Abstract
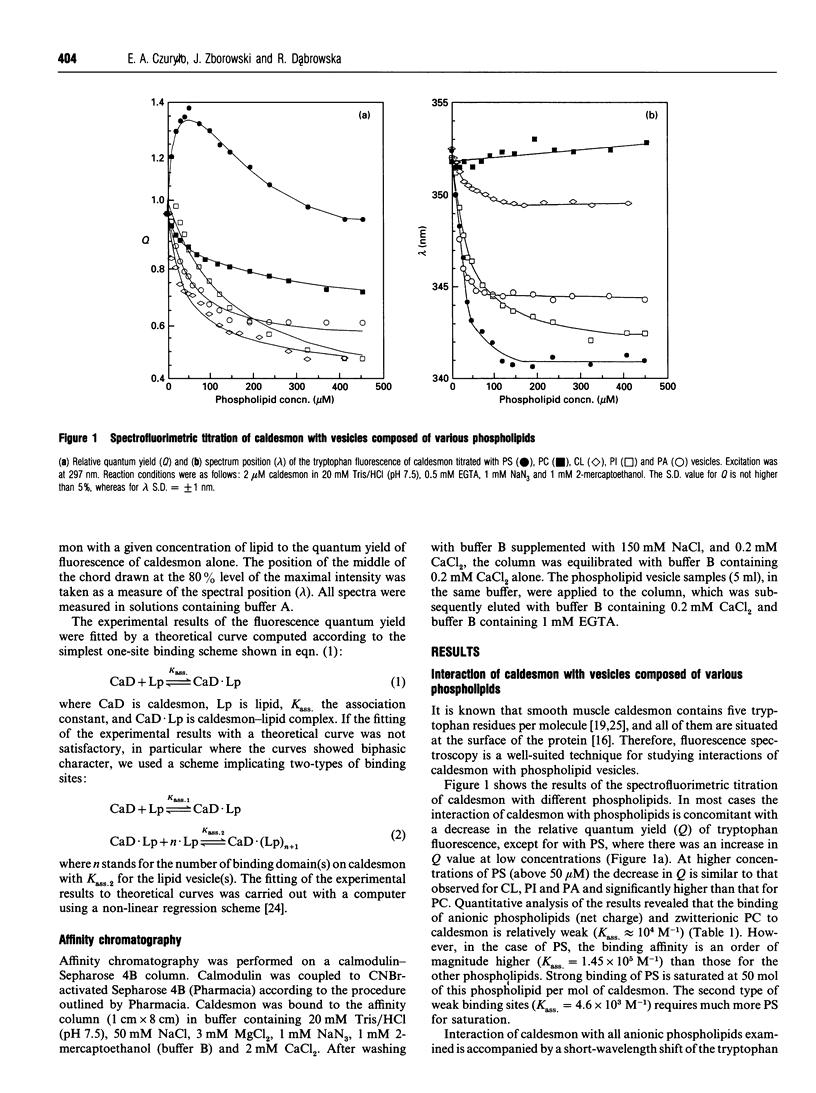
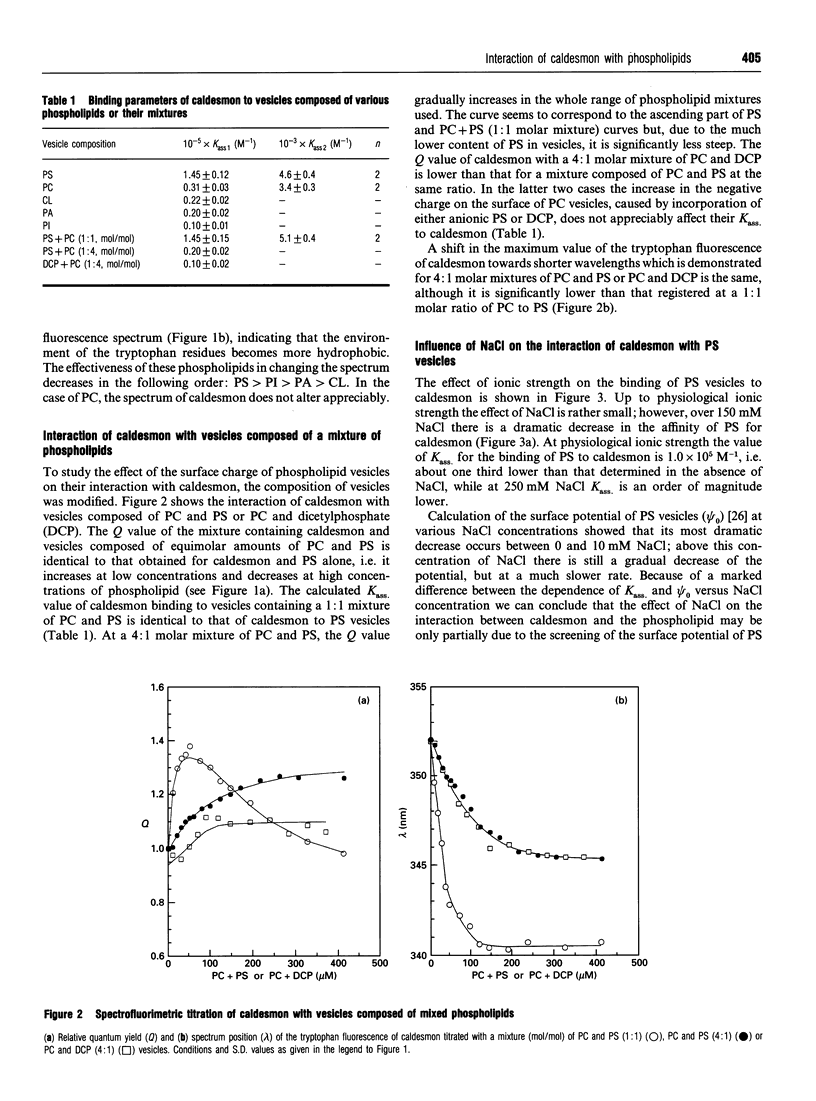
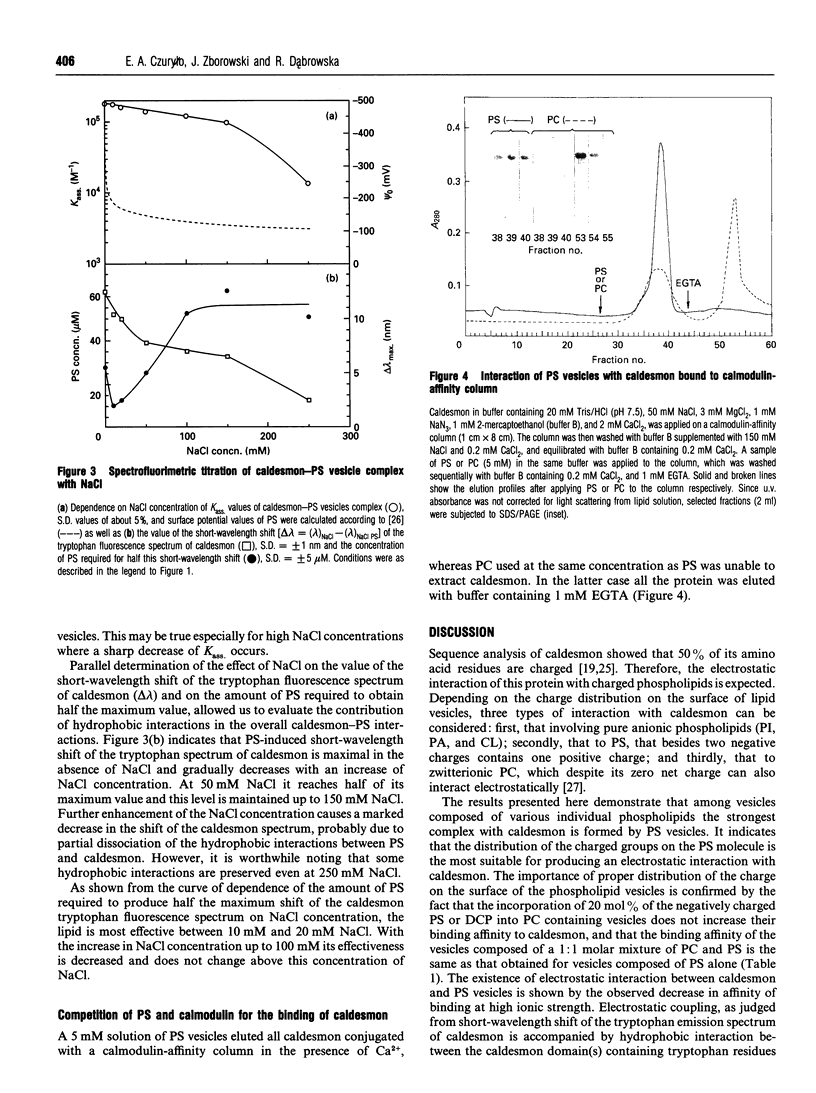
The interaction of caldesmon with liposomes composed of various phospholipids has been examined by tryptophan fluorescence spectroscopy. The results indicate that caldesmon makes its strongest complex with phosphatidylserine (PS) vesicles (Kass. = 1.45 x 10(5) M-1). Both electrostatic and hydrophobic interactions contribute to the stability of this complex. The site for strong binding of PS seems to be located in the N-terminal part of the 34 kDa C-terminal fragment of caldesmon. Binding of PS at this site results in displacement of calmodulin from its complex with caldesmon.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam L. P., Milio L., Brengle B., Hathaway D. R. Myosin light chain and caldesmon phosphorylation in arterial muscle stimulated with endothelin-1. J Mol Cell Cardiol. 1990 Sep;22(9):1017–1023. doi: 10.1016/0022-2828(90)91041-5. [DOI] [PubMed] [Google Scholar]
- Adams R. J., Pollard T. D. Binding of myosin I to membrane lipids. Nature. 1989 Aug 17;340(6234):565–568. doi: 10.1038/340565a0. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Lynch W. Identification and localization of immunoreactive forms of caldesmon in smooth and nonmuscle cells: a comparison with the distributions of tropomyosin and alpha-actinin. J Cell Biol. 1985 May;100(5):1656–1663. doi: 10.1083/jcb.100.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Bryan J., Imai M., Lee R., Moore P., Cook R. G., Lin W. G. Cloning and expression of a smooth muscle caldesmon. J Biol Chem. 1989 Aug 15;264(23):13873–13879. [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Norman K. M. Identification of a secretory granule-binding protein as caldesmon. Nature. 1986 Jan 2;319(6048):68–70. doi: 10.1038/319068a0. [DOI] [PubMed] [Google Scholar]
- Burstein E. A., Vedenkina N. S., Ivkova M. N. Fluorescence and the location of tryptophan residues in protein molecules. Photochem Photobiol. 1973 Oct;18(4):263–279. doi: 10.1111/j.1751-1097.1973.tb06422.x. [DOI] [PubMed] [Google Scholar]
- Chalovich J. M. Caldesmon and thin-filament regulation of muscle contraction. Cell Biophys. 1988 Jan-Jun;12:73–85. doi: 10.1007/BF02918351. [DOI] [PubMed] [Google Scholar]
- Comfurius P., Zwaal R. F. The enzymatic synthesis of phosphatidylserine and purification by CM-cellulose column chromatography. Biochim Biophys Acta. 1977 Jul 20;488(1):36–42. doi: 10.1016/0005-2760(77)90120-5. [DOI] [PubMed] [Google Scholar]
- Czuryło E. A., Emelyanenko V. I., Permyakov E. A., Dabrowska R. Spectrofluorimetric studies on C-terminal 34 kDa fragment of caldesmon. Biophys Chem. 1991 May;40(2):181–188. doi: 10.1016/0301-4622(91)87007-r. [DOI] [PubMed] [Google Scholar]
- Dingus J., Hwo S., Bryan J. Identification by monoclonal antibodies and characterization of human platelet caldesmon. J Cell Biol. 1986 May;102(5):1748–1757. doi: 10.1083/jcb.102.5.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gałazkiewicz B., Belagyi J., Dabrowska R. The effect of caldesmon on assembly and dynamic properties of actin. Eur J Biochem. 1989 May 15;181(3):607–614. doi: 10.1111/j.1432-1033.1989.tb14767.x. [DOI] [PubMed] [Google Scholar]
- Gałazkiewicz B., Mossakowska M., Osińska H., Dabrowska R. Polymerization of G-actin by caldesmon. FEBS Lett. 1985 May 6;184(1):144–149. doi: 10.1016/0014-5793(85)80671-2. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Anderson W. B. Ca2+-induced hydrophobic site on calmodulin: application for purification of calmodulin by phenyl-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1982 Jan 29;104(2):830–836. doi: 10.1016/0006-291x(82)90712-4. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Chambers K. A., Hopcia K. L., Kwiatkowski D. J. Association of profilin with filament-free regions of human leukocyte and platelet membranes and reversible membrane binding during platelet activation. J Cell Biol. 1989 Oct;109(4 Pt 1):1571–1579. doi: 10.1083/jcb.109.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Kanda K., Kimizuka F., Kato I., Sobue K. Primary structure and functional expression of h-caldesmon complementary DNA. Biochem Biophys Res Commun. 1989 Oct 16;164(1):503–511. doi: 10.1016/0006-291x(89)91748-8. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Hornick T. Determination of the phosphorylation sites of smooth muscle caldesmon by protein kinase C. Arch Biochem Biophys. 1991 Aug 1;288(2):538–542. doi: 10.1016/0003-9861(91)90232-8. [DOI] [PubMed] [Google Scholar]
- Isenberg G. Actin binding proteins--lipid interactions. J Muscle Res Cell Motil. 1991 Apr;12(2):136–144. doi: 10.1007/BF01774032. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Evidence that the phosphatidylinositol cycle is linked to cell motility. Exp Cell Res. 1988 Jan;174(1):1–15. doi: 10.1016/0014-4827(88)90136-x. [DOI] [PubMed] [Google Scholar]
- Litchfield D. W., Ball E. H. Phosphorylation of caldesmon77 by protein kinase C in vitro and in intact human platelets. J Biol Chem. 1987 Jun 15;262(17):8056–8060. [PubMed] [Google Scholar]
- Morrisett J. D., Jackson R. L., Gotto A. M., Jr Lipoproteins: structure and function. Annu Rev Biochem. 1975;44:183–207. doi: 10.1146/annurev.bi.44.070175.001151. [DOI] [PubMed] [Google Scholar]
- Op den Kamp J. A. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- Pritchard K., Moody C. J. Caldesmon: a calmodulin-binding actin-regulatory protein. Cell Calcium. 1986 Dec;7(5-6):309–327. doi: 10.1016/0143-4160(86)90035-7. [DOI] [PubMed] [Google Scholar]
- Reich J. G., Wangermann G., Falck M., Rohde K. A general strategy for parameter estimation from isosteric and allosteric-kinetic data and binding measurements. Eur J Biochem. 1972 Apr 11;26(3):368–379. doi: 10.1111/j.1432-1033.1972.tb01776.x. [DOI] [PubMed] [Google Scholar]
- Rodaway A. R., Sternberg M. J., Bentley D. L. Similarity in membrane proteins. Nature. 1989 Dec 7;342(6250):624–624. doi: 10.1038/342624a0. [DOI] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Shirinsky V. P., Bushueva T. L., Frolova S. I. Caldesmon-calmodulin interaction. Study by the method of protein intrinsic tryptophan fluorescence. Biochem J. 1988 Oct 1;255(1):203–208. [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Tanaka T., Kanda K., Ashino N., Kakiuchi S. Purification and characterization of caldesmon77: a calmodulin-binding protein that interacts with actin filaments from bovine adrenal medulla. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5025–5029. doi: 10.1073/pnas.82.15.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocanne J. F., Teissié J. Ionization of phospholipids and phospholipid-supported interfacial lateral diffusion of protons in membrane model systems. Biochim Biophys Acta. 1990 Feb 28;1031(1):111–142. doi: 10.1016/0304-4157(90)90005-w. [DOI] [PubMed] [Google Scholar]
- Vorotnikov A. V., Bogatcheva N. V., Gusev N. B. Caldesmon-phospholipid interaction. Effect of protein kinase C phosphorylation and sequence similarity with other phospholipid-binding proteins. Biochem J. 1992 Jun 15;284(Pt 3):911–916. doi: 10.1042/bj2840911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorotnikov A. V., Gusev N. B. Interaction of smooth muscle caldesmon with phospholipids. FEBS Lett. 1990 Dec 17;277(1-2):134–136. doi: 10.1016/0014-5793(90)80827-6. [DOI] [PubMed] [Google Scholar]
- Wang C. L., Wang L. W., Xu S. A., Lu R. C., Saavedra-Alanis V., Bryan J. Localization of the calmodulin- and the actin-binding sites of caldesmon. J Biol Chem. 1991 May 15;266(14):9166–9172. [PubMed] [Google Scholar]



