conversion of phytochrome Pr into the Ptr form.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anuntalabhochai S., Terryn N., Van Montagu M., Inzé D. Molecular characterization of an Arabidopsis thaliana cDNA encoding a small GTP-binding protein, Rha1. Plant J. 1991 Sep;1(2):167–174. [PubMed] [Google Scholar]
- Blum W., Hinsch K. D., Schultz G., Weiler E. W. Identification of GTP-binding proteins in the plasma membrane of higher plants. Biochem Biophys Res Commun. 1988 Oct 31;156(2):954–959. doi: 10.1016/s0006-291x(88)80936-7. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carricarte V. C., Bianchini G. M., Muschietti J. P., Téllez-Iñn M. T., Perticari A., Torres N., Flawiá M. M. Adenylate cyclase activity in a higher plant, alfalfa (Medicago sativa). Biochem J. 1988 Feb 1;249(3):807–811. doi: 10.1042/bj2490807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina J., Hildebrandt J. D., Sekura R. D., Birnbaumer M., Bryan J., Manclark C. R., Iyengar R., Birnbaumer L. Ns and Ni, the stimulatory and inhibitory regulatory components of adenylyl cyclases. Purification of the human erythrocyte proteins without the use of activating regulatory ligands. J Biol Chem. 1984 May 10;259(9):5871–5886. [PubMed] [Google Scholar]
- Das R., Sopory S. K. Evidence of regulation of calcium uptake by phytochrome in maize protoplasts. Biochem Biophys Res Commun. 1985 May 16;128(3):1455–1460. doi: 10.1016/0006-291x(85)91103-9. [DOI] [PubMed] [Google Scholar]
- Einspahr K. J., Peeler T. C., Thompson G. A. Phosphatidylinositol 4,5-Bisphosphate Phospholipase C and Phosphomonoesterase in Dunaliella salina Membranes. Plant Physiol. 1989 Jul;90(3):1115–1120. doi: 10.1104/pp.90.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspahr K. J., Thompson G. A. Transmembrane Signaling via Phosphatidylinositol 4,5-Bisphosphate Hydrolysis in Plants. Plant Physiol. 1990 Jun;93(2):361–366. doi: 10.1104/pp.93.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenschlos C., Flawiá M. M., Torruella M., Torres H. N. Interaction of Trypanosoma cruzi adenylate cyclase with liver regulatory factors. Biochem J. 1986 May 15;236(1):185–191. doi: 10.1042/bj2360185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettlinger C., Lehle L. Auxin induces rapid changes in phosphatidylinositol metabolites. Nature. 1988 Jan 14;331(6152):176–178. doi: 10.1038/331176a0. [DOI] [PubMed] [Google Scholar]
- Fairley-Grenot K., Assmann S. M. Evidence for G-Protein Regulation of Inward K+ Channel Current in Guard Cells of Fava Bean. Plant Cell. 1991 Sep;3(9):1037–1044. doi: 10.1105/tpc.3.9.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flawiá M. M., Kornblihtt A. R., Reig J. A., Torruella M., Torres H. N. Reconstitution of a hormone-sensitive adenylate cyclase with membrane extracts from Neurospora and avian erythrocytes. J Biol Chem. 1983 Jul 10;258(13):8255–8259. [PubMed] [Google Scholar]
- Goldsmith P., Backlund P. S., Jr, Rossiter K., Carter A., Milligan G., Unson C. G., Spiegel A. Purification of heterotrimeric GTP-binding proteins from brain: identification of a novel form of Go. Biochemistry. 1988 Sep 6;27(18):7085–7090. doi: 10.1021/bi00418a062. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Thelen M. P., Farndale R. W., Astle M. C., Rubery P. H. Specific guanine nucleotide binding by membranes from Cucurbita pepo seedlings. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1478–1484. doi: 10.1016/s0006-291x(88)81308-1. [DOI] [PubMed] [Google Scholar]
- Kornblihtt A. R., Flawia M. M., Torres H. N. Manganese ion dependent adenylate cyclase activity in rat testes: purification and properties. Biochemistry. 1981 Mar 3;20(5):1262–1267. doi: 10.1021/bi00508a033. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lam E., Benedyk M., Chua N. H. Characterization of phytochrome-regulated gene expression in a photoautotrophic cell suspension: possible role for calmodulin. Mol Cell Biol. 1989 Nov;9(11):4819–4823. doi: 10.1128/mcb.9.11.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linse K., Mandelkow E. M. The GTP-binding peptide of beta-tubulin. Localization by direct photoaffinity labeling and comparison with nucleotide-binding proteins. J Biol Chem. 1988 Oct 15;263(29):15205–15210. [PubMed] [Google Scholar]
- Ma H., Yanofsky M. F., Meyerowitz E. M. Molecular cloning and characterization of GPA1, a G protein alpha subunit gene from Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1990 May;87(10):3821–3825. doi: 10.1073/pnas.87.10.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M., Sasamoto S., Kunieda T., Nomura N., Ishizaki R. Cloning of ara, a putative Arabidopsis thaliana gene homologous to the ras-related gene family. Gene. 1989;76(2):313–319. doi: 10.1016/0378-1119(89)90171-6. [DOI] [PubMed] [Google Scholar]
- Melin P. M., Sommarin M., Sandelius A. S., Jergil B. Identification of Ca2+-stimulated polyphosphoinositide phospholipase C in isolated plant plasma membranes. FEBS Lett. 1987 Oct 19;223(1):87–91. doi: 10.1016/0014-5793(87)80515-x. [DOI] [PubMed] [Google Scholar]
- Morse M. J., Crain R. C., Satter R. L. Light-stimulated inositolphospholipid turnover in Samanea saman leaf pulvini. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7075–7078. doi: 10.1073/pnas.84.20.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschietti J. P., Bianchini G. M., Martinetto H. E., Carricarte V. C., Giusto N., Farber D. B., Torres H. N., Flawia M. M. Reconstitution of a light-stimulated adenylate cyclase from retina and Neurospora crassa preparations. Characterization of the heterologous systems using normal and degenerative retinas. Eur J Biochem. 1989 Oct 20;185(1):205–210. doi: 10.1111/j.1432-1033.1989.tb15103.x. [DOI] [PubMed] [Google Scholar]
- Northup J. K., Smigel M. D., Gilman A. G. The guanine nucleotide activating site of the regulatory component of adenylate cyclase. Identification by ligand binding. J Biol Chem. 1982 Oct 10;257(19):11416–11423. [PubMed] [Google Scholar]
- Oelze-Karow H., Mohr H. Phytochrome action on chlorophyll synthesis-a study of the escape from photoreversibility. Plant Physiol. 1982 Sep;70(3):863–866. doi: 10.1104/pp.70.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palme K., Diefenthal T., Vingron M., Sander C., Schell J. Molecular cloning and structural analysis of genes from Zea mays (L.) coding for members of the ras-related ypt gene family. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):787–791. doi: 10.1073/pnas.89.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffmann H., Hartmann E., Brightman A. O., Morré D. J. Phosphatidylinositol specific phospholipase C of plant stems : membrane associated activity concentrated in plasma membranes. Plant Physiol. 1987 Dec;85(4):1151–1155. doi: 10.1104/pp.85.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail P. H. Phytochrome: a light-activated molecular switch that regulates plant gene expression. Annu Rev Genet. 1991;25:389–409. doi: 10.1146/annurev.ge.25.120191.002133. [DOI] [PubMed] [Google Scholar]
- Romero L. C., Sommer D., Gotor C., Song P. S. G-proteins in etiolated Avena seedlings. Possible phytochrome regulation. FEBS Lett. 1991 May 6;282(2):341–346. doi: 10.1016/0014-5793(91)80509-2. [DOI] [PubMed] [Google Scholar]
- Schaltmann K., Pongs O. A simple procedure for blotting of proteins to study antibody specificity and antigen structure. Hoppe Seylers Z Physiol Chem. 1980;361(2):207–210. [PubMed] [Google Scholar]
- Simonds W. F., Goldsmith P. K., Codina J., Unson C. G., Spiegel A. M. Gi2 mediates alpha 2-adrenergic inhibition of adenylyl cyclase in platelet membranes: in situ identification with G alpha C-terminal antibodies. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7809–7813. doi: 10.1073/pnas.86.20.7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternweis P. C., Northup J. K., Smigel M. D., Gilman A. G. The regulatory component of adenylate cyclase. Purification and properties. J Biol Chem. 1981 Nov 25;256(22):11517–11526. [PubMed] [Google Scholar]
- Tate B. F., Schaller G. E., Sussman M. R., Crain R. C. Characterization of a Polyphosphoinositide Phospholipase C from the Plasma Membrane of Avena sativa. Plant Physiol. 1989 Dec;91(4):1275–1279. doi: 10.1104/pp.91.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewavas A., Gilroy S. Signal transduction in plant cells. Trends Genet. 1991 Nov-Dec;7(11-12):356–361. doi: 10.1016/0168-9525(91)90255-o. [DOI] [PubMed] [Google Scholar]
- Waldo G. L., Evans T., Fraser E. D., Northup J. K., Martin M. W., Harden T. K. Identification and purification from bovine brain of a guanine-nucleotide-binding protein distinct from Gs, Gi and Go. Biochem J. 1987 Sep 1;246(2):431–439. doi: 10.1042/bj2460431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner T. J., Ross J. D., Coombs J. Phytochrome Control of Maize Coleoptile Section Elongation : A RAPID LOSS OF PHOTOREVERSIBILITY. Plant Physiol. 1981 Feb;67(2):355–357. doi: 10.1104/pp.67.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warpeha K. M., Hamm H. E., Rasenick M. M., Kaufman L. S. A blue-light-activated GTP-binding protein in the plasma membranes of etiolated peas. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8925–8929. doi: 10.1073/pnas.88.20.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zherelova O. M. Protein kinase C is involved in regulation of Ca2+ channels in plasmalemma of Nitella syncarpa. FEBS Lett. 1989 Jan 2;242(2):330–332. doi: 10.1016/0014-5793(89)80495-8. [DOI] [PubMed] [Google Scholar]