Abstract
Background
Electroacupuncture (EA) has been shown to promote functional recovery after cerebral ischemia–reperfusion (I/R) injury. However, the contribution of mitochondrial dynamics to recovery remains unclear. The aim of this study was to investigate whether mitochondrial dynamics are involved in the effects of EA on cerebral I/R injury.
Methods
The rats with cerebral I/R injury were established by the middle cerebral artery occlusion/reperfusion. Subsequently, EA was applied to Baihui (GV20) and Dazhui (GV14) acupoints, with 2 Hz/5 Hz in frequency, 1.0 mA in intensity, 20 min each time, once a day for seven consecutive days. The therapeutic outcomes were assessed by modified neurological severity score (mNSS), 2,3,5-Triphenyte-trazolium chloride (TTC) staining, and hematoxylin-eosin (HE) staining. Mitochondrial morphology was observed under transmission electron microscopy. Adenosine triphosphate (ATP) content and ATP synthases (ATPases) activity were evaluated to measure mitochondrial function using ELISA. Finally, mitochondrial dynamics-related molecules, including dynamin-related protein 1 (Drp1), fission 1 (Fis1), mitofusin 1 (Mfn1), mitofusin 2 (Mfn2), and optic atrophy 1 (OPA1), were detected by Western blot and immunofluorescence staining.
Results
Cerebral I/R injury induced neurological dysfunction, cerebral infarction and neuronal injury, all of which were ameliorated by EA. And EA improved mitochondrial morphology and function. Moreover, EA altered the balance of mitochondrial dynamics. Specifically, the data showed a significant decrease in the expression of Drp1 and Fis1, leading to the inhibition of mitochondrial fission. Additionally, Mfn1, Mfn2 and Opa1, which are related to mitochondrial fusion, were effectively promoted after EA treatment. However, sham EA did not show any neuroprotective effects in rats with cerebral I/R injury.
Conclusions
In summary, our study indicates that the balance of mitochondrial dynamics is crucial for EA therapy to treat cerebral I/R injury.
Keywords: Cerebral ischemia–reperfusion injury, Electroacupuncture, Mitochondrial dynamics, Neurological deficits
1. Introduction
Ischemic stroke is prevalent cerebrovascular accidents leading to mortality and permanent disability worldwide [1]. Thrombolytic therapy such as recombinant tissue plasminogen activators, the preferred therapeutic approach for acute ischemic stroke, is capable of restoring the cerebral blood flow [2]. However, the rapid reperfusion of ischemic brain may subsequently aggravate cerebral damage and cause neurological deficits, which was known as cerebral ischemia-reperfusion (I/R) injury [3]. Studies indicate that cerebral I/R injury initiates a sequential series of responses such as oxidative stress, excitotoxicity and impaired mitochondrial processes [4], ultimately leading to the cell death and irreversible neurological dysfunction [5]. As a result, there is still a need for effective strategies in the neurological recovery of cerebral I/R injury.
As an alternative and complementary medicine, acupuncture therapy has been extensively practiced for nervous disorders in Asia for centuries [6]. Electroacupuncture (EA), an innovative therapeutic method combining traditional acupuncture with modern electrostimulation, is proved to be effective for clinical treatment of stroke patients [[7], [8], [9], [10]]. Baihui (GV20) and Dazhui (GV14) are acupoints commonly used for stroke recovery, both located on the “Du meridian”, which are in close contact with the brain [11,12]. According to the theory of traditional Chinese medicine, stimulation of these two points may facilitate the upward flow of Yang Qi to the head, exerting a range of effects on physiological functions and pathological processes. Previous studies have demonstrated that EA at GV20 and GV14 may promote neurological recovery and repair the damaged brain tissue in laboratory models [13,14]. However, the mechanisms underlying the effect of EA treatment on cerebral I/R injury have not been fully elucidated.
Mitochondria, intracellular organelles producing energy, are essential mediators of metabolic homeostasis following cerebral I/R injury [15,16]. Mitochondrial dynamics refers to mitochondrial fusion/fission processes that are critical for mitochondrial morphology and function [17]. Therefore, it is necessary to clarify the biological processes involved in mitochondrial dynamics to better understand cerebral I/R injury. In the mitochondrial fusion/fission processes, precise protein regulation is necessary for the reorganization of the mitochondrial membrane. On the one hand, dynamin-related protein 1 (Drp1) and Fission 1 (Fis1) serve as main proteins responsible for mitochondrial fission. Drp1 is the primary regulator of mitochondrial fission and mainly expressed in cytoplasm [18]. Fis1, a receptor for Drp1, is capable of recruiting Drp1 from the cytoplasm to the outer mitochondrial membrane [19]. Then Drp1 accumulates on the outer mitochondrial membrane and forms a spiral ring, resulting in the separation of the mitochondria into two mitochondria. On the other hand, mitochondrial fusion involves three crucial proteins: mitofusin 1 (Mfn1), mitofusin 2 (Mfn2), and optic atrophy 1 (Opa1). Mfn1 and Mfn2, situated on the outer mitochondrial membrane, promote the degradation of GTP enzyme and ultimately lead to the fusion of the outer membranes of the two mitochondria [20]. In cells lacking either Mfn1 or Mfn2, the degree of mitochondrial fusion is greatly reduced, resulting in a complete loss of mitochondrial tubular structure and impaired mitochondrial function [21,22]. In contrast to Mfn1/Mfn2, Opa1is primarily involved in the fusion of the mitochondrial inner membrane [23]. It has been reported that the imbalance of mitochondrial dynamics after cerebral I/R injury causes excessive mitochondrial fragments, with consequent pathological conditions such as metabolic disorders and oxidative stress, culminating in cell programmed death [24]. Moreover, previous pharmacological researches also suggested the mitochondrial dynamics has been considered as a new therapeutic target for cerebral I/R injury [[25], [26], [27]]. In recent years, some studies have demonstrated that mitochondrial disorders are involved in the development of cerebral I/R injury [28,29], and EA is reported having the potential to maintain mitochondrial quality [30,31]. Nevertheless, the role of mitochondrial dynamics in EA against cerebral I/R injury remains unclear.
Based on the known interactions between mitochondrial dynamics and cerebral I/R injury as reported above, it is speculated that there may be a potential correlation between EA treatment and mitochondrial dynamics in cerebral I/R injury. The purpose of the present study is to investigate whether the EA treatment is achieved by regulating mitochondrial dynamics.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats (220 g–250 g, 8 weeks old) were obtained from Liaoning Changsheng Biotechnology Co., Ltd. [Experimental animal certificate number: SCXK(Liao)2020-0001] and raised in the Experimental Animal Center of Anhui University of Chinese Medicine. The rats were housed in conditions with 12 h of daylight exposure, temperature ranging between 21°C and 23 °C, humidity maintained at 70 %, and with free access to food and water. All rats were handled and treated in accordance with the Guidelines for the Human Treatment of Laboratory Animals promulgated by the Ministry of Science and Technology of the People's Republic of China in 2006. This study was approved by the Experimental Animal Care and Use Committee of Anhui University of Chinese Medicine (approval number: AHUCM-rats-2022158).
2.2. Experimental protocol
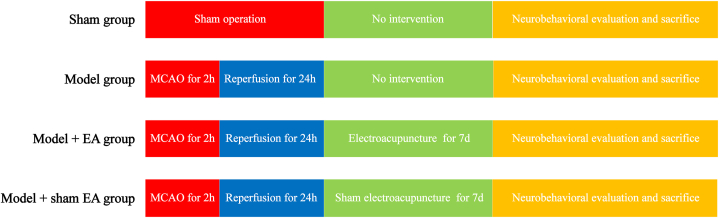
Sixty rats were randomly assigned into four groups: sham group, model group, model + EA group, and model + sham EA group. The cerebral I/R injury model was established by middle cerebral artery occlusion followed by reperfusion in the model group, model + EA group, and model + sham EA group. Rats in the model + EA group received EA at GV20 and GV14, while the rats in the model + sham EA group underwent a similar procedure at non-acupoints without electrical stimulation. After neurobehavioral impairment conditions were evaluated, rats were sacrificed and brain tissues were collected for multiplex analysis.
The experimental protocol is shown in Fig. 1.
Fig. 1.
The experimental protocol for the four groups.
2.3. Model establishment
Cerebral I/R injury models were established as previously described [32]. In brief, rats were anesthetized by pentobarbital sodium (40 mg/kg, i. p.) and fixed in the supine position. The right common carotid artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA) were exposed, with the CCA and ECA being tied off. And a nylon suture was inserted into the ICA from the incision on CCA and moved forward for approximately 18 mm in order to occlude the middle cerebral artery for a duration of 120 min. Afterward, the nylon suture was removed for reperfusion, and the skin on the neck was sutured. Longa score of 1–3 were regarded to be successfully established and included into the experiment.
In the sham group, rats underwent the same procedure as other groups except for the insertion of nylon suture.
2.4. EA treatment
On the first day after surgery, EA treatment was performed as reported [33]. In the model + EA group, stainless-steel acupuncture needles were inserted into GV 20 (located in the median of the parietal) and GV 14 (located below the spinous process of the seventh cervical vertebra) at a depth of 3 mm. The electrical current range was set at 1.0 mA lasting for 20 min, with a controlled frequency of 2Hz/5 Hz using an electric acupuncture apparatus (SDZ-IV, Huatuo, Suzhou, China) [33]. While the stainless-steel acupuncture needles were inserted superficially into the non-acupoints of GV20 and GV 14 (5 mm to the right of correct position) without electrical stimulation in model + sham EA group. These procedures were repeated once daily for seven consecutive days.
2.5. Neurobehavioral evaluation
After completion of intervention, neurological functions of rats in each group were evaluated by modified neurological severity score (mNSS) as reported [34]. In brief, this test was composed of assessments for motor functions, sensory functions, balance abilities, and reflexes. The higher score indicates the more severe neurological deficits.
2.6. TTC staining
The volume of cerebral infarct was evaluated by 2,3,5-Triphenyte-trazolium chloride (TTC) staining. In brief, fresh brains were washed with saline and frozen at - 20 °C for 20 min and were cut into slices of 2 mm in thickness. The slices were stained using a 2 % TTC solution (Solarbio, 20220525, Beijing, China) and incubated for 20 min at 37 °C. After staining, normal brain tissue turns red while infarcted tissue turns white. Then, the brain slices were photographed, and the infarcted and normal tissues were measured using the Image J software (NIH, Bethesda, USA). The infarct volume was calculated by the following formula: infarct volume (%) = [ infarct tissues/(infarct tissues + normal tissues)] × 100.
2.7. HE staining
The pathological injury of neurons was observed by hematoxylin-eosin (HE) staining. In brief, paraffin sections were dewaxed in water and then immersed in a hematoxylin staining solution (ebiogo, B006, California, USA) for 5 min. After rinsing with pure water, the sections were dehydrated in 70 % and 90 % ethanol for 10 min each. Following immersion in eosin staining solution (ebiogo, B006, California, USA) for 1 min, the sections were dehydrated in 100 % ethanol, washed with xylene, and observed under a microscope. Finally, the sample was sealed with neutral gum.
2.8. Transmission electron microscopy
The mitochondrial morphology of neurons was observed under transmission electron microscopy (TEM). A slice of 1 mm in thickness, taken from the cerebral cortex and measuring, was immersed in a 2.5 % glutaraldehyde solution at 4 °C for 24 h, and rinsed with PBS solution for three times. Then the tissue was dehydrated with a graded series of ethanol acetone, soaked and infiltrated with acetone, placed in pure epoxy resin, baked at 45 °C for 12 h and transferred to a 72 °C oven for 24 h. After embedding, the sample was made into slices of 70 nm by microtome (Leica, UC-7, skar-Barnack-Straße, Germany), stained with lead (TED PELLA INC, 19 312, California, USA) for 15 min, and dried at 37 °C. The neural morphological structure of the cerebral cortex was observed and photographed under TEM (JEOL, JEM1400, Tokyo, Japan) for image analysis.
2.9. Determination of ATP content and ATPases activity
Adenosine triphosphate (ATP) content and ATP synthases (ATPases) activity were determined to assess mitochondrial function. Tissue homogenate was prepared by lysis solution at a temperature of 4 °C. And mitochondria precipitate was obtained after centrifugations at 3000 r/min for 5 min and at 12 000 r/min for 30 min. ATP content and ATPases activity were measured using the Assay Kits.
2.10. Western blot
The relative expressions of mitochondrial dynamics-related proteins were identified by Western blot. Total proteins of brain tissues were extracted using RIPA lysis buffer (Beyotime Institute of Biotechnology, P0013B, Jiangsu, China) and subjected to centrifugation at 12 000 r/min for 15 min. Approximately 20 μL amount of protein samples were separated by 12 % SDS-PAGE (Solarbio, S8010, Beijing, China) and transferred onto PVDF membranes (Millipore, IPVH00010, Massachusetts, USA). After blocking in 5 % skim milk, the primary antibodies against Drp1 (1:2000, 12957-1-AP, Proteintech), Fis1 (1:1000, bsm-60551 M, Bioss), Opa1 (1:1000, bs-11764R, Bioss), Mfn1 (1:1000, SC-166644, Santa Cruz), Mfn2 (1:1000, SC-100560, Santa Cruz), and GAPDH (1:5000, TA-08, Zsbio) were applied at 4 °C overnight. The corresponding secondary antibodies were incubated for 1 h at 37 °C. The immune response was then purified using an ECL kit (Thermo, 340 958, Massachusetts, USA). Relative integrated density values were analyzed using Image J software.
2.11. Immunofluorescence staining
The expressions of Drp1 and Opa1 were detected by the immunofluorescence staining. The paraffin sections were immersed in xylene for dewaxing, and dipped in 95 %, 85 %, and 75 % ethanol for gradient ethanol hydration. Subsequently, the sections were subjected to microwave antigen retrieval and were blocked in goat serum. Following this, they were incubated with primary antibodies targeting Drp1 (1:200, PA5-105983, Thermofish), or Opa1 (1:200, MA5-32786, Thermofish) at 4 °C overnight. Then the samples were stained with a fluorescently-labeled secondary antibody (goat anti-rabbit IgG 1:400) and incubated at 37 °C for 30 min, followed by staining with DAPI. Finally, the sections were imaged under a digital scanner (Pannoramic MIDI, 3DHISTECH, Hungary).
2.12. Statistical analysis
All statistical analyses were conducted using SPSS 23.0 software. And the measurement data conforming to the normal distribution were expressed as mean ± SEM while one-way analysis of ANOVA followed by LSD post hoc test was used for comparison between multiple groups. P < 0.05 was considered as statistically significant.
3. Results
3.1. EA alleviated neurological deficits, infarct volume, and neuronal injury
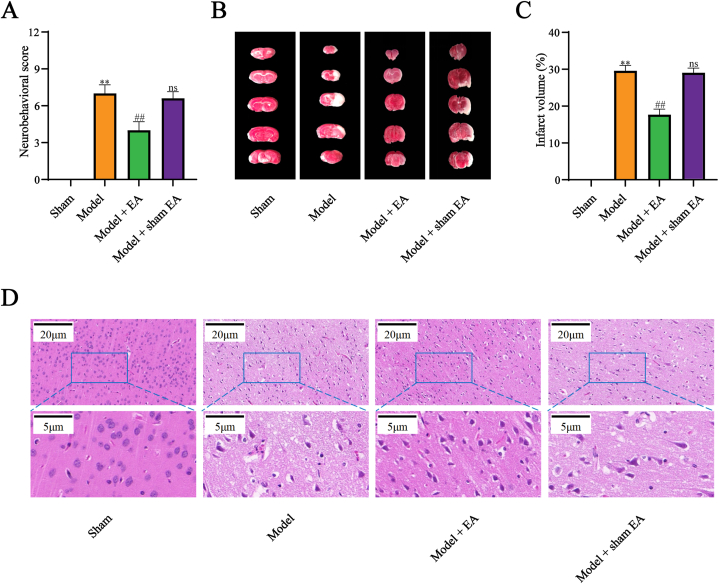
To prove the beneficial efforts of EA intervention on cerebral I/R injury, mNSS and TTC staining were respectively used to determine the neurological deficits and cerebral infarct at the beginning. The neurobehavioral score and infarct volume were increased by cerebral I/R injury, which was reduced after EA treatment (Fig. 2A–C). However, no statistical difference was detected between the model group and the model + sham EA group.
Fig. 2.
EA alleviated neurological deficits, infarct volume, and neuronal injury in cerebral I/R injury. (A) EA decreased neurobehavioral score in cerebral I/R injury (n=15). (B, C) EA reduced infarct volume in cerebral I/R injury (n=5). Red regions represented normal brain tissue while white regions represented infarcted brain tissue. (D) EA improved the pathological injury in cerebral I/R injury (n=5). All data are shown as mean ± SD. Statistical significance was determined using one-way ANOVA followed by LSD post hoc test. ** indicates P < 0.01, compared with sham group; ns indicates not significant, ## indicates P < 0.01, compared with model group. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The HE staining showed that the cell structure remained unblemished in the sham group, with sufficient cytoplasm and clear nuclei. In comparison to the sham group, the cortical neurons of the model and model + sham EA groups displayed irregular arrangements, with extended intercellular spaces, vacuoles, and pyknosis of nuclei. In contrast to the model group, the organization of cortical neurons in the model + EA group was moderately regular, accompanied by decreased intercellular spaces and clear nucleoli (Fig. 2D).
The preliminary data demonstrated that cerebral I/R injury prominently induced neurological deficits, cerebral infarct, and neuronal injury. Notably, EA not sham EA markedly exerted positive efforts on cerebral I/R injury.
3.2. EA improved mitochondrial morphology and function in cerebral I/R injury
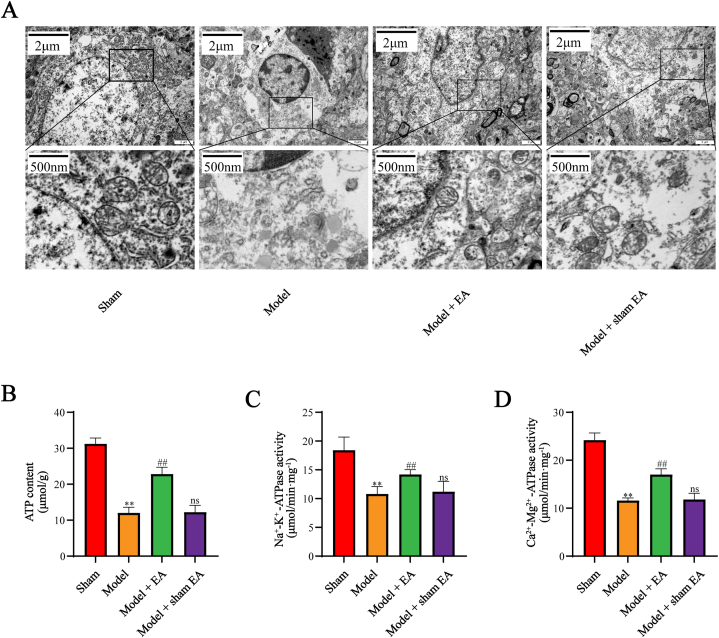
To probe the potential mitochondrial mechanisms of EA treatment, we focused on mitochondrial morphology and function in the next step. The mitochondrial morphology was observed under TEM. As shown in Fig. 3A, the degree of mitochondrial morphology was preliminary assessed by neuronal mitochondrial ultra-structure under TEM. Contrary to the sham group, the mitochondria in the model group and model + sham EA group showed a lack of typical tubular or elliptic morphology, disappearance of the double-membrane structure, addition of vacuoles, and swelling of cristae. In contrast, treatment with EA remarkably restrained the above changes. Since ATP content and ATPases activity are important indications of mitochondrial function, we further examine the effect of EA on mitochondrial function. As presented in Fig. 3B–D, the ATP content and ATPases activity were enhanced by EA treatment compared with the model group. Collectively, EA intervention improved mitochondrial morphology and function in cerebral I/R injury.
Fig. 3.
EA improved mitochondrial morphology and function in cerebral I/R injury. (A) EA improved mitochondrial morphology in cerebral I/R injury. (B, C, D) EA improved mitochondrial function in cerebral I/R injury. The experiments were repeated three times in each group. All data are shown as mean ± SD. Statistical significance was determined using one-way ANOVA followed by LSD post hoc test. ** indicates P < 0.01, compared with sham group; ns indicates not significant, ## indicates P < 0.01, compared with model group.
3.3. EA altered the balance of mitochondrial dynamics in cerebral I/R injury
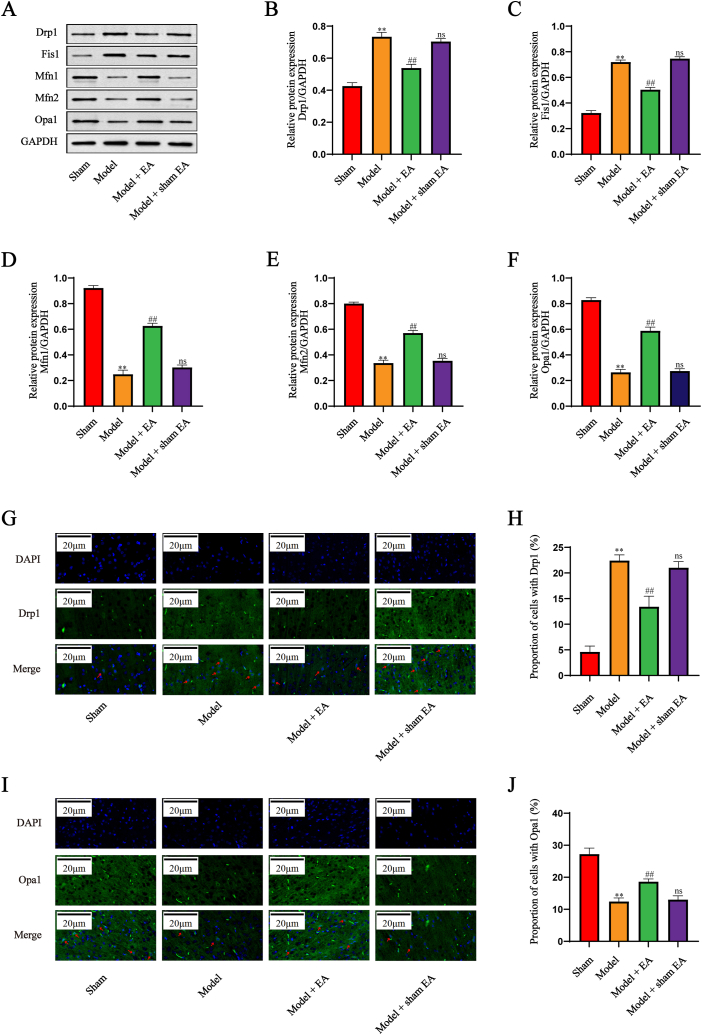
To explore the role of the mitochondrial dynamics in the EA treatment, the related proteins of mitochondrial fission and fusion were identified by Western blot. As illustrated in Fig. 4A–F, the increase of Drp1 and Fis1 and the decrease of Mfn1, Mfn1, and Opa1 were induced by cerebral I/R injury, which were reversed by intervention with EA not sham EA. In addition, the immunofluorescence staining for Drp1 and Opa1 was performed to verify the correlation between mitochondrial dynamics and EA therapy. As shown in Fig. 4G–J, the expression of Drp1 was significantly elevated and Opa1 markedly suppressed in the model group relative to that in the sham group, with these effects notably prevented by EA but not by the sham EA. These results indicated that EA treatment altered the balance of mitochondrial dynamics induced by cerebral I/R injury.
Fig. 4.
EA altered the balance of mitochondrial dynamics in cerebral I/R injury. (A–F) Western blot and quantitative analysis of Drp1 and Fis1, Mfn2, Mfn2 and Opa1 (n = 5). GAPDH was used as an internal control. The experiments were repeated three times in each group. (G–J) Immunofluorescence staining and quantitative analysis of Drp1 and Opa1 (n = 5). The nuclei were stained with DAPI (blue). The target proteins were stained green. The representative positive cells were marked with red arrows. Drp1: dynamin-related protein 1; Fis1; fission 1; Mfn1: mitofusin 1; Mfn1: mitofusin 2; Opa1: optic atrophy 1. All data are shown as mean ± SD. Statistical significance was determined using one-way ANOVA followed by LSD post hoc test. ** indicates P < 0.01, compared with sham group; ns indicates not significant, ## indicates P < 0.01, compared with model group. The original versions of Fig. 4A are presented as supplementary data. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Ischemic stroke is primarily characterized by poor prognosis and high cost, which imposes immense burdens to families and society [35]. Although thrombolytic therapy is able to restore blood flow to the ischemic tissue, the resupply of blood could deteriorate neuronal injury and cerebral infraction [36]. A considerable body of literature has reported that EA treatment substantially improved neurological outcomes in cases of cerebral I/R injury [37,38]. Here, we investigated the potential mechanism of application of EA for cerebral I/R injury. The following findings were illustrated by our results: (1) EA intervention reduces neurological score, cerebral infarct volume, and neuronal injury; (2) mitochondria are involved in the effect of EA treatment; (3) EA exerts neuroprotective effects and improves neuronal survival by reducing mitochondrial fission and promoting mitochondrial fusion.
EA is a contemporary acupuncture therapy in the clinical treatment of ischemic stroke to improve quality of life and alleviate inconvenience [7,39]. Therefore, the models were established by middle cerebral artery occlusion followed by reperfusion in order to assess the clinical effect of EA intervention at first. Our study showed that the neurobehavioral score, cerebral infarct volume, and neuronal injury in the model group were more severe than those in the sham group, which indicated that the rat models of cerebral I/R injury were successfully established. Moreover, the rats in the EA group but not the sham EA group showed improved outcomes, suggesting that EA had beneficial effects on cerebral I/R injury, similar to the results of the previous study [40,41].
The structure and function of mitochondria are fundamental to the physiological activities of neurons, but mitochondria are highly vulnerable to damage in the presence of various stressors [42]. In order to gain insight into the mechanisms by which EA against cerebral I/R injury, the mitochondrial morphology and function were observed in this study. The data showed that EA treatment ameliorated severe structural abnormalities and decreased ATP generation induced by cerebral I/R injury. Mitochondrial dynamics, consisting of the processes of mitochondrial fission and fusion, play an important role in the mitochondrial morphology and function [43]. Several studies have demonstrated that inhibition of mitochondrial fission and enhancement of mitochondrial fusion could help reduce neuronal damage [[44], [45], [46]], so we speculated that mitochondrial dynamics might be a potential perspective to understand EA improving mitochondrial disorders. To investigate the involvement of mitochondrial dynamics in EA treatment alleviating cerebral I/R injury, various related molecules were examined in the present study. According to our results, there was an increase in mitochondrial fission-related proteins (Drp1 and Fis1) and a decrease in mitochondrial fusion-related proteins (Mfn2, Mfn2, and Opa1) following cerebral I/R injury. And both Western blot and immunofluorescence staining revealed that EA, but not sham EA, downregulated the expression of mitochondrial fission-related proteins while upregulating those involved in mitochondrial fusion. The results showed that EA treatment counteracted the enhancement of mitochondrial fission and reduction of mitochondrial fusion, indicating EA treatment after cerebral I/R injury could be achieved by regulating mitochondrial dynamics.
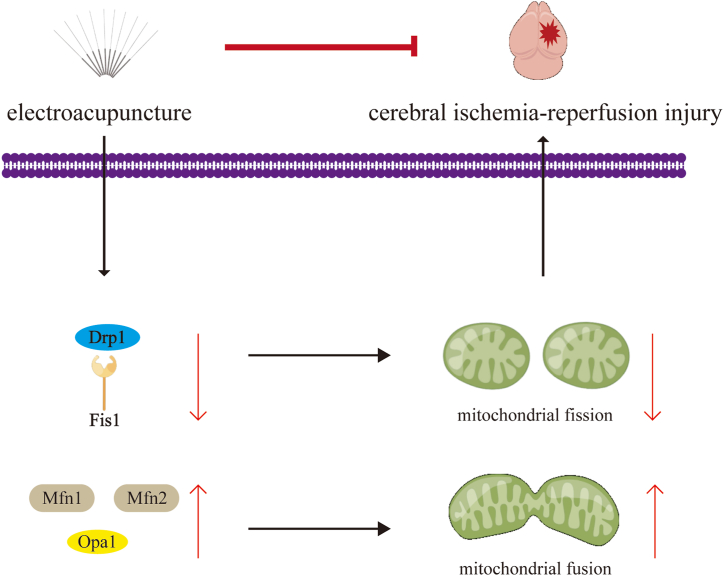
Taken together, EA may protect against cerebral I/R injury through mitochondrial dynamics, including the inhibition of Drp1/Fis1-mediated mitochondrial fission and the elevation of Mfn2/Mfn2/Opa1-mediated mitochondrial fusion (Fig. 5). The findings provide a novel view on mitochondrial dynamics in cerebral I/R injury ameliorated by EA. However, this study is just a preliminary experiment due to the limited sample size and data available for analysis, and the molecular interactions have not yet been adequately explored. Subsequently, the potential molecular mechanism between EA stimulation and mitochondrial dynamics in cerebral I/R injury will be investigated. Recent studies have demonstrated that the fat mass and obesity-associated protein, a recognized m6A demethylase, is involved in the suppression of Drp1 activity, both in vivo and in vitro [47,48]. Thus, the role of m6A modification in EA treatment would be a promise direction for further research, which might present new experimental basis for promoting the clinical application.
Fig. 5.
EA treatment alleviates cerebral I/R injury by regulating the balance of mitochondrial dynamics, including decreasing mitochondrial fission and increasing mitochondrial fusion.
Ethics declarations
This study was reviewed and approved by the Experimental Animal Care and Use Committee of Anhui University of Chinese Medicine with the approval number: AHUCM-rats-2022158, dated February 20, 2023.
Funding
The study was supported by the National Natural Science Foundation of China (No. 81973933), Anhui Provincial University Scientific Research Programmes (No. 2023AH040111, No. 2023AH050739, No. 2023AH050816, No. 2022AH050503), Anhui Key R&D Programmes (No. 202304295107020103), Open Fund Project of Anhui Acupuncture and Moxibustion Clinical Medicine Research Center (No. 2021zjzx02), the 13th “115” Industrial Innovation Team of Anhui Province (No. 2020-4), Anhui Provincial Famous Expert of TCM Studio (No. 2022-5) and Guangzhou Municipal Three-class Famous Expert of TCM Studio (No. 2023-2515-43).
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Cheng-long Li: Writing – original draft, Formal analysis, Data curation. Wei Mao: Writing – original draft, Formal analysis, Data curation. Li-da Zhang: Methodology, Investigation. Hai-sheng Ji: Investigation, Data curation. Ting-ting Tong: Investigation. Jun-li Wang: Investigation. Xiao-qing Wu: Investigation. Kui-wu Li: Investigation. Hai-yang Wu: Data curation. Guo-qing Zhang: Investigation. Jun-yu Zhang: Investigation. Wei Han: Writing – review & editing, Supervision, Data curation. Ying Wang: Writing – review & editing, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e34986.
Contributor Information
Wei Han, Email: 13956060099@139.com.
Ying Wang, Email: zhenjiu205@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Feigin V.L., Brainin M., Norrving B., Martins S., Sacco R.L., Hacke W., Fisher M., Pandian J., Lindsay P. World stroke organization (WSO): global stroke fact sheet 2022. Int. J. Stroke. 2022;17(1):18–29. doi: 10.1177/17474930211065917. [DOI] [PubMed] [Google Scholar]
- 2.Yang N., Lee H., Wu C. Intravenous thrombolysis for acute ischemic stroke: from alteplase to tenecteplase. Brain Circ. 2023;9(2):61–63. doi: 10.4103/bc.bc_70_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y., He Y., Yan S., Chen L., Zhang R., Xu J., Hu H., Liebeskind D.S., Lou M. Reperfusion injury is associated with poor outcome in patients with recanalization after thrombectomy. Stroke. 2023;54(1):96–104. doi: 10.1161/STROKEAHA.122.039337. [DOI] [PubMed] [Google Scholar]
- 4.Li M., Tang H., Li Z., Tang W. Emerging treatment strategies for cerebral ischemia-reperfusion injury. Neuroscience. 2022;507:112–124. doi: 10.1016/j.neuroscience.2022.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q., Jia M., Wang Y., Wang Q., Wu J. Cell death mechanisms in cerebral ischemia-reperfusion injury. Neurochem. Res. 2022;47(12):3525–3542. doi: 10.1007/s11064-022-03697-8. [DOI] [PubMed] [Google Scholar]
- 6.Lu L., Zhang Y., Tang X., Ge S., Wen H., Zeng J., Wang L., Zeng Z., Rada G., Ávila C., Vergara C., Tang Y., Zhang P., Chen R., Dong Y., Wei X., Luo W., Wang L., Guyatt G., Tang C., Xu N. Evidence on acupuncture therapies is underused in clinical practice and health policy. Bmj. 2022;376 doi: 10.1136/bmj-2021-067475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duc Nguyen M., Van Tran T., Vinh Nguyen Q., Khac Nguyen N., Truong Vu S., Trong Nguyen L., Vu Phuong Dang L. Effectiveness on post-stroke hemiplegia in patients: electroacupuncture plus cycling electroacupuncture alone. J. Tradit. Chin. Med. 2023;43(2):352–358. doi: 10.19852/j.cnki.jtcm.2023.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu B.H., Xing Y., Zhang F. The therapeutic effect of electroacupuncture therapy for ischemic stroke. Evid Based Complement Alternat Med. 2020;2020 doi: 10.1155/2020/6415083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu A.J., Li J.H., Li H.Q., Fu D.L., Lu L., Bian Z.X., Zheng G.Q. Electroacupuncture for acute ischemic stroke: a meta-analysis of randomized controlled trials. Am. J. Chin. Med. 2015;43(8):1541–1566. doi: 10.1142/S0192415X15500883. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Yang X., Cao Y., Li X., Xu R., Yan J., Guo Z., Sun S., Sun X., Wu Y. Electroacupuncture alleviates early brain injury via modulating microglia polarization and suppressing neuroinflammation in a rat model of subarachnoid hemorrhage. Heliyon. 2023;9(3) doi: 10.1016/j.heliyon.2023.e14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F., He T., Xu Q., Lin L.T., Li H., Liu Y., Shi G.X., Liu C.Z. What is the Acupoint? A preliminary review of Acupoints. Pain Med. 2015;16(10):1905–1915. doi: 10.1111/pme.12761. [DOI] [PubMed] [Google Scholar]
- 12.Qiu K., Yin T., Hong X., Sun R., He Z., Liu X., Ma P., Yang J., Lan L., Li Z., Tang C., Cheng S., Liang F., Zeng F. Does the acupoint specificity exist? Evidence from functional neuroimaging studies. Curr Med Imaging. 2020;16(6):629–638. doi: 10.2174/1573405615666190220113111. [DOI] [PubMed] [Google Scholar]
- 13.Yao L.L., Yuan S., Wu Z.N., Luo J.Y., Tang X.R., Tang C.Z., Cui S., Xu N.G. Contralateral S1 function is involved in electroacupuncture treatment-mediated recovery after focal unilateral M1 infarction. Neural Regen Res. 2022;17(6):1310–1317. doi: 10.4103/1673-5374.327355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu F., Lu Z., Li Z., Wang S., Zhuang L., Hong M., Huang K. Electroacupuncture improves cerebral ischemic injury by enhancing the EPO-JAK2-STAT5 pathway in rats. Neuropsychiatric Dis. Treat. 2021;17:2489–2498. doi: 10.2147/NDT.S316136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardoso A.R., Queliconi B.B., Kowaltowski A.J. Mitochondrial ion transport pathways: role in metabolic diseases. Biochim. Biophys. Acta. 2010;1797(6–7):832–838. doi: 10.1016/j.bbabio.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Park E., Choi S.K., Kang S.W., Pak Y.K., Lee G.J., Chung J.H., Park H.K. Cerebral ischemia-induced mitochondrial changes in a global ischemic rat model by AFM. Biomed. Pharmacother. 2015;71:15–20. doi: 10.1016/j.biopha.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Giacomello M., Pyakurel A., Glytsou C., Scorrano L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 2020;21(4):204–224. doi: 10.1038/s41580-020-0210-7. [DOI] [PubMed] [Google Scholar]
- 18.Losón O.C., Song Z., Chen H., Chan D.C. Fis1, mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell. 2013;24(5):659–667. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James D.I., Parone P.A., Mattenberger Y., Martinou J.C. hFis1, a novel component of the mammalian mitochondrial fission machinery. J. Biol. Chem. 2003;278(38):36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- 20.Chan D.C. Mitochondrial fusion and fission in mammals. Annu. Rev. Cell Dev. Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 21.Gao S., Hu J. Mitochondrial fusion: the machineries in and out. Trends Cell Biol. 2021;31(1):62–74. doi: 10.1016/j.tcb.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Cerveny K.L., Tamura Y., Zhang Z., Jensen R.E., Sesaki H. Regulation of mitochondrial fusion and division. Trends Cell Biol. 2007;17(11):563–569. doi: 10.1016/j.tcb.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Alexander C., Votruba M., Pesch U.E., Thiselton D.L., Mayer S., Moore A., Rodriguez M., Kellner U., Leo-Kottler B., Auburger G., Bhattacharya S.S., Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat. Genet. 2000;26(2):211–215. doi: 10.1038/79944. [DOI] [PubMed] [Google Scholar]
- 24.Huang J., Chen L., Yao Z.M., Sun X.R., Tong X.H., Dong S.Y. The role of mitochondrial dynamics in cerebral ischemia-reperfusion injury. Biomed. Pharmacother. 2023;162 doi: 10.1016/j.biopha.2023.114671. [DOI] [PubMed] [Google Scholar]
- 25.Wu Q., Liu J., Mao Z., Tian L., Wang N., Wang G., Wang Y., Seto S. Ligustilide attenuates ischemic stroke injury by promoting Drp1-mediated mitochondrial fission via activation of AMPK. Phytomedicine. 2022;95 doi: 10.1016/j.phymed.2021.153884. [DOI] [PubMed] [Google Scholar]
- 26.Huang Q., Li J., Chen J., Zhang Z., Xu P., Qi H., Chen Z., Liu J., Lu J., Shi M., Zhang Y., Ma Y., Zhao D., Li X. Ginsenoside compound K protects against cerebral ischemia/reperfusion injury via Mul1/Mfn2-mediated mitochondrial dynamics and bioenergy. J Ginseng Res. 2023;47(3):408–419. doi: 10.1016/j.jgr.2022.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali M., Tabassum H., Alam M.M., Parvez S. N-acetyl-L-cysteine ameliorates mitochondrial dysfunction in ischemia/reperfusion injury via attenuating Drp-1 mediated mitochondrial autophagy. Life Sci. 2022;293 doi: 10.1016/j.lfs.2022.120338. [DOI] [PubMed] [Google Scholar]
- 28.Xu Y., Chen B., Yi J., Tian F., Liu Y., Ouyang Y., Yuan C., Liu B. Buyang Huanwu Decoction alleviates cerebral ischemic injury through modulating caveolin-1-mediated mitochondrial quality control. Front. Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1137609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng H., Sun L.K., Su J., Yan W.Y., Jin Y., Luo X., Jiang X.R., Wang H.L. Serine protease HtrA2/Omi regulates adaptive mitochondrial reprogramming in the brain cortex after ischemia/reperfusion injury via UCP2-SIRT3-PGC1 axis. Hum. Cell. 2022;35(1):63–82. doi: 10.1007/s13577-021-00610-3. [DOI] [PubMed] [Google Scholar]
- 30.Ding Z., Gao J., Feng Y., Wang M., Zhao H., Wu R., Zheng X., Feng X., Lai M. Electroacupuncture ameliorates depression-like behaviors in post-stroke rats via activating AMPK-mediated mitochondrial function. Neuropsychiatric Dis. Treat. 2023;19:2657–2671. doi: 10.2147/NDT.S436177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mu R., Li N., Yu J.B., Gong L.R., Dong S.A., Shi J., Zhang Y., Xie Z.L. Electroacupuncture relieves hippocampal injury by heme oxygenase-1 to improve mitochondrial function. J. Surg. Res. 2022;273:15–23. doi: 10.1016/j.jss.2021.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Longa E.Z., Weinstein P.R., Carlson S., Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 33.Tong T.T., Wang Y., Li K.W., Zhang L.D., Wu X.Q., Wang J.L., Li C.L., Zhang G.Q., Zhang J.Y., Han W. Effect of Tongdu Tiaoshen electroacupuncture pretreatment on PPARγ-mediated pyroptosis of cerebral cortex in rats with cerebral ischemia reperfusion injury. Zhongguo Zhen Jiu. 2023;43(7):783–792. doi: 10.13703/j.0255-2930.20221010-k0004. [DOI] [PubMed] [Google Scholar]
- 34.Chen J., Li Y., Wang L., Zhang Z., Lu D., Lu M., Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 35.Martínez-Vila E., Irimia P. The cost of stroke. Cerebrovasc. Dis. 2004;17(Suppl 1):124–129. doi: 10.1159/000074804. [DOI] [PubMed] [Google Scholar]
- 36.Mao R., Zong N., Hu Y., Chen Y., Xu Y. Neuronal death mechanisms and therapeutic strategy in ischemic stroke. Neurosci. Bull. 2022;38(10):1229–1247. doi: 10.1007/s12264-022-00859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deng P., Wang L., Zhang Q., Chen S., Zhang Y., Xu H., Chen H., Xu Y., He W., Zhang J., Sun H. Therapeutic potential of a combination of electroacupuncture and human iPSC-derived small extracellular vesicles for ischemic stroke. Cells. 2022;11(5) doi: 10.3390/cells11050820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S., Zhao X., Lin F., Ni X., Liu X., Kong C., Yao X., Mo Y., Dai Q., Wang J. Gut flora mediates the rapid tolerance of electroacupuncture on ischemic stroke by activating melatonin receptor through regulating indole-3-propionic acid. Am. J. Chin. Med. 2022;50(4):979–1006. doi: 10.1142/S0192415X22500409. [DOI] [PubMed] [Google Scholar]
- 39.Kim M.S., Moon B.S., Ahn J.Y., Shim S.S., Yun J.M., Joo M.C. Elucidating the mechanisms of post-stroke motor recovery mediated by electroacupuncture using diffusion tensor tractography. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.888165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao F., Xu Y., Zhang M., Li X., Chen Y., Zhi M., Li Y. Baihui (DU20), Shenmen (HT7) and Sanyinjiao (SP6) target the cAMP/CREB/BDNF and PI3K/Akt pathways to reduce central nervous system apoptosis in rats with insomnia. Heliyon. 2022;8(12) doi: 10.1016/j.heliyon.2022.e12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiuping L., Pan P., Zhenzhen L., Zhen Z., Xuezhu Z., Shuting L. Acupuncture regulates the Th17/Treg balance and improves cognitive deficits in a rat model of vascular dementia. Heliyon. 2023;9(2) doi: 10.1016/j.heliyon.2023.e13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nusir A., Sinclair P., Kabbani N. Mitochondrial proteomes in neural cells: a systematic review. Biomolecules. 2023;13(11) doi: 10.3390/biom13111638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vongsfak J., Pratchayasakul W., Apaijai N., Vaniyapong T., Chattipakorn N., Chattipakorn S.C. The alterations in mitochondrial dynamics following cerebral ischemia/reperfusion injury. Antioxidants. 2021;10(9) doi: 10.3390/antiox10091384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C., Yuan X.R., Li H.Y., Zhao Z.J., Liao Y.W., Wang X.Y., Su J., Sang S.S., Liu Q. Downregualtion of dynamin-related protein 1 attenuates glutamate-induced excitotoxicity via regulating mitochondrial function in a calcium dependent manner in HT22 cells. Biochem. Biophys. Res. Commun. 2014;443(1):138–143. doi: 10.1016/j.bbrc.2013.11.072. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Y.X., Cui M., Chen S.F., Dong Q., Liu X.Y. Amelioration of ischemic mitochondrial injury and Bax-dependent outer membrane permeabilization by Mdivi-1. CNS Neurosci. Ther. 2014;20(6):528–538. doi: 10.1111/cns.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grohm J., Kim S.W., Mamrak U., Tobaben S., Cassidy-Stone A., Nunnari J., Plesnila N., Culmsee C. Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ. 2012;19(9):1446–1458. doi: 10.1038/cdd.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du Y.D., Guo W.Y., Han C.H., Wang Y., Chen X.S., Li D.W., Liu J.L., Zhang M., Zhu N., Wang X. N6-methyladenosine demethylase FTO impairs hepatic ischemia-reperfusion injury via inhibiting Drp1-mediated mitochondrial fragmentation. Cell Death Dis. 2021;12(5):442. doi: 10.1038/s41419-021-03622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y., Gong X. Fat mass and obesity associated protein inhibits neuronal ferroptosis via the FYN/Drp1 axis and alleviate cerebral ischemia/reperfusion injury. CNS Neurosci. Ther. 2024;30(3) doi: 10.1111/cns.14636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.