Abstract
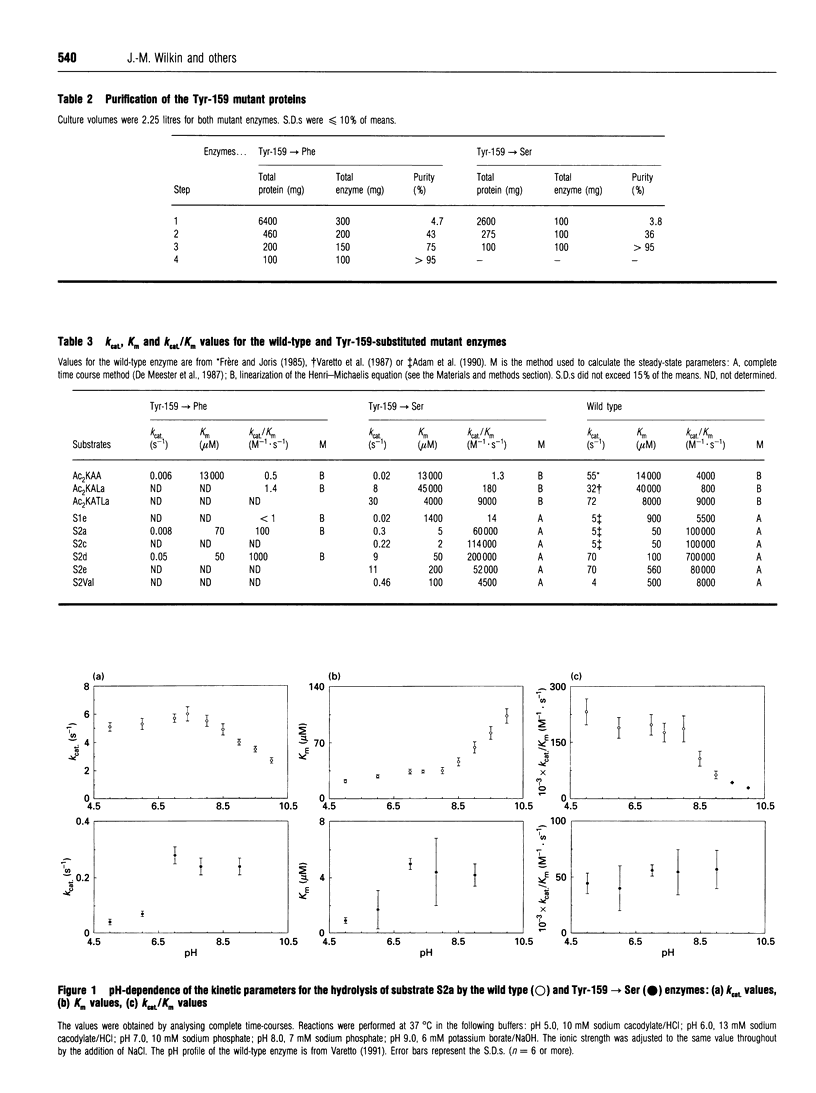
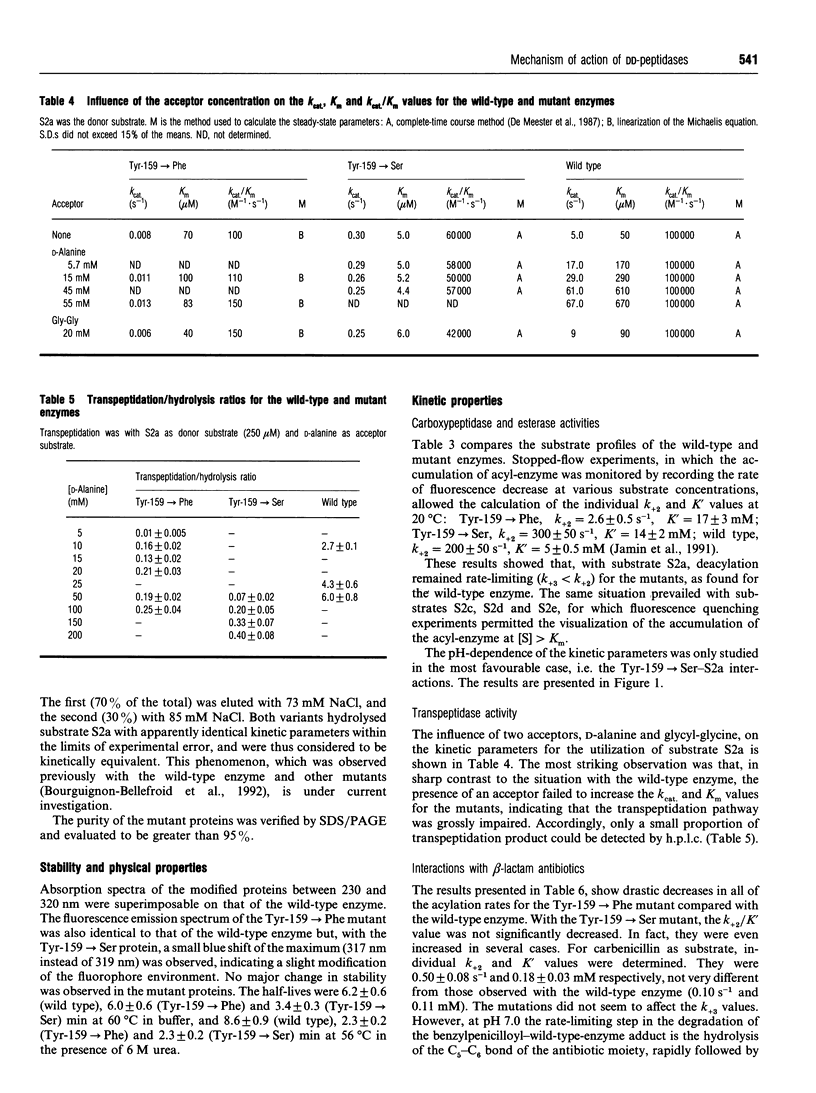
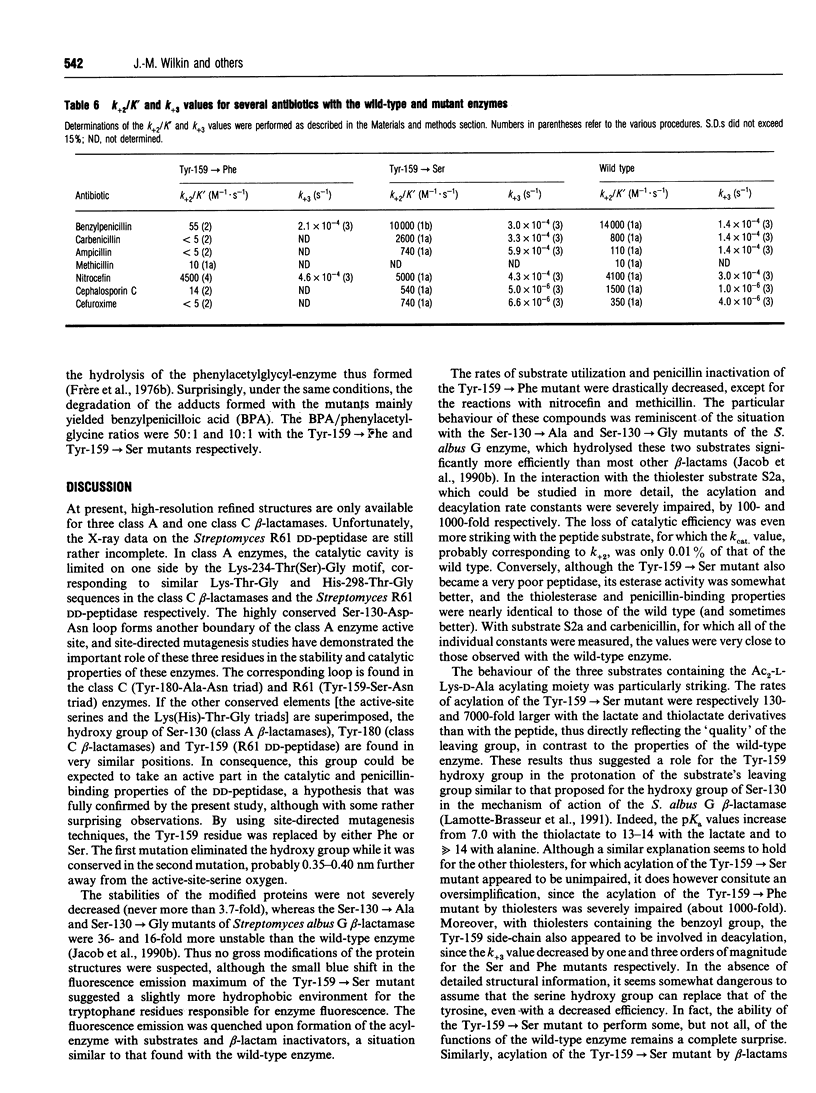
Tyrosine-159 of the Streptomyces R61 penicillin-sensitive DD-peptidase was replaced by serine or phenylalanine. The second mutation yielded a very poorly active protein whose rate of penicillin binding was also drastically decreased, except for the reactions with nitrocefin and methicillin. The consequences of the first mutation were more surprising, since a large proportion of the thiolesterase activity was retained, together with the penicillin-binding capacity. Conversely, the peptidase properties was severely affected. In both cases, a drastic decrease in the transferase activity was observed. The results are compared with those obtained by mutation of the corresponding residue in the class A beta-lactamase of Streptomyces albus G.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam M., Damblon C., Jamin M., Zorzi W., Dusart V., Galleni M., el Kharroubi A., Piras G., Spratt B. G., Keck W. Acyltransferase activities of the high-molecular-mass essential penicillin-binding proteins. Biochem J. 1991 Oct 15;279(Pt 2):601–604. doi: 10.1042/bj2790601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam M., Damblon C., Plaitin B., Christiaens L., Frère J. M. Chromogenic depsipeptide substrates for beta-lactamases and penicillin-sensitive DD-peptidases. Biochem J. 1990 Sep 1;270(2):525–529. doi: 10.1042/bj2700525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon-Bellefroid C., Wilkin J. M., Joris B., Aplin R. T., Houssier C., Prendergast F. G., Van Beeumen J., Ghuysen J. M., Frère J. M. Importance of the two tryptophan residues in the Streptomyces R61 exocellular DD-peptidase. Biochem J. 1992 Mar 1;282(Pt 2):361–367. doi: 10.1042/bj2820361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H., Martin M. T., Waley S. G. Beta-lactamases as fully efficient enzymes. Determination of all the rate constants in the acyl-enzyme mechanism. Biochem J. 1990 Mar 15;266(3):853–861. [PMC free article] [PubMed] [Google Scholar]
- De Meester F., Joris B., Reckinger G., Bellefroid-Bourguignon C., Frère J. M., Waley S. G. Automated analysis of enzyme inactivation phenomena. Application to beta-lactamases and DD-peptidases. Biochem Pharmacol. 1987 Jul 15;36(14):2393–2403. doi: 10.1016/0006-2952(87)90609-5. [DOI] [PubMed] [Google Scholar]
- Duez C., Piron-Fraipont C., Joris B., Dusart J., Urdea M. S., Martial J. A., Frère J. M., Ghuysen J. M. Primary structure of the Streptomyces R61 extracellular DD-peptidase. 1. Cloning into Streptomyces lividans and nucleotide sequence of the gene. Eur J Biochem. 1987 Feb 2;162(3):509–518. doi: 10.1111/j.1432-1033.1987.tb10669.x. [DOI] [PubMed] [Google Scholar]
- Frere J., Ghuysen J., Vanderhaeghe H., Adriaens P., Degelaen J., De Graeve J. Fate of thiazolidine ring during fragmentation of penicillin by exocellular DD-carboxypeptidase-transpeptidase of Streptomyces R61. Nature. 1976 Apr 1;260(5550):451–454. doi: 10.1038/260451a0. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Duez C., Ghuysen J. M., Vandekerkhove J. Occurrence of a serine residue in the penicillin-binding site of the exocellular DD-carboxy-peptidase-transpeptidase from Streptomyces R61. FEBS Lett. 1976 Nov;70(1):257–260. doi: 10.1016/0014-5793(76)80770-3. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Ghuysen J. M., Perkins H. R. Interaction between the exocellular DD-carboxypeptidase-transpeptidase from Streptomyces R61, substrate and beta-lactam antibiotics. A choice of models. Eur J Biochem. 1975 Sep 15;57(2):353–359. doi: 10.1111/j.1432-1033.1975.tb02308.x. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Ghuysen J. M., Perkins H. R., Nieto M. Kinetics of concomitant transfer and hydrolysis reactions catalysed by the exocellular DD-carboxypeptidase-transpeptidase of streptomyces R61. Biochem J. 1973 Nov;135(3):483–492. doi: 10.1042/bj1350483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frère J. M., Joris B. Penicillin-sensitive enzymes in peptidoglycan biosynthesis. Crit Rev Microbiol. 1985;11(4):299–396. doi: 10.3109/10408418409105906. [DOI] [PubMed] [Google Scholar]
- Ghuysen J. M. Serine beta-lactamases and penicillin-binding proteins. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- Hadonou A. M., Jamin M., Adam M., Joris B., Dusart J., Ghuysen J. M., Frère J. M. Importance of the His-298 residue in the catalytic mechanism of the Streptomyces R61 extracellular DD-peptidase. Biochem J. 1992 Mar 1;282(Pt 2):495–500. doi: 10.1042/bj2820495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F., Joris B., Dideberg O., Dusart J., Ghuysen J. M., Frère J. M. Engineering a novel beta-lactamase by a single point mutation. Protein Eng. 1990 Oct;4(1):79–86. doi: 10.1093/protein/4.1.79. [DOI] [PubMed] [Google Scholar]
- Jacob F., Joris B., Lepage S., Dusart J., Frère J. M. Role of the conserved amino acids of the 'SDN' loop (Ser130, Asp131 and Asn132) in a class A beta-lactamase studied by site-directed mutagenesis. Biochem J. 1990 Oct 15;271(2):399–406. doi: 10.1042/bj2710399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamin M., Adam M., Damblon C., Christiaens L., Frère J. M. Accumulation of acyl-enzyme in DD-peptidase-catalysed reactions with analogues of peptide substrates. Biochem J. 1991 Dec 1;280(Pt 2):499–506. doi: 10.1042/bj2800499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris B., Ghuysen J. M., Dive G., Renard A., Dideberg O., Charlier P., Frère J. M., Kelly J. A., Boyington J. C., Moews P. C. The active-site-serine penicillin-recognizing enzymes as members of the Streptomyces R61 DD-peptidase family. Biochem J. 1988 Mar 1;250(2):313–324. doi: 10.1042/bj2500313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris B., Ledent P., Dideberg O., Fonzé E., Lamotte-Brasseur J., Kelly J. A., Ghuysen J. M., Frère J. M. Comparison of the sequences of class A beta-lactamases and of the secondary structure elements of penicillin-recognizing proteins. Antimicrob Agents Chemother. 1991 Nov;35(11):2294–2301. doi: 10.1128/aac.35.11.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. A., Dideberg O., Charlier P., Wery J. P., Libert M., Moews P. C., Knox J. R., Duez C., Fraipont C., Joris B. On the origin of bacterial resistance to penicillin: comparison of a beta-lactamase and a penicillin target. Science. 1986 Mar 21;231(4744):1429–1431. doi: 10.1126/science.3082007. [DOI] [PubMed] [Google Scholar]
- Kelly J. A., Waley S. G., Adam M., Frère J. M. Crystalline enzyme kinetics: activity of the Streptomyces R61 D-alanyl-D-alanine peptidase. Biochim Biophys Acta. 1992 Mar 12;1119(3):256–260. doi: 10.1016/0167-4838(92)90211-u. [DOI] [PubMed] [Google Scholar]
- Lamotte-Brasseur J., Dive G., Dideberg O., Charlier P., Frère J. M., Ghuysen J. M. Mechanism of acyl transfer by the class A serine beta-lactamase of Streptomyces albus G. Biochem J. 1991 Oct 1;279(Pt 1):213–221. doi: 10.1042/bj2790213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra K. T., Nicholas R. A. Substitution of lysine 213 with arginine in penicillin-binding protein 5 of Escherichia coli abolishes D-alanine carboxypeptidase activity without affecting penicillin binding. J Biol Chem. 1992 Jun 5;267(16):11386–11391. [PubMed] [Google Scholar]
- Nieto M., Perkins H. R., Frère J. M., Ghuysen J. M. Fluorescence and circular dichroism studies on the Streptomyces R61 DD-carboxypeptidase-transpeptidase. Penicillin binding by the enzyme. Biochem J. 1973 Nov;135(3):493–505. doi: 10.1042/bj1350493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oefner C., D'Arcy A., Daly J. J., Gubernator K., Charnas R. L., Heinze I., Hubschwerlen C., Winkler F. K. Refined crystal structure of beta-lactamase from Citrobacter freundii indicates a mechanism for beta-lactam hydrolysis. Nature. 1990 Jan 18;343(6255):284–288. doi: 10.1038/343284a0. [DOI] [PubMed] [Google Scholar]
- Samraoui B., Sutton B. J., Todd R. J., Artymiuk P. J., Waley S. G., Phillips D. C. Tertiary structural similarity between a class A beta-lactamase and a penicillin-sensitive D-alanyl carboxypeptidase-transpeptidase. 1986 Mar 27-Apr 2Nature. 320(6060):378–380. doi: 10.1038/320378a0. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varetto L., Frère J. M., Nguyen-Distèche M., Ghuysen J. M., Houssier C. The pH dependence of the active-site serine DD-peptidase of Streptomyces R61. Eur J Biochem. 1987 Feb 2;162(3):525–531. doi: 10.1111/j.1432-1033.1987.tb10671.x. [DOI] [PubMed] [Google Scholar]


