Abstract
Background:
In 2002 CDC initiated the Anthrax Vaccination Program (AVP) to provide voluntary pre-exposure vaccination with Anthrax Vaccine Adsorbed (AVA) for persons at high risk of exposure to B. anthracis spores. There has been concern that AVA could be associated with long term impairment of mental and/or physical health.
Objectives:
To ascertain whether physical and mental functional status, as measured by the SF-36v2 health survey (Medical Outcomes Trust, Boston, MA), of AVA recipients and controls changed differently over time.
Methods:
We enrolled 437 exposed (received AVA) and 139 control subjects. The exposed group received AVA under then-current Advisory Committee on Immunization Practices (ACIP) recommendations of 0.5 mL doses given SQ at 0, 2, and 4 weeks, and 6, 12, and 18 months, followed by annual boosters. SF-36v2 surveys were completed just before injection at 0, 12, and 30 months. The subjects’ answers to the survey questions were summarized into a physical and a mental score at each of the three time points. SF-36v2 physical and mental scores both range from 0-100 with an estimated national average of 50 points. We assessed change in physical and mental score from baseline at 12 and 30 months, and examined whether there was a difference between the exposed and control subjects; positive changes in the scores indicated improving, and negative changes worsening, physical or mental function.
Results:
At baseline, average physical scores were 55.4 among exposed and 54.5 among controls (p=0.07); mental scores were 55.0 among exposed and 51.4 among controls (p<0.0001). For physical scores, the average change from baseline was −0.53 for exposed vs. −0.67 for controls at 12 months (p=0.80) and −1.09 for exposed vs. −1.97 for controls at 30 months (p=0.23). For mental scores, the average change from baseline was −1.50 for exposed vs. −1.64 for controls at 12 months (p=0.86) and −2.11 for exposed vs. −0.24 for controls at 30 months (p=0.06). When adjusting for demographic and employment factors in multivariate linear models, the difference in mental score change between exposed vs. controls at 30 months was less pronounced (p=0.37 than it had been in univariate analyses, but other findings were similar to univariate analyses.
Conclusions:
We found no evidence that change in physical scores differed between the exposed and control groups at 12 or 30 months. Although the unadjusted change in mental score in exposed vs. control approached statistical significance at 30 months, the magnitude of this difference was small and a significant difference was not found in the the multivariable analysis. These results do not favor an association between receipt of AVA and an altered health related quality of life over a 30-month period.
Keywords: Health Related Quality of Life, Health Survey, AVA, AVP
INTRODUCTION
Anthrax is a zoonotic disease caused by the bacterium Bacillus anthracis. Historically, the route of transmission to humans was from soil to grazing livestock to persons working with livestock carcasses or live infected animals, such as tanners, wool workers, and veterinarians 1. The potential for B. anthracis to be used as a biological terrorism agent and the attacks that occurred in the U.S. in 2001 have led to vaccination programs. Currently, the Advisory Committee on Immunization Practices (ACIP) considers persons at occupational risk of exposure to include laboratory and environmental remediation workers who might handle specimens for research or in support of investigations.
Anthrax Vaccine Adsorbed (AVA, BioThrax®, Emergent BioSolutions, Lansing, MI 2) is the only licensed anthrax vaccine in the U.S. When licensed in 1970, doses were to be given subcutaneously at 0, 2, and 4 weeks, and 6, 12, and 18 months, followed by annual boosters. An AVA clinical trial 3,4 conducted during 2002-2005 showed that intramuscular injections had non-inferior immunological priming compared to subcutaneous injections and had significantly fewer adverse events following injection. The interim report from this trial also showed non-inferiority up to month 7 between the full dosing schedule and exclusion of the week 2 dose.
To date, research suggests that AVA has an acceptable safety profile. In 2002 an Institute of Medicine (IOM) committee concluded that AVA is reasonably safe; while the committee found no convincing evidence that AVA recipients face elevated risks of developing long-term sequelae, they also stated that the data were limited in this regard, and recommended monitoring for later-onset health conditions 4. However, concerns have been expressed regarding adverse effects of AVA, sometimes related to long-term and non-specific symptoms such as Gulf War and chronic fatigue syndromes 5,6, but also with potential high rates of local adverse events 1,7, and reproductive toxicity 8,9.
In response to the potential of anthrax to be used as a bioterrorism agent, in 1998 the Department of Defense (DoD) initiated a program to administer mandatory vaccinations to U.S. military personnel with AVA. Although temporarily halted by a court injunction, this program was restarted initially under an Emergency Use Authorization as a voluntary program in 2004 and since early 2007 again as a mandatory program.
In 1999, the Laboratory Response Network (LRN) was established to provide laboratory testing support for detecting possible biological and chemical weapons attacks 10,11. The LRN tested more than 125,000 samples during the investigation ensuing from the 2001 anthrax attacks in the U.S 10. Following these attacks, in 2002, the ACIP revised their recommendations to include pre-exposure vaccinations with AVA of certain LRN workers and environmental remediation personnel who repeatedly enter contaminated sites 12.
In 2002, the CDC initiated the Anthrax Vaccination Program (AVP) to provide voluntary pre-exposure AVA to persons included in the ACIP’s recommendations 13. At its onset, the AVP provided an opportunity to follow AVA exposed and unexposed individuals working in the same laboratories over a prolonged period of time. However, due to the above-mentioned court injunction primarily aimed at the military AVA vaccination program, the AVP was halted in May 2005 and was not permitted to restart. The currently-reported study is an evaluation of persons involved in the AVP. Our objective was to determine whether physical and mental functional status, as measured by the SF-36v2 health survey (Medical Outcomes Trust, Boston, MA), of AVA recipients and controls changed differently over time.
METHODS
This study was performed among LRN workers. The vaccine exposed group included those who were enrolled in the AVP, and the control group included LRN workers from the same laboratories in which the exposed subjects worked but who were ineligible for the AVP. Each laboratory’s manager submitted lists of all employees potentially eligible to receive AVA; these lists were then screened by staff at the CDC’s Bioterrorism Preparedness and Response Program to confirm eligibility according to the ACIP recommendations. Eligible participants included LRN staff handling environmental specimens (especially powders) and performing confirmatory testing for anthrax, and environmental clean-up teams working at multiple contaminated sites in succession. The unvaccinated cohort consisted of laboratory personnel working in similar occupational settings as the participants enrolled in the AVP. AVP eligibility requirements are described further elsewhere 13.
At the time of enrollment, each subject was provided with study educational materials, signed an informed consent form, and completed a brief demographic questionnaire. The AVA-exposed group was administered AVA under then-current ACIP recommendations of 0.5 mL doses given subcutaneously at 0, 2, and 4 weeks, and 6, 12, and 18 months, followed by annual boosters. Since publication of the report of the interim analysis of the CDC AVA human clinical trial 3, the current FDA licensed schedule for AVA excludes the original 2-week dose, and injections are administered intramuscularly 14,15.
This was an observational, prospective study in which subjects completed the SF-36v2 health survey (described below) at 0, 12, and 30 months after enrollment. Surveys were filled out by exposed and control subjects just before injections were given to AVP participants at the time of the first, 12-month, and first annual booster doses, which correspond to the 0-, 12-, and 30-month time points, respectively; a study nurse collected the completed surveys on site at the laboratories. Due to the AVP’s termination before our study’s planned 30-month follow up, the vaccination clinics were no longer available to coordinate administration of the surveys. We thus mailed the 30-month survey and a prepaid envelope to all study participants from October 2006 through March 2007.
The SF-36v2 survey is a validated instrument for measuring an individual’s self reported functional status from the patient’s point of view 16. This survey is comprised of 36 questions which yield an eight-scale profile of scores 17. These scales can be combined into two summary measures of mental and physical health, called physical and mental component summary. Physical and mental component scores can range from 0 to 100, with larger values corresponding to more favorable status, and are estimated to have an overall mean of 50 and standard deviation of 10 points in the 1998 U.S. population 17.
Data on demographic and employment variables were obtained at baseline via a questionnaire, while height and weight were measured by a nurse. For analysis, each participant’s age at baseline was categorized as <30, 30-39, 40-49, and ≥50 years of age, although some ages were unknown. Race was categorized as black, other, white, and unknown. Body Mass Index (BMI) was computed using body weight and height at enrollment and categorized using CDC cutoffs: underweight (<18.5), normal (18.5-24.9), overweight (25-29.9), and obese (≥30). Also available were sex, educational attainment (college degree, post-graduate college degree), and smoking status (self-reported at enrollment as smoker or non-smoker). Given that there could be differences among the participants completing the mailed 30-month survey and those who did not, we also included the number of subsequent surveys completed (1 or 2) as an analysis factor.
We first summarized baseline demographic, employment, and SF-36v2 score data on exposed vs. control subjects. We performed a Chi-square test to assess whether the distribution of the study participants was different between the exposed and control groups across each factor’s levels using OpenEpi, Version 2, open source calculator (http://www.openepi.com/OE2.3/RbyC/RbyC.htm); “Unknown” responses were excluded. The difference in physical and mental scores at baseline between the exposed vs. control groups was assessed with t-tests. For statistical testing of the vaccine effect on physical and mental scores, we subtracted each subject’s baseline score from subsequent scores to produce the physical score difference and mental score difference. We evaluated each analysis factor using univariable linear models wherein the response variable was difference in physical or mental score at 12 or 30 months from baseline score, and one factor was included as the independent variable. We also used multivariable linear regression having the same response variable but with all factors included as main effects. We performed this analysis both by leaving in and omitting “Unknown” levels of factors from the univariable and multivariable models; similar results were found, and only the latter are reported.
Significance was assessed using a Type-I error rate of 0.05; no adjustments were made for multiple comparisons. SAS® version 9.2 (SAS Institute, Inc., Cary, NC) was used for the regression analyses. We used the R statistical computing software 18 to produce figures, including the beanplot package for the empirical distribution of scores.
RESULTS
We analyzed SF-36v2 survey results of 576 individuals from 59 LRN laboratories in 35 states, including 437 vaccine exposed and 139 control subjects. All participants completed the survey at baseline, but due largely to the unplanned halt in the AVP, only 547 completed the survey at 12 months (411 exposed, 136 controls) and 344 participants at 30 months (250 exposed, 94 controls).
At baseline, differences in demographic characteristics between the exposed and control groups included a higher percentage of white subjects in the exposed (82%) than control group (71%) (p=0.01 for heterogeneity of race), a lower percentage of females in the exposed (55%) than control (71%) group (p<0.001), and a lower percentage of subjects in the normal BMI class in the exposed (39%) than the control group (55%) (p=0.0015 for heterogeneity among BMI groups) (Table 1). The two groups also differed significantly in occupational and educational groups.
Table 1.
Number (%) of Subjects by Characteristic at Baseline, Anthrax Vaccination Program, CDC, 2002 – 2005.
| Vaccinated | Controls | |||||
|---|---|---|---|---|---|---|
| Characteristic | Level | n = 437 | n = 139 | p-value* | ||
| Age group (years) | <30 | 66 | (15) | 27 | (19) | 0.21 |
| 30-39 | 99 | (23) | 24 | (17) | ||
| 40-49 | 153 | (35) | 39 | (28) | ||
| ≥50 | 105 | (24) | 38 | (27) | ||
| Unknown | 14 | (3) | 11 | (8) | ||
|
| ||||||
| Race | Black | 31 | (7) | 16 | (12) | 0.01 |
| Other | 44 | (10) | 25 | (18) | ||
| White | 357 | (82) | 98 | (71) | ||
| Unknown | 5 | (1) | 0 | (0) | ||
|
| ||||||
| Sex | Female | 242 | (55) | 99 | (71) | <0.005 |
| Male | 195 | (45) | 40 | (29) | ||
|
| ||||||
| Occupation group | Laboratory management | 133 | (30) | 24 | (17) | <0.005 |
| Laboratory technician | 210 | (48) | 70 | (50) | ||
| Other | 94 | (22) | 45 | (32) | ||
|
| ||||||
| Education group | College degree | 247 | (57) | 97 | (70) | 0.01 |
| Post-graduate degree | 180 | (41) | 42 | (30) | ||
| Unknown | 10 | (2) | 0 | (0) | ||
|
| ||||||
| Smoking status | Non smoker | 286 | (65) | 93 | (67) | 0.27 |
| Smoker | 138 | (32) | 35 | (25) | ||
| Unknown | 13 | (3) | 11 | (8) | ||
|
| ||||||
| BMI group | Underweight | 5 | (1) | 4 | (3) | <0.005 |
| Normal | 172 | (39) | 77 | (55) | ||
| Overweight | 176 | (40) | 35 | (25) | ||
| Obese | 83 | (19) | 22 | (16) | ||
| Unknown | 1 | (0) | 1 | (1) | ||
|
| ||||||
| Number of subsequent survey forms completed | 1 | 213 | (49) | 48 | (35) | <0.005 |
| 2 | 224 | (51) | 91 | (65) | ||
|
| ||||||
| ≥1 characteristic was “Unknown”? | Yes | 27 | (6) | 23 | (17) | <0.005 |
| No | 410 | (94) | 116 | (83) | ||
Chi-square test that the distribution of the study participants was different between the exposed and control groups, excluding “Unknown” categories.
At baseline, the mean physical component score was 54.5 for the control group and 55.4 for the exposed group (p=0.07); mean scores changed little at 12 and 30 months (Figure 1a). The mean baseline mental scores were 51.4 among the controls and 55.0 among exposed (p<0.0001) and again changed little at 12 and 30 months. These scores were negatively skewed (i.e., had an asymmetric distribution with long tail towards smaller scores) with modes above the reported national average of 50 (Figure 2).
Figure 1.
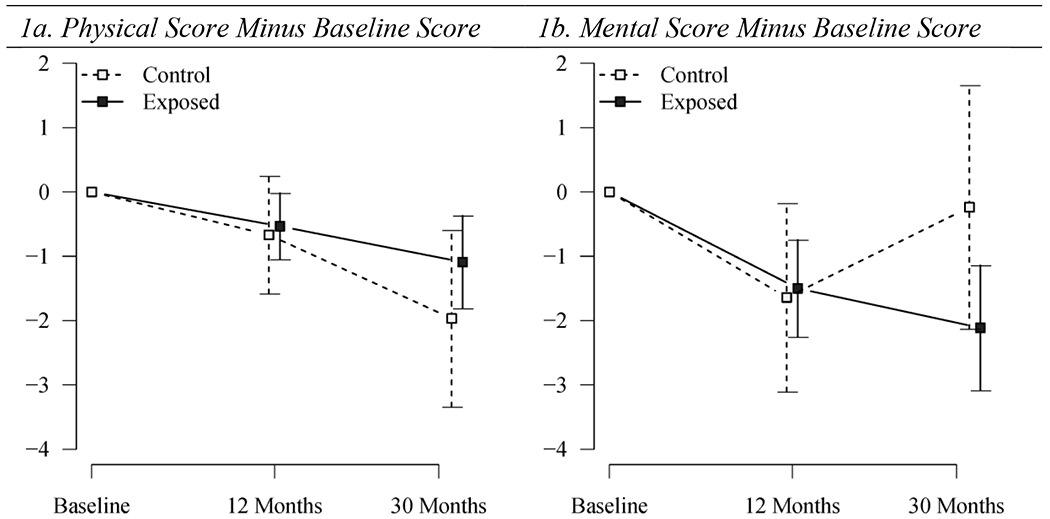
Arithmetic averages and 95% confidence intervals from one-sample t-test of difference in subsequent physical or mental scores from baseline by time-point and exposure group, Anthrax Vaccination Program, CDC, 2002 – 2005.
Figure 2.
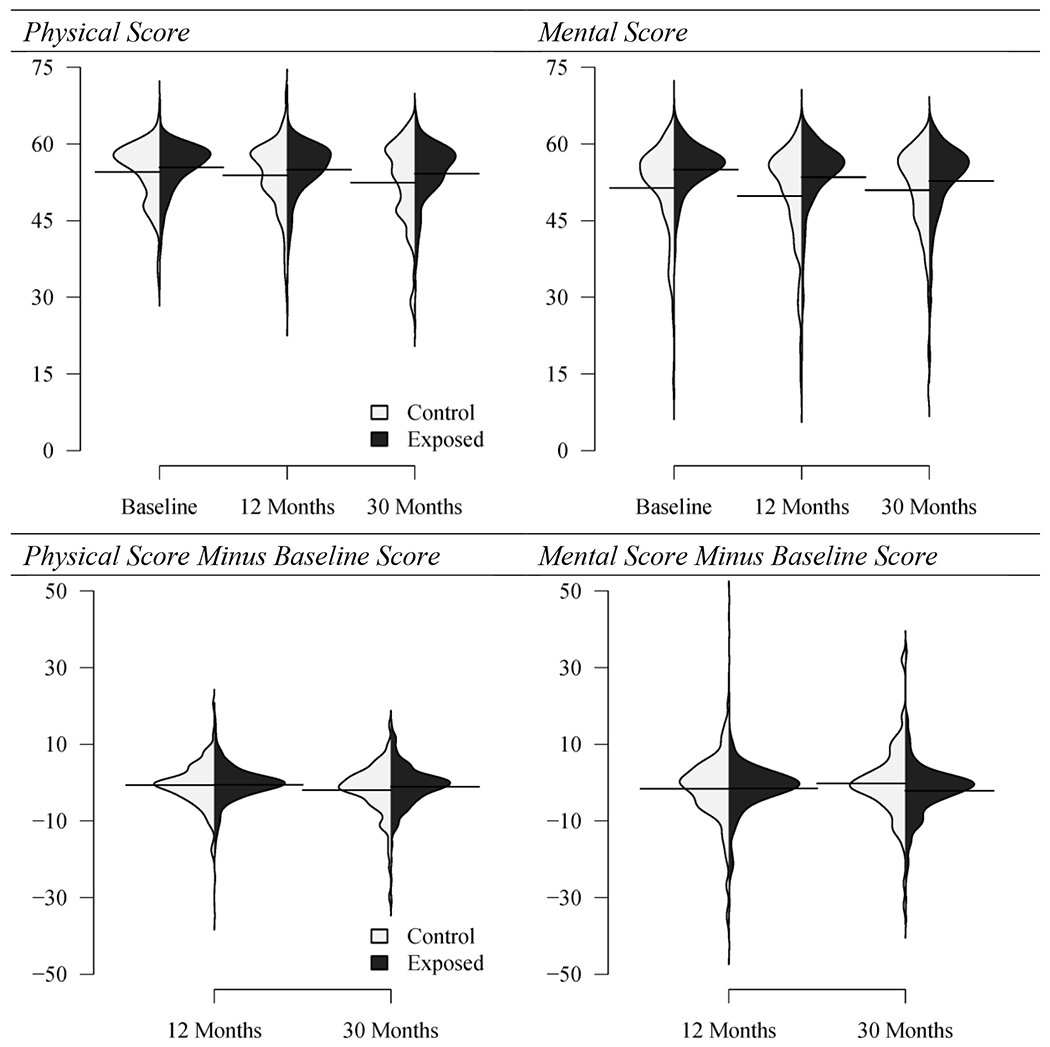
Distribution of physical and mental component scores (top), and of subsequent score minus baseline (bottom; these are smoothed histograms rotated to a vertical orientation – numbers on vertical axes correspond to survey scores and survey score difference from baseline. Solid horizontal lines: arithmetic average of the values in the respective histograms. Anthrax Vaccination Program, CDC, 2002 – 2005.
Next, to analyze change from baseline, we subtracted each baseline score from subsequent scores to produce the physical and mental score differences from baseline. Positive scores for physical and mental score differences indicate that physical or mental function improved over time, whereas negative values indicate that function is worsened. Subtraction of the baseline scores acted as a normalizing factor: the distribution of the physical and mental score differences from baseline are more bell-shaped than the crude physical and mental scores (Figure 2). At 12 months, the physical score difference from baseline was −0.53 for exposed vs. −0.67 for controls (p=0.8), and at 30 months the physical score difference from baseline was −1.09 for exposed vs. −1.97 for controls (p=0.2) (Figure 1b). At 12 months, the mental score difference from baseline was −1.50 for exposed vs. −1.64 for controls (p=0.9), and at 30 months the mental score difference from baseline was −2.11 for exposed vs. −0.24 for controls (p=0.06).
The multivariable model controlling for the available factors confirmed that vaccination had no significant effect on the physical score difference from baseline at 12 months (p=0.97) or at 30 months (p=0.26) (Table 2). Among the factors analyzed, only BMI class significantly influenced physical score difference: underweight individuals demonstrated a significant decline in physical score compared to obese individuals (p=0.03), but this comparison was based on a small number of underweight participants; since weight was not collected over time, but rather only at baseline, any impact in changes in BMI over time on physical or mental scores could not be assessed. Similarly, the multivariable model of mental score difference from baseline showed no significant effect of vaccination at 12 months (p=0.47), and less evidence of statistical significance at 30 months than the univariable model (p=0.37) (Table 3). Among factors analyzed, mental score difference at 30 months in younger individuals decreased significantly more than the mental score in persons ≥50 years of age. A significant difference was again identified between obese and underweight individuals based on a small number of underweight participants.
Table 2.
Linear models of Differences from Baseline of Physical Summary Scores, Anthrax Vaccination Program, CDC, 2002 -- 2005.†
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Factor (reference) | 12 Months | 30 Months | 12 Months | 30 Months | ||||
| Exposure (Control) | ||||||||
| Vaccinated | 0.1 | (0.80) | 0.9 | (0.23) | 0.0 | (0.97) | 1.0 | (0.26) |
| Age group (≥50) | ||||||||
| <30 | −1.0 | (0.16) | −0.4 | (0.70) | −1.5 | (0.07) | −0.6 | (0.61) |
| 30-39 | −0.7 | (0.30) | −0.4 | (0.71) | −0.7 | (0.35) | −0.4 | (0.71) |
| 40-49 | −0.5 | (0.43) | −0.3 | (0.74) | −0.5 | (0.47) | −0.5 | (0.59) |
| Race (White) | ||||||||
| Black | −0.8 | (0.35) | 1.1 | (0.37) | −0.8 | (0.35) | 1.0 | (0.42) |
| Other | 0.7 | (0.36) | 0.5 | (0.63) | 0.6 | (0.48) | 0.2 | (0.86) |
| Sex (Male) | ||||||||
| Female | −0.4 | (0.37) | −0.8 | (0.26) | −0.5 | (0.37) | −0.5 | (0.54) |
| Occupation group (Other) | ||||||||
| Laboratory management | 0.6 | (0.37) | 0.2 | (0.87) | −0.1 | (0.89) | −0.5 | (0.60) |
| Laboratory technician | 0.7 | (0.25) | 0.8 | (0.34) | 0.4 | (0.56) | 0.8 | (0.38) |
| Education (College degree) | ||||||||
| Post-graduate degree | −0.2 | (0.73) | −0.3 | (0.62) | −0.2 | (0.74) | 0.0 | (0.97) |
| Smoking status (Smoker) | ||||||||
| Non smoker | 0.2 | (0.72) | −0.1 | (0.93) | 0.3 | (0.64) | −0.3 | (0.70) |
| BMI group (Obese) | ||||||||
| Underweight* | −0.9 | (0.62) | −6.7 | (<0.005) | 1.3 | (0.54) | −6.5 | (0.03) |
| Normal | 0.4 | (0.52) | 1.6 | (0.07) | 0.8 | (0.25) | 1.5 | (0.13) |
| Overweight | −0.1 | (0.83) | 0.8 | (0.40) | 0.0 | (0.97) | 0.5 | (0.63) |
| Surveys after baseline (2) | ||||||||
| 1 | 0.5 | (0.33) | −0.3 | (0.77) | 0.6 | (0.26) | −0.6 | (0.61) |
There were 9 Underweight participants at baseline and 12 months, and 7 at 30 months.
Values represent coefficients (p-values) from the linear models; estimates can be interpreted as mean changes from baseline in the physical component score.
Table 3.
Linear models of Differences from Baseline of Mental Summary Scores, Anthrax Vaccination Program, CDC, 2002 -- 2005.†
| Univariable | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Factor (reference) | 12 Months | 30 Months | 12 Months | 30 Months | ||||
| Exposure (Control) | ||||||||
| Vaccinated | 0.1 | (0.86) | −1.9 | (0.06) | 0.7 | (0.47) | −1.0 | (0.37) |
| Age group (≥50) | ||||||||
| <30 | 0.5 | (0.66) | −2.7 | (0.08) | 0.4 | (0.77) | −4.1 | (0.02) |
| 30-39 | 0.2 | (0.81) | −2.2 | (0.10) | 0.0 | (1.00) | −3.3 | (0.02) |
| 40-49 | −0.6 | (0.52) | −0.5 | (0.66) | −0.9 | (0.38) | −0.8 | (0.53) |
| Race (White) | ||||||||
| Black | 2.1 | (0.09) | 1.6 | (0.33) | 2.5 | (0.07) | 1.5 | (0.42) |
| Other | −1.3 | (0.25) | −0.6 | (0.65) | −1.6 | (0.19) | −0.1 | (0.94) |
| Sex (Male) | ||||||||
| Female | 0.0 | (0.98) | 0.7 | (0.44) | 0.0 | (0.97) | −0.1 | (0.95) |
| Occupation group (Other) | ||||||||
| Laboratory management | −0.1 | (0.90) | −1.1 | (0.37) | −0.3 | (0.81) | −2.0 | (0.16) |
| Laboratory technician | −0.1 | (0.90) | −0.6 | (0.56) | 0.0 | (0.99) | −0.8 | (0.54) |
| Education (College degree) | ||||||||
| Post-graduate degree | 0.3 | (0.66) | −0.2 | (0.84) | 0.5 | (0.52) | 0.4 | (0.73) |
| Smoking status (Smoker) | ||||||||
| Non smoker | 0.3 | (0.74) | 0.4 | (0.73) | 0.2 | (0.85) | 0.9 | (0.41) |
| BMI group (Obese) | ||||||||
| Underweight* | 3.2 | (0.25) | 8.1 | (0.01) | 3.7 | (0.26) | 11.1 | (0.01) |
| Normal | 0.4 | (0.70) | −1.3 | (0.28) | 0.1 | (0.91) | −1.2 | (0.40) |
| Overweight | 0.6 | (0.57) | −1.7 | (0.17) | 0.3 | (0.77) | −1.5 | (0.26) |
| Surveys after baseline (2) | ||||||||
| 1 | 0.0 | (0.99) | 0.4 | (0.80) | −0.2 | (0.76) | 1.6 | (0.34) |
There were 9 Underweight participants at baseline and 12 months, and 7 at 30 months.
Values represent coefficients (p-values) from the linear models; estimates can be interpreted as mean changes from baseline in the mental component score.
DISCUSSION
After 30 months of follow up, we found that AVA vaccine had no sustained impact on physical or mental functional status. Although results from the unadjusted linear regression model comparing mental score difference from baseline between the exposed vs. the control groups approached statistical significance (p=0.06), the magnitude of the difference between the groups was small, and this comparison was clearly non-significant (p=0.37) when adjusting for available factors in the multivariable model.
To our knowledge, this is the first published report of a completed study that incorporated repeated measurements with the SF-36v2 instrument to evaluate the long-term impact of anthrax vaccine exposure on functional impairment. The SF-36v2 survey is a widely employed clinical research tool for evaluating the possible impacts of a variety of different diseases or medical treatments on individuals’ self reported functional status 19–21. Researchers are collecting repeated measurements using the Veterans SF-36 self-reports as one of the measures incorporated in the ongoing Millennium Cohort Study, which is a large prospective cohort study exploring associations between military exposures (including AVA exposure) and important health outcomes including short-term and long term functional capacity and quality of life 22,23. Using the SF-36v2 survey provides a more global evaluation compared to traditional vaccine safety studies (e.g., human clinical trials employing subject diary cards) of the potential impact of a vaccine on a recipient’s health.
Our data showed that our study subjects had higher physical and mental component scores at baseline than the national averages, which are 50 for both scores. Also, mean baseline scores differed between the exposed and control subjects; in order to control for this difference, we analyzed change from baseline score for each participant. We hypothesized that a period of follow up <12 months might be insufficient to detect a significant impact on the physical and mental component scores from baseline. Therefore, we used measurements obtained one year and 30 months after baseline in our analysis, which in the licensed schedule for AVA at the time of this study corresponded to an individual receiving vaccine doses 5 and 7, respectively.
There are several limitations which may influence the interpretation of our results. It is unknown whether our study subjects were representative of all U.S. workers who are eligible for AVA. Although our unvaccinated controls worked in the same laboratories as the exposed subjects, they were also clearly different from the AVA vaccinated subjects since they were considered not to be at high risk for occupational exposure to B. anthracis and the effect of this difference on our analysis is unknown. BMI and smoking status on our subjects were obtained once at the time of enrollment and possible fluctuations in these covariates during follow up were not measured. Also, the effect of the termination of the AVP and to what extent the survey responses might differ when administered on-site versus through mail were factors not specifically measured, although our results do not show a significant difference between those completing all surveys or just the first two before the program was halted; while this cannot indicate whether or not participants responded differently when the survey was administered on-site versus through the mail, it does support the idea that the subjects choosing to participate after the unplanned halt are representative of the larger study population. As with any survey, there is a certain level of subjectivity embedded in the subjects’ responses. There were large changes in some individuals’ scores, as evidenced by the long tails in the distributions in the bottom of Figure 1. Unfortunately we did not have medical records available which might explain whether a medical illness or injury coincided with a large decrease in survey score in some participants.
The results of our analysis do not favor an association between physical or mental component scores and receipt of AVA over a 30-month timeframe and revealed no clear trends between the two exposure groups. The negative findings presented here are important to the understanding of the safety profile of AVA, and support its safe use for the period we studied. Our analysis identified only minor differences in physical or mental component scores between the levels of the available demographic factors. Most notably, younger participants had a greater decrease in mental score (i.e., worsening mental status) than older participants at 30 months. Since this difference was found in only mental score and only at one time point, it may have been due to chance. Unfortunately, the observational nature of this study is not well suited to rigorously examine these types of associations. While these results provide some level of reassurance related to long-term effects of AVA, studies involving experimental controls, randomization, and a longer timeframe would be useful additions to our findings.
ACKNOWLEDGEMENTS
The authors would like to thank the many volunteers who made this study possible. Also, we thank Lanaya Peterson (Logistics Health Inc.) for her assistance with the study survey and managing the database.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Mention of a product or company name does not constitute endorsement by the CDC. The protocol for this study was approved by an Institutional Review Board of the CDC. None of the authors have conflicts of interest to declare.
REFERENCES
- 1.Centers for Disease Control and Prevention. Use of anthrax vaccine in the United States: recommendations of the Advisory Committee on Immunization Practices (2009). MMWR 2010; 59(RR-6):1–34. [PubMed] [Google Scholar]
- 2.Food and Drug Administration. Biothrax. Available at http://www.fda.gov/Biologics
- 3.Marano N, Plikaytis BD, Martin SW, et al. Effects of a reduced dose schedule and intramuscular administration of anthrax vaccine adsorbed on immunogenicity and safety at 7 months: a randomized trial. JAMA 2008; 300(13):1532–1543. [DOI] [PubMed] [Google Scholar]
- 4.Committee to Assess the Safety and Efficacy of the Anthrax Vaccine, Medical Follow-up Agency. The Anthrax Vaccine: Is It Safe? Does It Work? Washington, DC: Institute of Medicine, National Academy Press; 2002. [Google Scholar]
- 5.Asa PB, Wilson RB, Garry RF. Antibodies to squalene in recipients of anthrax vaccine. Exp Mol Pathol 2002; 73(1):19–27. [DOI] [PubMed] [Google Scholar]
- 6.Petrik MS, Wong MC, Tabata RC, Garry RF, Shaw CA. Aluminum adjuvant linked to Gulf War illness induces motor neuron death in mice. Neuromolecular Med 2007; 9(1):83–100. [DOI] [PubMed] [Google Scholar]
- 7.Greidanus TG, Honl BA. Delayed-type hypersensitivity reaction to anthrax vaccine. Mil Med 2002; 167(1):74–75. [PubMed] [Google Scholar]
- 8.Prater MR, Johnson VJ, Germolec DR, Luster MI, Holladay SD. Maternal treatment with a high dose of CpG ODN during gestation alters fetal craniofacial and distal limb development in C57BL/6 mice. Vaccine 2006; 24(3):263–271. [DOI] [PubMed] [Google Scholar]
- 9.Franco C, Lewis E, Morseth S, Simon L, Waytes AT. Reproductive toxicity of BioThrax® in rabbits. Birth Defects Res B Dev Reprod Toxicol. 2009; 86(5):370–376. [DOI] [PubMed] [Google Scholar]
- 10.Morse SA, Kellogg RB, Perry S, Meyer RF, Bray D, Nichelson D, Miller JM. Detecting biothreat agents: the Laboratory Response Network. ASM News 2003; 69(9):433–437. [Google Scholar]
- 11.Gilchrist MJ. A national laboratory network for bioterrorism: evolution from a prototype network of laboratories performing routine surveillance. Mil Med 2000; 165(7):28–31. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Notice to readers: Use of anthrax vaccine in response to terrorism: supplemental recommendations of the Advisory Committee on Immunization Practices. MMWR 2002; 51(45):1024–1026. [PubMed] [Google Scholar]
- 13.Fowler GL, Baggs JM, Weintraub ES, Martin SW, McNeil MM, Gust DA. Factors influencing laboratory workers’ decisions to accept or decline anthrax vaccine adsorbed (AVA): results of a decision-making study in CDC’s Anthrax Vaccination Program. Pharmacoepidemiol Drug Saf 2006; 15:880–888. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Use of anthrax vaccine in the United States: recommendations of the Advisory Committee on Immunization Practices (2009). MMWR 2010; 59(RR-6):1–34. [PubMed] [Google Scholar]
- 15.Anthrax Vaccine Adsorbed (BIOTHRAX™) Package Insert. 2002. Manufactured by Bioport Corporation, Lansing Michigan, US Lic; No. 1260. [Google Scholar]
- 16.Brazier JE, Harper R, Jones NMB, O’Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Brit Med J 1992; 305:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware JE, Snow KK, Kosinski MA, Gandek B. SF-36 Health Survey, Manual and Interpretation Guides. The Health Institute, New England Medical Center: Boston, 2000. [Google Scholar]
- 18.R Development Core Team (2010). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- 19.Ware JE. SF-36 Health Survey update. Spine 2000; 25:3130–3139. [DOI] [PubMed] [Google Scholar]
- 20.Picavet HSJ, Hoeymans N. Health related quality of life in multiple musculoskeletal diseases: SF-36 and EQ-5D in the DMC3 study. Ann Rheum Dis 2004; 63:723–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang W, Wang Y, Du W, Cheng N, Chen B. Assessment of quality of life and relevant factors in elderly diabetic patients in the Shanghai community. Pharmacoepidemiol Drug Saf 2006; 15:123–130. [DOI] [PubMed] [Google Scholar]
- 22.Smith B, Leard CA, Smith TC, Reed RJ, Ryan MAK, Millennium Cohort Study Team. Anthrax vaccination in the Millennium Cohort: validation and measures of health. Am J Prev Med 2007;32:347–353. [DOI] [PubMed] [Google Scholar]
- 23.Kazis LE, Lee A, , Spiro A III, Rogers W, Ren XS, Miller DR, Selim A, Hamed A, Haffer SC. Measurement comparisons of the Medical Outcomes Study and Veterans SF-36® health survey. Health Care Financing Review 2004; 24(4):43–56. [PMC free article] [PubMed] [Google Scholar]


