Abstract
Snail flesh is a highly nutritious and easily digestible food commonly integrated into the human diet. In this study, snails belonging to the Helix aspersa Müller species were used to determine their chemical composition and evaluate the antioxidant and antibacterial activities of their flesh using successive maceration extractions with three solvents of different polarities. Biomolecules were analyzed spectrophotometrically, and their chemical compositions were determined by using gas chromatography coupled with mass spectroscopy. The antioxidant activity was assessed using three tests: DPPH, iron-reducing power test, and total antioxidant activity. The ethanol extract was found to be the most effective, with a high yield and high biomolecule content compared with other extracts. The extracts showed a significant amount of antioxidants, ranging from 3.14 to 7.04 mg AAE g–1 of dry matter, according to the total antioxidant activity assay. The DPPH scavenging capacity showed a reduction of the radical, with inhibitory concentrations ranging from 507.07 to 829.49 μg mL–1. In contrast, the iron-reducing power ranged from 67.98 to 424.74 μg mL–1. All of the strains studied responded favorably to the antimicrobial effects of H. aspersa extracts, with a zone of inhibition ranging from 8.48 to 15.53 mm. Additionally, at approximately 15 mg mL–1, the ethanolic extract had the lowest minimum inhibitory concentration against Pseudomonas aeruginosa. H. aspersa Müller flesh is rich in biomolecules with antioxidant and antibacterial activities, which could justify its use as a natural product and in therapeutic applications in the food industry.
1. Introduction
Morocco has a diverse range of biological resources, including mollusks. Mollusks, including a diverse group of organisms, are the second-largest phylum within the animal world, with a remarkable number of species exceeding 50,000. These organisms fulfill a significant ecological function and provide substantial nutritional value.1 Mollusks are a heterogeneous group with a great diversity of forms. Furthermore, they are a rich and varied source of bioactive compounds with diverse structures. These compounds are of great interest to the pharmaceutical and biomedical industries because of their beneficial properties.2
Mollusks contain many active compounds such as peptides, lipids, steroids, terpenes, nitrogenous chemicals, sterols, alkaloids, fatty acids, and their derivatives,3−7 which can be used to develop dietary supplements, functional foods, nutraceuticals, and medicines.5 In addition, highly effective mechanisms that are part of the innate immune system have been developed.8 Mollusks are a good source of biologically active secondary metabolites.9 The use of mollusks as a source of medicines has great potential for the development of new therapies for a wide range of diseases, such as ziconotide (Prialt), a peptide found in the marine cone snail, which was the first marine peptide approved by the FDA in 2004 as an analgesic,10 the sea hare’s Adcetris for the treatment of cancer.3
Natural substances that are important in medicine can be discovered by studying the physical and chemical traits of mollusks. These substances have potential for medical use, are relevant to the pharmaceutical industry, and have many applications. They also contain chemicals that are important in pharmacology.
Mollusks have again become popular because they enjoy the taste and the flavor. The demand for snails has led to shortages, and snail farming has become an important source. Currently, the garden snail Helix aspersa Müller is the most popular worldwide11 and is the preferred species for snail farming because it is well suited for commercial production. This is due to its ability to reproduce quickly, fast life cycle, taste, versatility, and ability to adapt to different regions and environments.12
Antioxidants are a class of substances capable of neutralizing free radicals, thereby preventing diseases related to oxidative stress. Among natural antioxidants are α-tocopherol, ascorbic acid, and phenolic compounds.13 They play a crucial role in inhibiting and eliminating free radicals, thereby protecting the human body against infections and degenerative diseases. Antioxidants can delay or prevent oxidative damage and, therefore, can be useful as therapeutic products or food additives.
Public health is threatened by antibiotic resistance.14 Therefore, it is critical to develop new medications to combat this resistance. The antibacterial, cytotoxic, anticancer, anti-inflammatory, antileukemic, antineoplastic, antioxidant, and antiviral properties of these bioactive substances have been previously studied.15 Eating snails has several health benefits, including the prevention of certain diseases.16,17
According to Zhang et al.,18 the type and polarity of the solvent used can affect the quality, quantity, extraction rate, and biological activity of an extract. Kang and Lee19 also found that antioxidant activity depends on the type and polarity of the extraction solvent.
Bibliographic research has shown that it is important to study terrestrial gastropods; there have been few studies on the flesh of the snail of H. aspersa Müller. Our study focuses on the exploration of the chemical compounds and biological activities of the flesh extracts of H. aspersa Müller and fills in the gaps; in particular, we consider the data on the chemical composition of the flesh of H. aspersa Müller. We report here the study of the three extracts using gas chromatography coupled to mass spectrometry (GC–MS) and quantify the biomolecules to elucidate their compositions. Second, this study focuses specifically on antioxidant and antibacterial activities, thus providing valuable information for health and medicine.
2. Results and Discussion
2.1. Extraction Yield
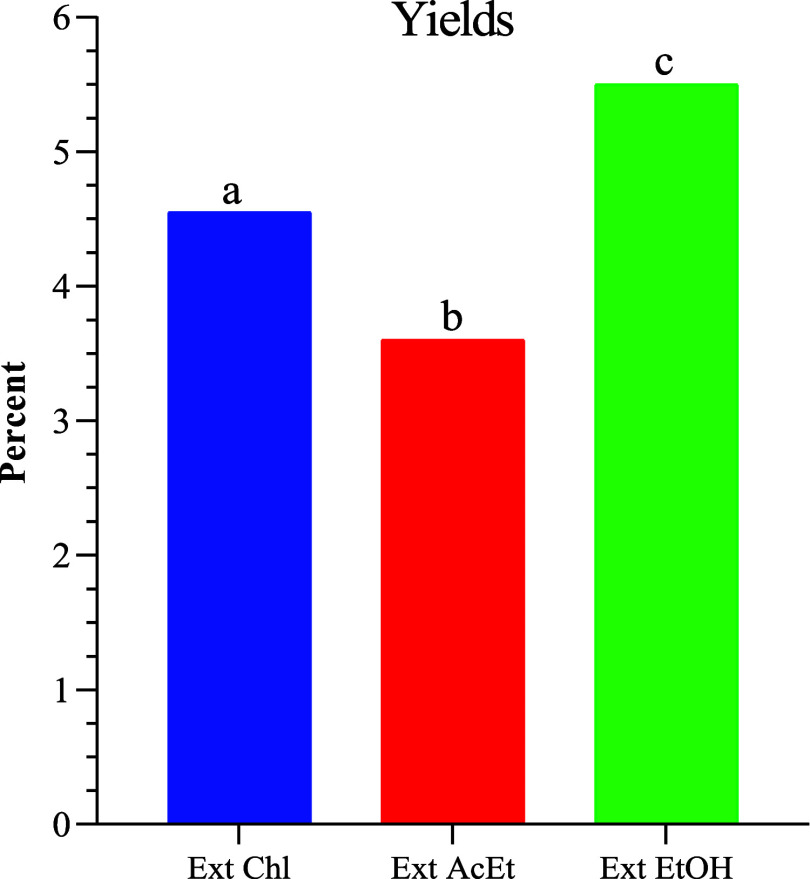
The key factors in the extraction process are the solvent used and its polarity.20Figure 1 shows the extraction yields of H. aspersa Müller snail powder. It is worth noting that the recorded values vary significantly. The ethanol extract yield is 5.50%, and the chloroformic extract yield is 4.55%, these two yields are greater than the ethyl acetate yield (3.60%). The polarity of the extraction solvent enhances the efficiency.
Figure 1.
Histogram of the extraction yields. Ex Chl: chloroform extract, Ex AcEt: ethyl acetate extract, and Ex EtOH: ethanol extract. The significant difference (p < 0.05) between the different extracts is illustrated by the letters a, b, and c.
2.2. Chemical Composition
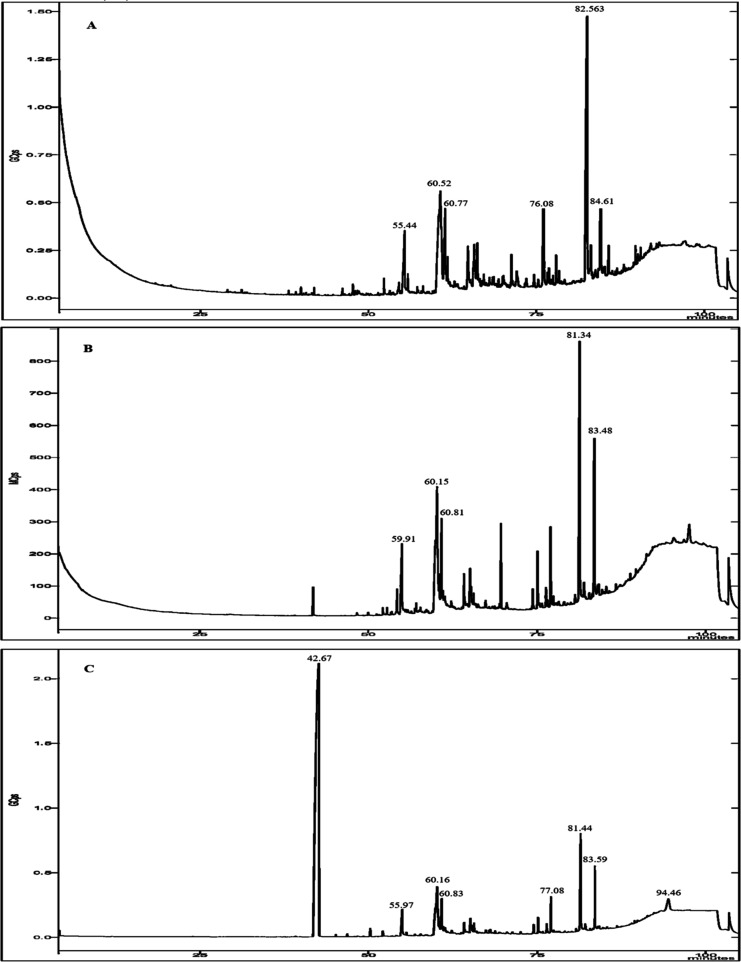
The GC–MS analysis of different extracts from the flesh of H. aspersa Müller revealed 45 potential bioactive compounds, including fatty acids, sterols, and other metabolites, that could contribute to the medicinal quality of the animal, which were identified by correlating their retention time (RT) and mass spectra fragmentation patterns to those of known compounds described in the National Institute of Standards and Technology (NISTII) library (Table 1 and Figure 2).
Table 1. GC–MS Analysis of Crude Extracts of H. aspersa Müller Flesh.
| compounds | chemical
class |
|||||
|---|---|---|---|---|---|---|
|
Ex Chl |
Ex AcEt |
Ex EtOH |
||||
| RT (min) | area % | RT (min) | area % | RT (min) | area % | |
| tricosane | 039.96 | 00.24 | ||||
| docosane | 046.13 | 00.24 | ||||
| nonadecane | 047.68 | 00.27 | ||||
| cyclohexadecane | 052.38 | 00.65 | 52.11 | 00.21 | ||
| hexadecanoic acid | 055.44 | 05.68 | 060.81 | 06.14 | 54.97 | 01.68 |
| pentadecanoic acid, ethyl ester | 066.24 | 01.73 | ||||
| (Z,Z) 9,12-octadecadienoic acid | 082.56 | 25.08 | 081.34 | 18.41 | 42.67 | 66.83 |
| oxirane, [(hexadecyloxy)methyl | 060.92 | 02.23 | ||||
| octadecanoic acid | 061.45 | 04.99 | 059.91 | 08.92 | 60.83 | 02.02 |
| heptadecanoic acid | 060.52 | 09.19 | 054.96 | 04.74 | 55.59 | 00.09 |
| octadecane | 062.39 | 00.05 | 66.03 | 00.16 | ||
| cis-5,8,11,14-eicosatetraenoic acid | 064.86 | 02.60 | 060.15 | 12.13 | 60.16 | 03.73 |
| 1,2-O-[2′-hydroxyoctadecyl]glycerol | 065.79 | 02.10 | 70.07 | 00.03 | ||
| hentriacontane | 066.63 | 00.15 | ||||
| (E)-butenedioic acid | 065.93 | 01.08 | 68.74 | 00.09 | ||
| cis-5,8,11,14,17-eicosapentaenoic acid | 070.12 | 00.82 | ||||
| (Z)-9-octadecenamide | 071.32 | 01.04 | 075.05 | 02.70 | 75.12 | 00.62 |
| elaidamide | 074.68 | 00.81 | 041.82 | 03.62 | 80.79 | 00.10 |
| cholesta-2,4-diene | 103.45 | 01.53 | ||||
| (3β)-cholest-5-en-3-ol | 060.77 | 09.18 | 076.99 | 03.78 | 77.08 | 01.33 |
| cholest-4-en-3-one | 084.61 | 07.94 | 083.48 | 12.58 | 83.59 | 02.51 |
| γ-sitostenone | 085.78 | 01.46 | 082.00 | 00.89 | 86.86 | 00.06 |
| clionasterol | 076.08 | 08.74 | 103.44 | 03.85 | ||
| 9,19-cyclolanost-24-en-3-ol | 076.89 | 00.78 | ||||
| 2-pentacosanone | 060.25 | 03.05 | ||||
| cholesta-3,5-diene | 069.64 | 03.40 | ||||
| cholesta-4,6-dien-3-one | 083.94 | 00.26 | ||||
| methyl 9,10-octadecadienoate | 58.41 | 00.02 | ||||
| 13-octadecenoic acid | 58.64 | 00.03 | ||||
| 16-methylheptadecanoic acid | 59.46 | 00.03 | ||||
| octanoic acid | 60.27 | 01.15 | ||||
| methyl dehydroabietate | 64.78 | 00.18 | ||||
| dehydroabietic acid | 67.39 | 00.16 | ||||
| abietic acid | 68.42 | 00.03 | ||||
| (3Z,13E)-2-methyloctadeca-3,13-dien-1-ol | 65.81 | 00.08 | ||||
| 8,11,14-eicosatrienoic acid | 81.44 | 04.93 | ||||
| psi.,.psi,-carotene, 1,1′,2,2′-tétrahydro-1,1′-diméthoxy | 78.33 | 00.06 | ||||
| dihydrobrassicasterol | 79.12 | 00.05 | ||||
| vitamin E | 81.74 | 00.01 | ||||
| cholesta-3,5-dien-7-one | 82.84 | 00.07 | ||||
| (3β)-ergost-5-en-3-ol | 83.27 | 00.09 | ||||
| β-sitosterol | 84.78 | 00.05 | ||||
| ergosta-4,6,8(14),22-tetraen-3-one | 85.03 | 00.02 | ||||
| 4-campestene-3-one | 85.40 | 00.06 | ||||
| docosanoic acid | 94.46 | 01.89 | ||||
| fatty acids | 51.17 | 50.34 | 81.36 | |||
| alkanes | 01.60 | 00.00 | 00.37 | |||
| ketones | 09.40 | 16.78 | 02.72 | |||
| aAmides | 01.85 | 06.32 | 00.72 | |||
| sterols | 10.27 | 07.25 | 00.14 | |||
| fatty alcohols | 12.06 | 03.78 | 01.44 | |||
| total identified | 88.58 | 84.47 | 84.15 | |||
| not identified | 11.42 | 15.53 | 15.85 | |||
Figure 2.
GC–MS chromatogram for the crude extract of the flesh of H. aspersa Müller. (A) Chloroform extract, (B) ethyl acetate extract, and (C) ethanolic extract.
Hexadecanoic acid, (Z,Z) 9,12-octadecadienoic acid, cis-5,8,11,14-eicosatetraenoic acid, heptadecanoic acid, clionasterol, cholest-4-en-3-one, and cholesterol were considered the most abundant. (Z,Z) 9,12-Octadecadienoic acid and hexadecanoic acid were the dominant ones in the chloroform extract. The predominant volatile compounds in the ethyl acetate extract were (Z,Z) 9,12-octadecadienoic acid and cholest-4-en-3-one (18.41 and 12.58%, respectively). The ethanol extract revealed the most diverse profile with the presence of 32 identified compounds, the highest content among which was established for (Z,Z) 9,12-octadecadienoic acid, at 66.83%.
Among the saturated fatty acids, octadecanoic acid stands out, with a range of 2.02 to 8.92%, followed by hexadecanoic acid, with a range of 1.68 to 4.14%. Among the polyunsaturated fatty acids, (Z,Z) 9,12-octadecadienoic acid had the highest percentage, with a range of 18.41 to 66.83%, and cis-5,8,11,14-eicosatetraenoic acid ranged from 2.60 to 12.13%.
The bioactive substances discovered by GC–MS analysis are reported in Table 2 and demonstrate various important biological activities for this investigation. The stated biological activities are based on Dr. Jim Duke of the Agricultural Research Service/USDA’s phytochemical and ethnobotanical databases.21
Table 2. Activities of Some Identified Compounds in the Extract of H. aspersa Müller.
| compounds name | Activities |
|---|---|
| hexadecanoic acid | antioxidant, flavour, antifibrinolytic, hypocholesterolemic, antiandrogenic, lubricant, hemolytic, 5-α reductase inhibitor, nematicide, antialopecic, antiandrogenic nematicide, antiandrogenic, hemolytic |
| methyl 11-octadecenoate | allelopathic, pesticide |
| 9-octadecenoic acid | anti-inflammatory, antialopecic, α-reductase inhibitor lubricant, antitumor, dermatitigenic, immunostimulant, antileucotriene-d4, lipoxygenase inhibitor, allergenic, flavor, hypocholesterolemic, insectifuge, percutaneo-stimulant, perfumery, and propecic |
| pentadecanoic acid | antioxidant |
| docosanoic acid | hair moisturizer |
| bonadecane | antimutagenic |
| vitamin E | cancer preventive |
| Oxirane | precursor of progesterone, antimicrobial |
| octadecanoic acid | antioxidant, hypoglycemic, and thyroid-inhibiting properties |
| (Z,Z) 9,12-octadecadienoic acid | anti-inflammatory, nematicide, insectifuge, hypocholesterolemic, cancer preventive, heptaoprotective, antistaminic, antiacne, antiarthritic, 5-α reductase inhibitor, anticoronary, anticancer |
2.3. Biochemical Analyses
Initially, our objective was to quantify the bioactive components in the flesh, which may have biological properties.
Protein, polyphenols, total sugars, and reducing sugars are measured in milligram equivalents of bovine albumin serum, gallic acid, and glucose per gram of dry matter (mg of BSA, GA, and GE g–1 of DM). The linear regression equation of the standard calibration curves is used to calculate the results. Table 3 indicates that all extracts contain organic molecules whose concentrations increase with the polarity of the extraction solvent. The results revealed that solvent polarity had a significant impact on total biomolecule content.
Table 3. Concentration of Biomolecules in H. aspersa Müller Crude Extracts.
| concentration (mg g–1 DM) |
|||||
|---|---|---|---|---|---|
| protein | polyphenols | total sugar | reducing sugar | total flavonoids | |
| Ex Chl | 1.34 ± 0.07a | 0.16 ± 0.03a | 1.16 ± 0.10a | 0.99 ± 0.10a | 0.04 ± 0.00a |
| Ex AcEt | 1.62 ± 0.09a | 0.32 ± 0.04a | 1.73 ± 0.24a | 1.39 ± 0.12ab | 0.06 ± 0.00a |
| Ex EtOH | 3.74 ± 0.18b | 1.26 ± 0.08b | 2.72 ± 0.11b | 2.07 ± 0.16b | 0.13 ± 0.00b |
The protein content of the ethanolic extract (3.74 ± 0.18 mg g–1 MS) is greater than that of the ethyl acetate and chloroform extracts (1.62 ± 0.09 and 1.34 ± 0.07 mg g–1 DM, respectively). These findings are compared to those of Gomot,22 who discovered that protein levels varied depending on the type of snail examined.
The values of the total sugar analysis show a significant variance in the various extracts. The ethyl extract has the highest level (2.72 ± 0.11 mg g–1 DM), followed by the ethyl acetate extract. Total carbohydrate levels have been reported.23
In addition, we notice that the content of reducing sugars is higher in the ethanolic extract (2.07 ± 0.16 mg g–1 of DM), while ethyl acetate and chloroform extracts showed the lowest levels. However, Gomot22 reported total monosaccharide levels in H. aspersa maxima.
The ethanol extract (1.26 ± 0.08, 0.32 ± 0.04, and 0.16 ± 0.03 mg GAE g–1 of DM) had the most polyphenols, followed by the ethyl acetate and chloroform extracts. The results show that the total polyphenol content increases with the polarity of the extracting solvent, and the difference between the three extracts is statistically significant (p < 0.05). Ghedadba et al.24 stated that the high content of phenolic compounds is related to the high solubility of phenols in polar solvents. A recent study reported 0.132 mg mL–1 of polyphenols.25
The results show that the average amount of flavonoids in the extracts of H. aspersa Müller made with chloroform, ethyl acetate, and ethanol is 0.04 ± 0.00, 0.06 ± 0.00, and 0.13 ± 0.00 mg QE g–1 of DM, respectively. The difference in flavonoid content between the three extracts is statistically significant (p < 0.05).
2.4. Determination of Antioxidant Activity
Various methods were employed to assess antioxidant activity designed to have varying levels of activity.
2.4.1. Total Antioxidant Capacity (TAC)
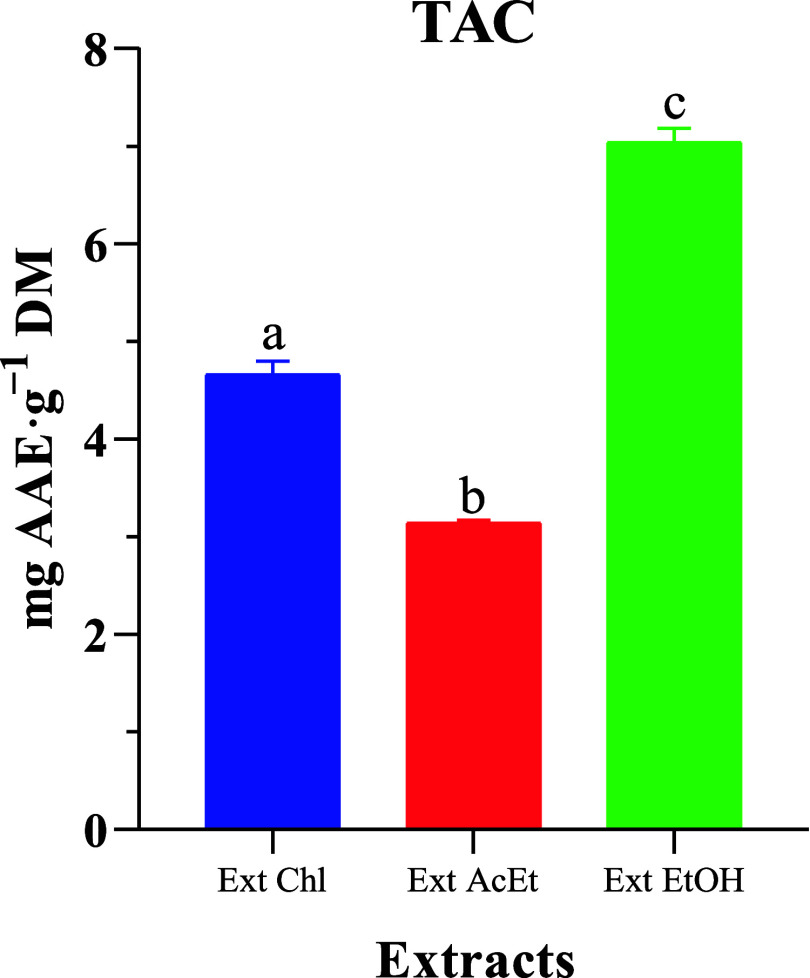
A standard ascorbic acid curve was established to evaluate the total antioxidant capacity. The results reveal that the three extracts showed different antioxidant activities, as shown in Figure 3. The ethanol extract had the highest TAC at approximately 7.04 ± 0.14 mg g–1 DM, followed by the chloroform extract at approximately 4.66 ± 0.14 mg g–1 DM, and finally, the ethyl acetate extract had a TAC of 3.14 ± 0.03 mg g–1 DM. The difference in TAC between the three extracts of H. aspersa Müller was statistically significant (p < 0.05). These results suggest that the flesh of H. aspersa Müller could serve as a natural source of antioxidants. Similar results were reported by Gogas et al.26 in H. aspersa Müller (0.587 ± 0.715) fed with different protein sources under intensive farming conditions.
Figure 3.
TAC of H. aspersa Müller crude extracts. Ex Chl: chloroform extract, Ex AcEt: ethyl acetate extract, and Ex EtOH: ethanol extract. The significant difference (p < 0.05) is illustrated by the letters a, b, and c.
In order to assess antioxidant capability, several researchers employ the phosphomolybdate method, which is one of the most reliable techniques.27 The extracts’ TAC differed widely among the several solvents, demonstrating that each solvent with a certain polarity may isolate particular components with a particular antioxidant ability.
2.4.2. Determination of Free Radical Scavenging Activity (DPPH)
The DPPH free radical method has been widely used for different natural and synthetic antioxidant compounds and potential free radical scavenging activity.27 First, the percentage of DPPH free radical inhibition was identified at different concentrations (200 to 1000 μg mL–1) of each extract.
Table 4 shows the results of the free radical inhibition test performed on the crude extracts of the H. aspersa Müller flesh. All three extracts showed dose-dependent inhibition. The free radical scavenging capacity of the crude extracts of H. aspersa Müller flesh was determined by using IC50 values. The ethanol extract exhibited higher scavenging activity than the ethyl acetate and chloroform extracts. The antioxidant activity of the crude ethanol extract was 89.36 ± 0.43%, while the ethyl acetate extract showed an activity of 71.51 ± 0.29% and the chloroform extract showed an activity of 60.12 ± 0.72% at the highest concentration.
Table 4. Comparison between the Effects of Antibiotics on Pathogenic Bacteria In Vitroa.
| antibiotics |
P. aeruginosa |
E. coli (ATCC 35218) |
L. monocytogenes (ATCC 7644) |
S. aureus (ATCC
29213) |
||||
|---|---|---|---|---|---|---|---|---|
| ZI (mm) | Pr | ZI (mm) | Pr | ZI (mm) | Pr | ZI (mm) | Pr | |
| AMP | 00.00 ± 0.00a | R | 00.00 ± 0.00a | R | 00.00 ± 0.00a | R | 00.00 ± 0.00a | R |
| COF | 09.60 ± 0.12b | S | 17.00 ± 0.00c | S | 10.00 ± 0.09b | S | 00.00 ± 0.00a | R |
| CUS | 10.00 ± 1.02b | S | 00.00 ± 0.00a | R | 28.00 ± 0.16d | ES | 00.00 ± 0.00a | R |
| ETM | 19.00 ± 0.24d | S | 00.00 ± 0.00a | R | 25.00 ± 0.05cd | ES | 20.00 ± 0.00bc | S |
| NM | 19.67 ± 0.10d | S | 13.50 ± 0.00b | S | 30.00 ± 0.37d | ES | 18.00 ± 0.00b | S |
| PRP | 00.00 ± 0.00a | R | 00.00 ± 0.00a | R | 17.00 ± 0.20bc | S | 00.00 ± 0.00a | R |
| TC | 00.00 ± 0.00a | R | 12.00 ± 0.00b | S | 00.00 ± 0.00a | R | 15.00 ± 0.00b | S |
ZI: zone of inhibition; Pr: profile; R: resistant; S: sensitive; ES: extremely sensitive; ER: extremely resistant. Means in the same column with the same letter do not differ significantly from each other at the 5% level of significance according to the Duncan’s test.
The IC50 values were 507.1 ± 1.2, 706.8 ± 8.03, and 829.5 ± 13.04 μg mL–1, respectively, for ethanol, ethyl acetate, and chloroform extracts. The antioxidant activity of all three extracts was lower than that of l-ascorbic acid, with an IC50 value of 41.55 ± 0.00 μg mL–1. The hydroxyl radical scavenging activity of l-ascorbic acid was 89.46% at the highest concentration (200 μg mL–1). IC50 values were significantly different in all extracts (p < 0.05), indicating that each solvent extracts a specific type of metabolite that could carry different levels of DPPH free radical scavenging activity.
Compared to the previous results, in their study, Borquaye et al.28 reported that the ethyl acetate extract of Galatea paradoxa had an IC50 of 1259.0 μg mL–1, while the methanolic extract displayed 370.0 μg mL–1. Similarly, the ethyl acetate extract of Littorina littorea showed an IC50 of 1065.0 μg mL–1, while the methanolic extract had a concentration of 780.0 μg mL–1. On the other hand, the methanolic extract of B. spinosa had an IC50 value of 39.43% at 10,000 μg mL–1.29
2.4.3. Determination of Reducing Power (FRAP)
Table 4 shows what happened when extracts of H. aspersa Müller flesh were used in the FRAP radical absorbance inhibition test. The IC50 values showed a statistically significant difference (p < 0.05) between ascorbic acid’s antioxidant activity and that of the three extracts. All tested extracts inhibited the FRAP absorbance, and this inhibition was dose-dependent for all three extracts. The ethanol extract showed significantly higher antioxidant activity (67.98 ± 4.31 μg mL–1) compared to the ethyl acetate (267.69 ± 19.83 μg mL–1) and chloroform (424.74 ± 14.33 μg mL–1) extracts but had lower antioxidant activity than ascorbic acid (53.24 ± 3.94 μg mL–1). This indicates that H. aspersa Müller extracts have important antioxidant characteristics. Similar results have been reported for marine species.30
Subavathy and Janet31 reported a maximum activity at 500 μg mL–1 in Turbo brunneus, Babylonia spirata, and Cypraea annulus of 73.35, 87.5, and 95.36%, respectively, and a minimum activity at 100 μg mL–1, of 52.07, 57.51, and 67.21%, respectively, in T. brunneus, B. spirata, and C. annulus.
The different amounts of biomolecules and their antioxidant capacity in terms of DPPH, FRAP, and total antioxidant capacity may be due to the polarity index of the molecules and their association with the polarity index of the solvent.
2.5. Antibacterial Activity
2.5.1. Disc Diffusion
In a previous study by Aouji et al.,32 the sensitivity of various bacterial strains to specific antibiotics was investigated. The results indicated that Escherichia coli was sensitive to cefotaxime sodium, netilmicin, and tetracycline, whereas Staphylococcus aureus was sensitive to erythromycin, netilmicin, and tetracycline. Additionally, Pseudomonas aeruginosa displayed resistance to ampicillin, piperacillin, and tetracycline, whereas L. monocytogenes exhibited resistance to ampicillin and tetracycline (Table 4).
Furthermore, the flesh extracts of H. aspersa Müller demonstrated antimicrobial effects against all bacterial strains examined (Table 5). Notably, the choice of solvent used for the extraction appeared to influence the inhibition of bacterial growth. Additionally, the findings indicated that the antimicrobial activity of H. aspersa Müller tended to increase with the increasing polarity of the solvent used for extraction.
Table 5. Average Diameter of Pathogen Inhibition Zones (ZI) Generated by H. aspersa Müller Flesh Extractsa.
| ZI (mm) |
||||
|---|---|---|---|---|
| P. aeruginosa | E. coli (ATCC 35218) | L. monocytogenes (ATCC 7644) | S. aureus (ATCC 29213) | |
| Ext EtOH | 15.26 ± 0.21a | 15.53 ± 0.21a | 12.30 ± 0.52a | 13.30 ± 0.23a |
| Ext AcEt | 10.47 ± 0.31b | 10.92 ± 0.13b | 09.98 ± 0.16b | 09.53 ± 0.15b |
| Ext Chl | 08.07 ± 0.15c | 09.77 ± 0.25c | 09.60 ± 0.22b | 08.48 ± 0.38c |
Ex Chl: chloroform extract, Ex AcEt: ethyl acetate extract and Ex EtOH: ethanol extract. Means in the same column with the same letter do not differ significantly from each other at the 5% significance level according to Duncan’s test.
The ethanol extract exhibited the highest inhibitory diameters, measuring 15.53, 15.26, 12.30, and 13.30 mm against E. coli, P. aeruginosa, S. aureus, and L. monocytogenes, respectively. In contrast, the chloroform extract exhibited the lowest inhibitory diameters, with inhibition zones of 8.07 and 8.48 mm against P. aeruginosa and S. aureus, respectively. The statistical analysis indicated that there were significant differences in the inhibitory effects of the three extracts on the bacterial strains that were tested (p ≥ 0.05). Nevertheless, while examining L. monocytogenes, there was no notable disparity seen between the ethyl acetate and chloroform extracts (p < 0.05). In conclusion, the results of this study indicate that extracts obtained from the H. aspersa Müller flesh have antibacterial activities against pathogenic microorganisms (Table 6).
Table 6. Minimal Inhibitory Concentration of Various Extracts of H. aspersa Müller Flesh against Bacteriaa.
| CMI (mg mL–1) |
||||
|---|---|---|---|---|
| P. aeruginosa | E. coli (ATCC 35218) | L. monocytogenes (ATCC 7644) | S. aureus (ATCC 29213) | |
| Ext Chl | 30 | 45 | 40 | 30 |
| Ext AcEt | 20 | 30 | 25 | 35 |
| Ext EtOH | 15 | 20 | 25 | 20 |
Ex Chl: chloroform extract, Ex AcEt: ethyl acetate extract, and Ex EtOH: ethanol extract.
Anand and Edward15 performed a comparative study on the antibacterial properties of ethanolic extracts obtained from the gastropod species B. spirata and T. brunneus. The researchers observed an increase in the antibacterial efficacy against E. coli, K. pneumoniae, Pseudomonas vulgaris, and Salmonella typhi.
2.5.2. Determination of Minimum Inhibitory Concentration by the Broth Dilution Method
The minimum inhibitory concentration (MIC) refers to the lowest concentration of an antibacterial agent that is necessary to effectively eradicate certain bacteria.33 In general, the findings of the study indicate the clear presence of antibacterial activity in the three extracts derived from H. aspersa Müller flesh. The MIC values of the extracts obtained from the H. aspersa Müller flesh were determined.
Our results revealed a significant level of antibacterial efficacy against the examined bacterial strains. The MIC values for the tested extracts varied when they were assessed against the target microorganisms. Specifically, the ethanol extract displayed an MIC of 15 mg mL–1 against P. aeruginosa, whereas the chloroform extract had an MIC of 45 mg mL–1 against E. coli.
The MIC values recorded for the ethanol extract against the four bacterial strains ranged from 15 to 25 mg mL–1. P. aeruginosa displayed the lowest MIC at 15 mg mL–1, followed by S. aureus, E. coli, and L. monocytogenes at concentrations of 20, 20, and 25 mg mL–1, respectively. The ethyl acetate extract exhibited MIC values of 20, 30, 25, and 35 mg mL–1 against P. aeruginosa, E. coli, L. monocytogenes, and S. aureus, respectively. In contrast, the chloroform extract displayed the lowest efficacy, with MIC values ranging from 30 to 45 mg mL–1. Specifically, the MIC was determined to be 30 mg mL–1 against both S. aureus and P. aeruginosa, while L. monocytogenes exhibited a MIC of 40 mg mL–1, and E. coli demonstrated a MIC of 45 mg mL–1.
Discrepancies in MIC values can be attributed to variations in the chemical composition of the extracts. The ethanol extract, characterized by a high concentration of polar compounds such as phenols and flavonoids, known for their potent antibacterial properties, displayed more significant antimicrobial effects. Conversely, the chloroform extract contained a higher proportion of nonpolar molecules, resulting in relatively weaker antibacterial activity.
Rota et al.34 have elucidated that the antibacterial action of phenolic compounds arises from the presence of numerous hydroxyl groups, which have the capacity to interact with bacterial cell membranes, inducing alterations in their structure and composition. Helal et al.35 proposed that phenolic compounds can modulate the bacterial cell membrane structure, diminish lipid content, and ultimately hinder bacterial growth. Prashar et al.36 suggested that phenolic substances can inflict damage on or permeate bacterial cell lipid structures through membrane saturation.
Furthermore, Ulagesan and Kim37 demonstrated the antibacterial activity of crude protein derived from six distinct snail species against specific bacterial and fungal strains. Our findings indicate that extracts sourced from the H. aspersa Müller flesh have the potential to serve as a novel reservoir of antibacterial agents.
2.6. Correlation between Different Parameters
Table 7 presents the Pearson correlation coefficients between the different parameters of the chloroform extract, where some parameters are positively correlated and others are negatively correlated. Starting with the positively correlated parameters, namely, Pr with Ts, E. coli, L. monocytogenes, S. aureus, and FRAP (r = 0.828, 0.949, 0.949, 0.747, and 0.886, respectively), Rs with Ts, Pp, FRAP, and TAC (r = 0.217, 0.956, 0.106, and 0.775, respectively), Ts with FRAP (r = 0.994), Pp with TAC (r = 0.927), Ft with DPPH (r = 0.549), and finally DPPH with TAC (r = 0.273). Those that are negatively correlated are TAC with FRAP (r = −0.547), FRAP with DPPH (r = −0.955), DPPH with Pr and Rs, Ts and Pp (r = −0.707, −0.397, −0.982, and −0.109, respectively), Pp with Pr and Ts (r = −0.626 and −0.080, respectively), Pr with Rs (r = −0.368), and finally FRAP with TAC (r = −0.824), P. aeruginosa with S. aureus (r = −1.000).
Table 7. Pearson Correlation Coefficients between Different Parameters of the Chloroform.
| Pr | Rs | Ts | Pp | Ft | DPPH | FRAP | TAC | P. aeruginosa | E. coli | L. monocytogenes | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rs | –0.368 | ||||||||||
| Ts | 0.828 | 0.217 | |||||||||
| Pp | –0.626 | 0.956 | –0.080 | ||||||||
| Ft | 0.202 | –0.985 | –0.382 | –0.891 | |||||||
| DPPH | –0.707 | –0.397 | –0.982 | –0.109 | 0.549 | ||||||
| FRAP | 0.886 | 0.106 | 0.994 | –0.192 | –0.276 | –0.955 | |||||
| TAC | –0.873 | 0.775 | –0.449 | 0.927 | –0.654 | 0.273 | –0.547 | ||||
| P. aeruginosa | –0.747 | –0.343 | –0.991 | –0.051 | 0.500 | 0.998 | –0.970 | 0.328 | |||
| E. coli | 0.949 | –0.642 | 0.609 | –0.839 | 0.500 | –0.449 | 0.695 | –0.982 | –0.500 | ||
| L. monocytogenes | 0.949 | –0.642 | 0.609 | –0.839 | 0.500 | –0.449 | 0.695 | –0.982 | –0.500 | 1.000 | |
| S. aureus | 0.747 | 0.343 | 0.991 | 0.051 | –0.500 | –0.998 | 0.970 | –0.328 | –1.000 | 0.500 | 0.500 |
According to the results presented in Table 8, it is evident that a number of factors studied in this research have shown a positive correlation between them. FRAP shows a positive correlation with Pr, Rs, Ts, and Ft (r = 0.982, 0.910, 0.882, and 0.185, respectively), while TAC shows a positive correlation with Pr, Rs, Ts, and FRAP (r = 0.759, 0.996, 0.532, and 0.868, respectively) and S. aureus with Ts and FRAP (r = 0.988 and 0.944). Furthermore, a negative correlation has been observed between DPPH and Pr, Rs, Ts, and Ft (r = −0.996, −0.759, −0.978, and −0.449, respectively) and Pr with Pp (r = −0.986).
Table 8. Pearson Correlation Coefficients between Different Parameters of the Ethyl Acetate Extract.
| Pr | Rs | Ts | Pp | Ft | DPPH | FRAP | TAC | P. aeruginosa | E. coli | L. monocytogenes | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rs | 0.815 | ||||||||||
| Ts | 0.955 | 0.607 | |||||||||
| Pp | –0.986 | –0.900 | –0.893 | ||||||||
| Ft | 0.367 | –0.240 | 0.626 | –0.208 | |||||||
| DPPH | –0.996 | –0.759 | –0.978 | 0.967 | –0.449 | ||||||
| FRAP | 0.982 | 0.910 | 0.882 | –1.000 | 0.185 | –0.961 | |||||
| TAC | 0.759 | 0.996 | 0.532 | –0.856 | –0.327 | –0.697 | 0.868 | ||||
| P. aeruginosa | –0.367 | 0.240 | –0.626 | 0.208 | –1.000 | 0.449 | –0.185 | 0.327 | |||
| E. coli | –0.783 | –0.277 | –0.932 | 0.669 | –0.866 | 0.836 | –0.652 | –0.189 | 0.866 | ||
| L. monocytogenes | –0.367 | 0.240 | –0.626 | 0.208 | –1.000 | 0.449 | –0.185 | 0.327 | 1.000 | 0.866 | |
| S. aureus | 0.989 | 0.721 | 0.988 | –0.951 | 0.500 | –0.998 | 0.944 | 0.655 | –0.500 | –0.866 | –0.500 |
The coefficients of Pearson correlation between the various examined characteristics showed that numerous factors correlated favorably (Table 9), including Ft and FRAP (r = 0.509), Pp with TAC and S. aureus (r = 0.191 and 0.999), and Pr with Pp, DPPH, and TAC (r = 0.216, 0.681, and 1.000, respectively). FRAP showed a positive correlation with Rs, Ts, Pp, and Ft (r = 0.646, 0.874, 0.143, and 0.509, respectively). On the other hand, Ts is strongly negatively correlated with DPPH (r = −0.999), the latter is negatively correlated with FRAP, Rs, Pp, and Ft (r = −0.896, −0.918, −0.567, and −0.075, respectively), and L. monocytogenes with Pp (r = −0.999).
Table 9. Pearson Correlation Coefficients between Different Parameters of the Ethanolic Extract.
| Pr | Rs | Ts | Pp | Ft | DPPH | FRAP | TAC | P. aeruginosa | E. coli | L. monocytogenes | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rs | –0.334 | |||||||||||
| Ts | –0.645 | 0.936 | ||||||||||
| Pp | 0.216 | 0.848 | 0.606 | |||||||||
| Ft | –0.781 | –0.327 | 0.027 | –0.779 | ||||||||
| DPPH | 0.681 | –0.918 | –0.999 | –0.567 | –0.075 | |||||||
| FRAP | –0.935 | 0.646 | 0.874 | 0.143 | 0.509 | –0.896 | ||||||
| TAC | 1.000 | –0.359 | –0.665 | 0.191 | –0.764 | 0.700 | –0.944 | |||||
| P. aeruginosa | –0.182 | –0.866 | –0.634 | –0.999 | 0.756 | 0.596 | –0.178 | –0.156 | ||||
| E. coli | –0.942 | 0.000 | 0.353 | –0.530 | 0.945 | –0.397 | 0.763 | –0.933 | 0.500 | |||
| L. monocytogenes | –0.182 | –0.866 | –0.634 | –0.999 | 0.756 | 0.596 | –0.178 | –0.156 | 1.000 | 0.500 | ||
| S. aureus | 0.182 | 0.866 | 0.634 | 0.999 | –0.756 | –0.596 | 0.178 | 0.156 | –1.000 | –0.500 | –1.000 |
3. Conclusions
This study investigated the biomolecule content and the antioxidant and antibacterial potential of raw extracts from the flesh of H. aspersa Müller. These extracts have a high abundance of molecules and significant antioxidant activity. The extracts also showed antibacterial activity against E. coli, P. aeruginosa, S. aureus, and L. monocytogenes. The GC–MS analysis identified 45 compounds, including octadecanoic and hexadecanoic acids, which may contribute to the observed activities. The results show that the extraction process and the polarity of the solvent significantly influence the yields of bioactive compounds and the biological activities of the H. aspersa Müller flesh. The ethanol extract contains the highest concentrations of proteins, sugars, polyphenols, and flavonoids. This enriched profile contributed to the superior biological properties observed compared with ethyl acetate and chloroform extracts. The ethanol extract presented a significant advantage in terms of total antioxidant capacity, DPPH radical scavenging activity, and iron reduction capacity. In addition, it has shown the most powerful antibacterial efficacy, with the largest areas of inhibition against the bacterial strains tested. The study highlights the importance of solvent selection to maximize extraction of valuable bioactive compounds.
These results underline the potential of the flesh of H. aspersa Müller as a rich source of natural antioxidants and antibacterial agents. However, further research is needed to elucidate the specific compounds responsible for the antioxidant and antimicrobial effects. Future studies should focus on the isolation, purification, and identification of key bioactive molecules. In addition, in vitro and in vivo studies are necessary to confirm the efficacy and safety of these extracts for potential applications in the food and pharmaceutical industries.
4. Materials and Methods
4.1. Snail Collection and Preparation of the Flesh Powder
Uninfected H. aspersa Müller snails were collected in the spring from the Moulay Bousselham region of Morocco (34° 52′ 43″ N, 6° 17′ 36″ O). These were immediately transferred to the laboratory, where healthy individuals were housed in rectangular plastic boxes (24 × 32 × 12 cm) with a sponge, moistened soil, and food (lettuce, carrot, and spinach). Boxes containing 50 snails were sprayed with water daily to keep them moist. It is important to note that the snails were treated as part of a scientific experiment that followed animal care guidelines.
150 snails were cleaned under running water and then removed from their shells, and the visceral masses were removed. The feet (with the head) were recovered and cleaned of mud with a NaCl solution (1.2%) and were washed with distilled water and dried on absorbent paper. The samples were dried in an oven (60 °C for 48 h) and then ground and sieved for recovery.38
4.2. Extracts of Molecules
A sequential maceration approach was used with three organic solvents, chosen based on increasing polarity: chloroform (boiling point: 61.2 °C), ethyl acetate (boiling point: 77.1 °C), and ethanol (boiling point: 78.3 °C) (starting with chloroform). At a rate of 10 g of flesh in 100 mL of solvent, extraction was performed at room temperature with constant agitation and light protection for 48 h (repeated twice).
To obtain a concentrated filtrate, traces of the solvent were removed using a rotating evaporator (HEI-VAP Core, HEIDOLPH) after filtering through a filter paper. The crystals were then crushed again. The dried marc (residue) was then extracted again under the same conditions but with different solvents (ethyl acetate and ethanol).
Three raw organic extracts were obtained from this extraction series: chloroform extract (Ex Chl), ethyl acetate extract (Ex AcEt), and ethanol extract (Ex EtOH). They were recovered in sterile glass vials, securely sealed, and refrigerated at 4 °C until use.
Using the following formula, the extraction yield (EY) was calculated in relation to the dry matter weight
4.3. GC–MS Analysis of the Chemical Content of Snail Powder Extracts
The H. aspersa Müller flesh extracts were analyzed by gas chromatography–mass spectrometry under the following conditions: the injector port temperature was 250 °C. The starting oven temperature was 40 °C, and the temperature was gradually increased at 8 °C min–1 for 18 min until it reached 260 °C. The BR-5 ns FS capillary column (30 m × 0.25 mm ID × 0.25 m) was utilized. In the undivided mode, the injection volume of helium was 1.0 mL min–1. The whole analysis took 105 min. The mass spectrometry detector (MSD) was set to electronic impact ionization mode, with an ionizing energy of 70 eV and an m/z scan range of 50 to 500. The temperature of the ion source was 230 °C, and it then quadrupled to 150 °C. The electron multiplier voltage (EM voltage) was kept at 1100 V above the self-regulatory level, with a 3 min solvent delay.38
4.4. Quantification of Bioactive Contents
Protein and carbohydrate contents were determined using the Deepachandi method39 and the Nielsen method,40 respectively, with the reagents Folin–Ciocalteu, sulfuric acid, and phenol.
In contrast, reducing sugars are quantified using the Negrulescu method,41 which uses dinitro-salicylic acid as a reagent. The Zargoosh et al.42 methods were modified to determine phenolic compounds; the reduction of the Folin–Ciocalteu reagent during polyphenol oxidation is the basis for these analytical methods. The researchers modified the methods described in the paper by Zirari et al.43 to measure the amount of flavonoid compounds in their samples.
4.5. Antioxidant Activity
The antioxidant activity of crude extracts from H. aspersa Müller was measured by looking at total antioxidant activity, DPPH radical scavenging activity, and total reducing power, using the protocols described in our previous study.44
4.6. Antibacterial Assays
4.6.1. Bacterial Strains
We determined the antibacterial properties of the crude extract of H. aspersa Müller flesh against the bacterium P. aeruginosa, which was obtained from the Plant, Animal and Agro-Industry Productions Laboratory (PAAP lab) at Ibn Tofail University. We also used other reference strains of bacteria in our study, including L. monocytogenes (ATCC 7644), S. aureus (ATCC 29213), and E. coli (ATCC 35218). To prepare the bacteria for testing, 1 mL of the stored bacterial suspension was mixed with 2 mL of nutrient broth. This broth provides the bacteria with the nutrients they need to grow and multiply.
4.6.2. Antibiotics
The antibiotics used were ampicillin (AMP) (10 μg), cefuroxime sodium (CUS) (30 μg), cefotaxime sodium (COF) (30 μg), erythromycin (ETM) (25 μg), netilmicin (NM) (30 μg), piperacillin (PRP) (100 μg), and tetracycline (TC) (30 μg).
4.6.3. Disc Diffusion Method
The Kirby–Bauer disk diffusion technique was used in this investigation to assess the antibacterial activity of H. aspersa Müller flesh extracts.45 Whatman N°3 paper disks (6 mm) were sanitized, sealed in sterile glass vials, and boiled for 30 min to eliminate any chemicals that would limit microbial growth in order to measure this activity. Then, different amounts of H. aspersa Müller flesh extracts were applied to each disk, and antibiotic disks were positioned on the MH medium’s surface after being preinoculated by swabbing with bacterial suspensions (108 cfu). The infected Petri plates were then incubated at 37 °C in the dark. The inhibitory zone diameter was evaluated 24 h after incubation. For employing the latter, the bacteria have been gathered together.46
The results were interpreted as follows:
Resistant: diameter ≤8 mm;
Moderately sensitive: diameter between 9 and 14 mm;
Sensitive: diameter between 15 and 19 mm;
Extremely sensitive: diameter >20 mm.
4.6.4. Determination of the MIC
Using the Wiegand et al.47 method, we calculated the lowest concentration of the snail flesh extract of H. aspersa Müller that inhibited the growth of the chosen bacterial strains. We added 20 mL of fresh bacterial culture (1 × 104 cfu) to different concentrations of the snail flesh extract (10, 20, 40, 60, 80, and 100 mg mL–1). MIC was defined as the lowest concentration of the H. aspersa Müller flesh extract that prevented the formation of turbidity. Dimethyl sulfoxide was used as a negative control.
4.7. Statistical Analysis
The data was subjected to a one-way ANOVA followed by Duncan’s test (α = 5) for multiple comparisons and determination of significance levels using statistical software (SPSS, ver. 20). The average value of three repetitions is presented. Values of p < 0.05 were considered statistically significant.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project (RSP2024R119), King Saud University, Riyadh, Saudi Arabia, for funding this work.
Glossary
Abbreviations
- H. aspersa
Helix aspersa
- GC–MS
gas chromatography–mass spectrometry
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- FRAP
ferric reducing antioxidant power
- TAC
total antioxidant capacity
- cfu
colony forming unit
- ANOVA
analysis of variance
- Ex Chl
chloroform extract
- Ex AcEt
ethyl acetate extract
- Ex EtOH
ethanol extract
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Luo F. L.; Xing R.; Wang X. Q.; Peng Q.; Li P. Proximate composition, amino acid and fatty acid profiles of marine snail Rapana venosa meat, visceral mass and operculum. J. Sci. Food Agric. 2017, 97, 5361–5368. 10.1002/jsfa.8425. [DOI] [PubMed] [Google Scholar]
- Shanmugam M.; Mody K. H. Heparinoid-active sulphated polysaccharides from marine algae as potential blood anticoagulant agents. Curr. Sci. 2000, 79, 1672–1683. [Google Scholar]
- Cheung R. C.; Ng T. B.; Wong J. H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. 10.3390/md13074006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukova N. V. Lipids and fatty acids of nudibranch mollusks: potential sources of bioactive compounds. Mar. Drugs 2014, 12 (8), 4578–4592. 10.3390/md12084578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkendorff K. Molluscan biological and chemical diversity: Secondary metabolites and medicinal resources produced by marine molluscs. Biol. Rev. 2010, 85 (4), 757–775. 10.1111/j.1469-185X.2010.00124.x. [DOI] [PubMed] [Google Scholar]
- Wendel M.; Heller A. R. Anticancer actions of omega-3 fatty acids-current state and future perspectives. Anti-Cancer Agents Med. Chem. 2009, 9 (4), 457–470. 10.2174/1871520610909040457. [DOI] [PubMed] [Google Scholar]
- Blunt J. W.; Copp B. R.; Hu W. P.; Munro M. H. G.; Northcote P. T.; Prinsep M. R. Marine natural products. Nat. Prod. Rep. 2007, 24, 31. 10.1039/B603047P. [DOI] [PubMed] [Google Scholar]
- Tincu J. A.; Taylor S. W. Antimicrobial peptides from marine invertebrates. Antimicrob. Agents Chemother. 2004, 48, 3645–3654. 10.1128/aac.48.10.3645-3654.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagash Y. S.; Nazeer R. A.; Kumar N. S. In vitro antioxidant activity of solvent extracts of molluscs (Loligo duvauceli and Donax strateus) from India. World J. Fish Mar. Sci. 2010, 2, 240–245. 10.3233/s12349-011-0088-1. [DOI] [Google Scholar]
- Olivera B. M. Conus peptides: biodiversity-based discovery and exogenomics. J. Biol. Chem. 2006, 281 (42), 31173–31177. 10.1074/jbc.R600020200. [DOI] [PubMed] [Google Scholar]
- Matusiewicz M.; Kosieradzka I.; Niemiec T.; Grodzik M.; Antushevich H.; Strojny B.; Gołębiewska M. In Vitro Influence of Extracts from Snail Helix aspersa Muller on the Colon Cancer Cell Line Caco-2. Int. J. Mol. Sci. 2018, 19, 1064. 10.3390/ijms19041064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilla A.; LaJeunesse C.; McMaster D.; Morgan D.. Feasibility of Snail Farming as a Model for Small Urban Farms to Expand into Niche Markets for Increased Profitability; Worcester Polytechnic Institute: Worcester, MA, USA, 2016. [Google Scholar]
- Kulawik P.; Özogul F.; Glew R.; Özogul Y. Significance of antioxidants for seafood safety and human health. J. Agric. Food Chem. 2013, 61 (3), 475–491. 10.1021/jf304266s. [DOI] [PubMed] [Google Scholar]
- Arias C. A.; Murray B. E. Antibiotic-Resistant Bugs in the 21st Century-A Clinical Super-Challenge. N. Engl. J. Med. 2009, 360, 439–443. 10.1056/NEJMp0804651. [DOI] [PubMed] [Google Scholar]
- Anand T. P.; Edward J. K. P. Antimicrobial activity in the tissue extracts of five species of cowries cypraea spp. (Mollusca: Gastropoda) and an ascidian didemnum psammathodes (Tunicata: Didemnidae). Ind. J. Mar. Sci. 2002, 31, 239–242. [Google Scholar]
- Szkucik K.; Ziomek M.; Paszkiewicz W.; Drozd Ł.; Gondek M.; Knysz P. Fatty acid profile in fat obtained from edible part of land snails harvested in Poland. J. Vet. Res. 2018, 62, 519–526. 10.2478/jvetres-2018-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Rochow V. B. Therapeutic arthropods and other, largely terrestrial, folk-medicinally important invertebrates: a comparative survey and review. J. Ethnobiol. Ethnomed. 2017, 13 (1), 9–31. 10.1186/s13002-017-0136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Birch J.; Pei J.; Mohamed Ahmed I. A.; Yang H.; Dias G.; Abd El-Aty A. M.; Bekhit A. E. D. Identification of six phytochemical compounds from Asparagus officinalis L. root cultivars from New Zealand and China using UAE-SPE-UPLC-MS/MS: effects of extracts on H2O2-induced oxidative stress. Nutrients 2019, 11, 107–117. 10.3390/nu11010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D. G.; Lee H. S. An improved method in screening of superoxide and hydroxyl radical scavenging activities of plant medicinal extracts. Korean J. Pharmacog. 2001, 32, 253–256. [Google Scholar]
- Rafińska K.; Pomastowski P.; Rudnicka J.; Krakowska A.; Maruśka A.; Narkute M.; Buszewski B. Effect of solvent and extraction technique on composition and biological activity of Lepidium sativum extracts. Food Chem. 2019, 289, 16–25. 10.1016/j.foodchem.2019.03.025. [DOI] [PubMed] [Google Scholar]
- USDA Agricultural Research Service . 1992–2016 Dr. Duke’s Phytochemical and Ethnobotanical Databases, 2016. [Google Scholar]
- Gomot A. Biochemical composition of Helix snails: influence of genetic and physiological factors. J. Molluscan Stud. 1998, 64 (2), 173–181. 10.1093/mollus/64.2.173. [DOI] [Google Scholar]
- Özogul Y.; Özogul F.; Olgunoglu A. I. Fatty acid profile and mineral content of the wild snail (Helix pomatia) from the region of the south of the Turkey. Eur. Food Res. Technol. 2005, 221 (3–4), 547–549. 10.1007/s00217-005-1191-7. [DOI] [Google Scholar]
- Ghedadba N.; Bousselsela H.; Hambaba L.; Benbia S.; Mouloud Y. Evaluation of the antioxidant and antimicrobial activities of the leaves and flowered tops of Marrubium vulgare L. Phytothérapie 2014, 12 (1), 15–24. 10.1007/s10298-014-0832-z. [DOI] [Google Scholar]
- Kouachi M.; Naimi D.; Djadouri D.; Bendahra I. Preventive effect of Helix aspersa crude extract against chemo-induced colonic damages in rats. Int. J. Pharma Sci. Res. 2017, 8 (6), 2458–2468. 10.13040/IJPSR.0975-8232.8(6).2458-68. [DOI] [Google Scholar]
- Gogas A.; Laliotis G. P.; Ladoukakis E.; Trachana V. Chemical composition and antioxidant profile of snails (<i>Cornu aspersum aspersum</i>) fed diets with different protein sources under intensive rearing conditions. J. Anim. Feed Sci. 2021, 30 (4), 391–398. 10.22358/jafs/143107/2021. [DOI] [Google Scholar]
- Chekroun-Bechlaghem N.; Belyagoubi-Benhammou N.; Belyagoubi L.; Gismondi A.; Nanni V.; Di Marco G.; Canuti L.; Canini A.; El Haci I. A.; Atik Bekkara F. Phytochemical analysis and antioxidant activity of Tamarix africana, Arthrocnemum macrostachyum and Suaeda fruticosa, three halophyte species from Algeria. Plant Biosyst. 2019, 153 (6), 843–852. 10.1080/11263504.2018.1555191. [DOI] [Google Scholar]
- Borquaye L. S.; Darko G.; Oklu N.; Anson-Yevu C.; Ababio A. Antimicrobial and antioxidant activities of ethyl acetate and methanol extracts of Littorina littorea and Galatea paradoxa. Cogent Chem. 2016, 2 (1), 1161865. 10.1080/23312009.2016.1161865. [DOI] [Google Scholar]
- Subhapradha N.; Ramasamy P.; Sudharsan S.; Seedevi P.; Moovendhan M.; Srinivasan A.; Shanmugam V.; Shanmugam A. Preparation of phosphorylated chitosan from gladius of the squid Sepioteuthis lessoniana (Lesson, 1830) and it’s in vitro antioxidant activity. Bioact. Carbohydr. Diet. Fibre 2013, 1, 148–155. 10.1016/j.bcdf.2013.03.001. [DOI] [Google Scholar]
- Nazeer R. A.; Naqash S. Y. In vitro antioxidant activity of two molluscs, Loligo duvauceli Orbigny and Donax cuneatus Linnaeus, by solvent extraction methods. Mediterr. J. Nutr. Metabol. 2013, 6, 17–21. 10.1007/s12349-011-0088-1. [DOI] [Google Scholar]
- Subavathy P.; Janet S. M. B. Molecular characterization of protein and antioxidant capacity of Turbo brunneus R. Cypraea annulus L. and Babylonia spirata L. Int. J. Pharm. Sci. Res. 2020, 11, 3285–3293. 10.13040/IJPSR.0975-8232.11(7).3285-93. [DOI] [Google Scholar]
- Aouji M.; Rkhaila A.; Bouhaddioui B.; Zirari M.; Harifi H.; Taboz Y.; Lrhorfi L. A.; Bengueddour R. Chemical composition, mineral profile, anti-bacterial, and wound healing properties of snail slime of Helix aspersa Müller. Biomed 2023, 13 (4), 10–19. 10.37796/2211-8039.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More K.; Tayade S.; Gawande P.; Manik S.; Shelke D. Antioxidant and antimicrobial potential of Canavalia gladiata (Jacq.) DC. leaves and seeds: GC-MS based metabolic profiling. Ind. J. Nat. Prod. Res. 2022, 13 (2), 163–169. 10.56042/ijnpr.v13i2.47499. [DOI] [Google Scholar]
- Rota M. C.; Herrera A.; Martínez R. M.; Sotomayor J. A.; Jordán M. J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19 (7), 681–687. 10.1016/j.foodcont.2007.07.007. [DOI] [Google Scholar]
- Helal G. A.; Sarhan M. M.; Abu Shahla A. N. K.; Abou El-Khair E. K. Effects of Cymbopogon Citratus L. Essential Oil on the Growth, Lipid Content and Morphogenesis of Aspergillus Niger ML2-Strain. J. Basic Microbiol. 2006, 46, 456–469. 10.1002/jobm.200510106. [DOI] [PubMed] [Google Scholar]
- Prashar A.; Hili P.; Veness R. G.; Evans C. S. Antimicrobial Action of Palmarosa Oil (Cymbopogon Martinii) on Saccharomyces Cerevisiae. Phytochemistry 2003, 63, 569–575. 10.1016/S0031-9422(03)00226-7. [DOI] [PubMed] [Google Scholar]
- Ulagesan S.; Kim H. J. Antibacterial and antifungal activities of proteins extracted from seven different snails. Appl. Sci. 2018, 8, 1362. 10.3390/app8081362. [DOI] [Google Scholar]
- Aouji M.; Imtara H.; Rkhaila A.; Bouhaddioui B.; Alahdab A.; Parvez M. K.; Saleh Alzahrani M.; Aicha Lrhorfi L.; Bengueddour R. Nutritional Composition, Fatty Acids Profile, Mineral Content, Antioxidant Activity and Acute Toxicity of the Flesh of Helix aspesra Müller. Molecules 2023, 28, 6323. 10.3390/molecules28176323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepachandi B.; Weerasinghe S.; Andrahennadi T. P.; Karunaweera N. D.; Wickramarachchi N.; Soysa P.; Siriwardana Y. Quantification of Soluble or Insoluble Fractions of Leishmania Parasite Proteins in Microvolume Applications: A Simplification to Standard Lowry Assay. Int. J. Anal. Chem. 2020, 2020, 1–8. 10.1155/2020/6129132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S. S.Phenol-Sulfuric Acid Method for Total Carbohydrates. In Food Analysis Laboratory Manual; Nielsen S. S., Ed.; Springer: Boston, 2010; pp 47–53. [Google Scholar]
- Negrulescu A.; Patrulea V.; Mincea M. M.; Ionascu C.; Vlad-Oros B. A.; Ostafe V. Adapting the reducing sugars method with dinitrosalicylic acid to microtiter plates and microwave heating. J. Braz. Chem. Soc. 2012, 23, 2176–2182. 10.1590/S0103-50532013005000003. [DOI] [Google Scholar]
- Zargoosh Z.; Ghavam M.; Bacchetta G.; Tavili A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci. Rep. 2019, 9, 16021. 10.1038/s41598-019-52605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirari M.; Aouji M.; Zouarhi̇ M.; Dermaj A.; Erramli̇ H.; Hmouni̇ D.; El Mejdoub N. Antioxidant activity, phytochemical screening and GC-MS profile of Abies marocana Trab. Int. J. Sec. Metab. 2024, 11 (1), 121–133. 10.21448/ijsm.1372709. [DOI] [Google Scholar]
- Aouji M.; Rkhaila A.; Bouhaddioui B.; Khalid G.; Lrhorfi L. A.; Bengueddour R. Antioxidant activity, biochemical composition and physicochemical properties of Helix aspersa Müller snail slime. Int. J. Chem. Bioch. Sci. 2023, 23 (3), 53–62. [Google Scholar]
- Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 1–23. [Google Scholar]
- EL Moussaoui A.; Bourhia M.; Jawhari F. Z.; Salamatullah A. M.; Ullah R.; Bari A.; Majid Mahmood H.; Sohaib M.; Serhii B.; Rozhenko A.; Aboul-Soud M. A. M.; Ezzeldin E.; Mostafa G. A. E.; Bousta D.; Bari A. Chemical Profiling, Antioxidant, and Antimicrobial Activity against Drug-Resistant Microbes of Essential Oil from Withania frutescens L. Appl. Sci. 2021, 11, 5168. 10.3390/app11115168. [DOI] [Google Scholar]
- Wiegand I.; Hilpert K.; Hancock R. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]