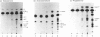Abstract
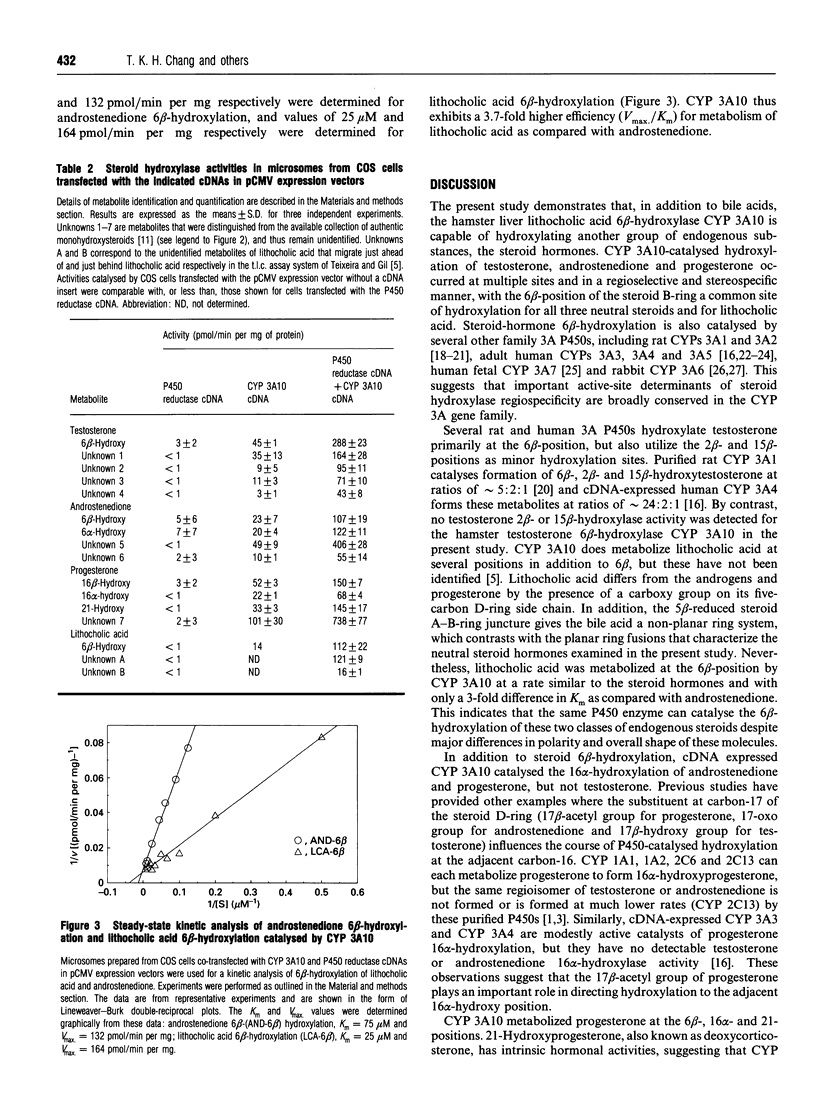
CYP 3A10 is a hamster liver cytochrome P-450 (P450) that encodes lithocholic acid 6 beta-hydroxylase, an enzyme that plays an important role in the detoxification of the cholestatic secondary bile acid lithocholate. Western-blot analysis revealed that the expression of CYP 3A10 protein is male-specific in hamster liver microsomes, a finding that is consistent with earlier analysis of CYP 3A10 mRNA. Since it has not been established whether the specificities of bile acid hydroxylase P450s, such as CYP 3A10, are restricted to their anionic bile acid substrates, we investigated the role of CYP 3A10 in the metabolism of a series of neutral steroid hormones using cDNA directed-expression in COS cells. The steroid hormones examined, testosterone, androstenedione and progesterone, were each metabolized by the expressed CYP 3A10, with 6 beta-hydroxylation corresponding to a major activity in all three instances. CYP 3A10-dependent steroid hydroxylation was increased substantially when the microsomes were prepared from COS cells co-transfected with NADPH:P450 reductase cDNA. In this case, the expressed P450 actively catalysed the 6 beta-hydroxylation of testosterone (288 +/- 23 pmol of product formed/min per mg of COS-cell microsomal protein), androstenedione (107 +/- 19 pmol/min per mg) and progesterone (150 +/- 7 pmol/min per mg). Other major CYP 3A10-mediated steroid hydroxylase activities included androstenedione 16 alpha-hydroxylation, progesterone 16 alpha- and 21-hydroxylation, and the formation of several unidentified products. CYP 3A10 exhibited similar Vmax. values for the 6 beta-hydroxylation of androstenedione and lithocholic acid (132 and 164 pmol/min per mg respectively), but metabolized the bile acid with a 3-fold lower Km (25 microM, as against 75 microM for androstenedione). Together, these studies establish that the substrate specificity of the bile acid hydroxylase CYP 3A10 is not restricted to bile acids, and further suggest that CYP 3A10 can play a physiologically important role in the metabolism of two classes of endogenous P450 substrates:steroid hormones and bile acids.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Bishop R. W., Russell D. W. Expression cloning and regulation of steroid 5 alpha-reductase, an enzyme essential for male sexual differentiation. J Biol Chem. 1989 Sep 25;264(27):16249–16255. [PMC free article] [PubMed] [Google Scholar]
- Aoyama T., Yamano S., Waxman D. J., Lapenson D. P., Meyer U. A., Fischer V., Tyndale R., Inaba T., Kalow W., Gelboin H. V. Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver. cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine. J Biol Chem. 1989 Jun 25;264(18):10388–10395. [PubMed] [Google Scholar]
- Björkhem I., Danielsson H., Wikvall K. Hydroxylations of bile acids by reconstituted systems from rat liver microsomes. J Biol Chem. 1974 Oct 25;249(20):6439–6445. [PubMed] [Google Scholar]
- Brian W. R., Sari M. A., Iwasaki M., Shimada T., Kaminsky L. S., Guengerich F. P. Catalytic activities of human liver cytochrome P-450 IIIA4 expressed in Saccharomyces cerevisiae. Biochemistry. 1990 Dec 25;29(51):11280–11292. doi: 10.1021/bi00503a018. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J. Molecular genetics of the P-450 superfamily. Pharmacol Ther. 1990;45(1):1–38. doi: 10.1016/0163-7258(90)90006-n. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Shimada T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P-450 enzymes. Chem Res Toxicol. 1991 Jul-Aug;4(4):391–407. doi: 10.1021/tx00022a001. [DOI] [PubMed] [Google Scholar]
- Halvorson M., Greenway D., Eberhart D., Fitzgerald K., Parkinson A. Reconstitution of testosterone oxidation by purified rat cytochrome P450p (IIIA1). Arch Biochem Biophys. 1990 Feb 15;277(1):166–180. doi: 10.1016/0003-9861(90)90566-h. [DOI] [PubMed] [Google Scholar]
- Imaoka S., Terano Y., Funae Y. Constitutive testosterone 6 beta-hydroxylase in rat liver. J Biochem. 1988 Sep;104(3):481–487. doi: 10.1093/oxfordjournals.jbchem.a122494. [DOI] [PubMed] [Google Scholar]
- Kitada M., Kamataki T., Itahashi K., Rikihisa T., Kanakubo Y. Significance of cytochrome P-450 (P-450 HFLa) of human fetal livers in the steroid and drug oxidations. Biochem Pharmacol. 1987 Feb 15;36(4):453–456. doi: 10.1016/0006-2952(87)90350-9. [DOI] [PubMed] [Google Scholar]
- Kronbach T., Larabee T. M., Johnson E. F. Hybrid cytochromes P-450 identify a substrate binding domain in P-450IIC5 and P-450IIC4. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8262–8265. doi: 10.1073/pnas.86.21.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc G. A., Waxman D. J. Interaction of anticancer drugs with hepatic monooxygenase enzymes. Drug Metab Rev. 1989;20(2-4):395–439. doi: 10.3109/03602538909103550. [DOI] [PubMed] [Google Scholar]
- Miyal K., Mayr W. W., Richardson A. L. Acute cholestasis induced by lithocholic acid in the rat. A freeze-fracture replica and thin section study. Lab Invest. 1975 Apr;32(4):527–535. [PubMed] [Google Scholar]
- Morgan E. T., Coon M. J. Effects of cytochrome b5 on cytochrome P-450-catalyzed reactions. Studies with manganese-substituted cytochrome b5. Drug Metab Dispos. 1984 May-Jun;12(3):358–364. [PubMed] [Google Scholar]
- Nagata K., Gonzalez F. J., Yamazoe Y., Kato R. Purification and characterization of four catalytically active testosterone 6 beta-hydroxylase P-450s from rat liver microsomes: comparison of a novel form with three structurally and functionally related forms. J Biochem. 1990 May;107(5):718–725. doi: 10.1093/oxfordjournals.jbchem.a123115. [DOI] [PubMed] [Google Scholar]
- Porter T. D., Coon M. J. Cytochrome P-450. Multiplicity of isoforms, substrates, and catalytic and regulatory mechanisms. J Biol Chem. 1991 Jul 25;266(21):13469–13472. [PubMed] [Google Scholar]
- Potenza C. L., Pendurthi U. R., Strom D. K., Tukey R. H., Griffin K. J., Schwab G. E., Johnson E. F. Regulation of the rabbit cytochrome P-450 3c gene. Age-dependent expression and transcriptional activation by rifampicin. J Biol Chem. 1989 Sep 25;264(27):16222–16228. [PubMed] [Google Scholar]
- Ryan D. E., Levin W. Purification and characterization of hepatic microsomal cytochrome P-450. Pharmacol Ther. 1990;45(2):153–239. doi: 10.1016/0163-7258(90)90029-2. [DOI] [PubMed] [Google Scholar]
- Sundseth S. S., Waxman D. J. Sex-dependent expression and clofibrate inducibility of cytochrome P450 4A fatty acid omega-hydroxylases. Male specificity of liver and kidney CYP4A2 mRNA and tissue-specific regulation by growth hormone and testosterone. J Biol Chem. 1992 Feb 25;267(6):3915–3921. [PubMed] [Google Scholar]
- Swinney D. C. Progesterone metabolism in hepatic microsomes. Effect of the cytochrome P-450 inhibitor, ketoconazole, and the NADPH 5 alpha-reductase inhibitor, 4-MA, upon the metabolic profile in human, monkey, dog, and rat. Drug Metab Dispos. 1990 Nov-Dec;18(6):859–865. [PubMed] [Google Scholar]
- Swinney D. C., Ryan D. E., Thomas P. E., Levin W. Regioselective progesterone hydroxylation catalyzed by eleven rat hepatic cytochrome P-450 isozymes. Biochemistry. 1987 Nov 3;26(22):7073–7083. doi: 10.1021/bi00396a032. [DOI] [PubMed] [Google Scholar]
- Teixeira J., Gil G. Cloning, expression, and regulation of lithocholic acid 6 beta-hydroxylase. J Biol Chem. 1991 Nov 5;266(31):21030–21036. [PubMed] [Google Scholar]
- Trant J. M., Lorence M. C., Johnson E. F., Shackleton C. H., Mason J. I., Estabrook R. W. Characterization of the steroid-metabolizing capacity of the hepatic cytochrome P450IIC5 expressed in COS-1 cells: 3 beta-hydroxysteroid dehydrogenase/delta 5----4 isomerase type activity. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9756–9760. doi: 10.1073/pnas.87.24.9756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Attisano C., Guengerich F. P., Lapenson D. P. Human liver microsomal steroid metabolism: identification of the major microsomal steroid hormone 6 beta-hydroxylase cytochrome P-450 enzyme. Arch Biochem Biophys. 1988 Jun;263(2):424–436. doi: 10.1016/0003-9861(88)90655-8. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Dannan G. A., Guengerich F. P. Regulation of rat hepatic cytochrome P-450: age-dependent expression, hormonal imprinting, and xenobiotic inducibility of sex-specific isoenzymes. Biochemistry. 1985 Jul 30;24(16):4409–4417. doi: 10.1021/bi00337a023. [DOI] [PubMed] [Google Scholar]
- Waxman D. J. Interactions of hepatic cytochromes P-450 with steroid hormones. Regioselectivity and stereospecificity of steroid metabolism and hormonal regulation of rat P-450 enzyme expression. Biochem Pharmacol. 1988 Jan 1;37(1):71–84. doi: 10.1016/0006-2952(88)90756-3. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Ko A., Walsh C. Regioselectivity and stereoselectivity of androgen hydroxylations catalyzed by cytochrome P-450 isozymes purified from phenobarbital-induced rat liver. J Biol Chem. 1983 Oct 10;258(19):11937–11947. [PubMed] [Google Scholar]
- Waxman D. J., Lapenson D. P., Aoyama T., Gelboin H. V., Gonzalez F. J., Korzekwa K. Steroid hormone hydroxylase specificities of eleven cDNA-expressed human cytochrome P450s. Arch Biochem Biophys. 1991 Oct;290(1):160–166. doi: 10.1016/0003-9861(91)90602-f. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Lapenson D. P., Nagata K., Conlon H. D. Participation of two structurally related enzymes in rat hepatic microsomal androstenedione 7 alpha-hydroxylation. Biochem J. 1990 Jan 1;265(1):187–194. doi: 10.1042/bj2650187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., LeBlanc G. A., Morrissey J. J., Staunton J., Lapenson D. P. Adult male-specific and neonatally programmed rat hepatic P-450 forms RLM2 and 2a are not dependent on pulsatile plasma growth hormone for expression. J Biol Chem. 1988 Aug 15;263(23):11396–11406. [PubMed] [Google Scholar]
- Waxman D. J. P450-catalyzed steroid hydroxylation: assay and product identification by thin-layer chromatography. Methods Enzymol. 1991;206:462–476. doi: 10.1016/0076-6879(91)06115-j. [DOI] [PubMed] [Google Scholar]
- Waxman D. J. Rat hepatic P450IIA and P450IIC subfamily expression using catalytic, immunochemical, and molecular probes. Methods Enzymol. 1991;206:249–267. doi: 10.1016/0076-6879(91)06095-k. [DOI] [PubMed] [Google Scholar]
- Yeowell H. N., Waxman D. J., LeBlanc G. A., Linko P., Goldstein J. A. Suppression of male-specific cytochrome P450 2c and its mRNA by 3,4,5,3',4',5'-hexachlorobiphenyl in rat liver is not causally related to changes in serum testosterone. Arch Biochem Biophys. 1989 Jun;271(2):508–514. doi: 10.1016/0003-9861(89)90302-0. [DOI] [PubMed] [Google Scholar]
- Yousef I. M., Kakis G., Fisher M. M. Bile acid metabolism in mammals. 3. Sex difference in the bile acid composition of rat bile. Can J Biochem. 1972 Apr;50(4):402–408. doi: 10.1139/o72-054. [DOI] [PubMed] [Google Scholar]
- Zaphiropoulos P. G., Mode A., Norstedt G., Gustafsson J. A. Regulation of sexual differentiation in drug and steroid metabolism. Trends Pharmacol Sci. 1989 Apr;10(4):149–153. doi: 10.1016/0165-6147(89)90167-3. [DOI] [PubMed] [Google Scholar]
- Zimniak P., Holsztynska E. J., Lester R., Waxman D. J., Radominska A. Detoxification of lithocholic acid. Elucidation of the pathways of oxidative metabolism in rat liver microsomes. J Lipid Res. 1989 Jun;30(6):907–918. [PubMed] [Google Scholar]
- Zimniak P., Holsztynska E. J., Radominska A., Iscan M., Lester R., Waxman D. J. Distinct forms of cytochrome P-450 are responsible for 6 beta-hydroxylation of bile acids and of neutral steroids. Biochem J. 1991 Apr 1;275(Pt 1):105–111. doi: 10.1042/bj2750105. [DOI] [PMC free article] [PubMed] [Google Scholar]