Abstract
Colorectal cancer (CRC) is highly prevalent and is a major cause of cancer-related deaths worldwide. The incidence rate of CRC remains alarmingly high despite screening measures. The main curative treatment for CRC is a surgical resection of the diseased bowel segment. Postoperative complications usually involve a weakened gut barrier and a dissemination of bacterial proinflammatory lipopolysaccharides. Herein we discuss how gut microbiota and microbial metabolites regulate basal inflammation levels in the gut and the healing process of the bowel after surgery. We further elaborate on the restoration of the gut barrier function in patients with CRC and how this potentially impacts the dissemination and implantation of CRC cells in extracolonic tissues, contributing therefore to worse survival after surgery.
Keywords: colorectal cancer, gut microbiome, gut barrier, colorectal surgery, anastomotic leak
INTRODUCTION
Colorectal cancer (CRC) is among the most prevalent and deadliest cancers worldwide [1, 2]. Screening methods like fecal immunochemical tests (FIT) and colonoscopies allow for the early detection and excision of premalignant tumors – polyps – but the incidence of this cancer remains alarmingly high despite such measures [1–4].
The cornerstone of CRC treatment is surgery, in which the diseased segment of the bowel is resected and the remaining bowel ends are reconnected with an anastomosis. What used to be initially an invasive abdominal procedure has evolved into a minimally invasive operation in most patients using techniques like laparoscopy and robotic surgery [5]. Coupled with “enhanced recovery after surgery” (ERAS) protocols, these novel surgical techniques have decreased postoperative complications and allowed patients to recover more quickly after surgery [6, 7]. Nevertheless, improved surgical technique and perioperative care have eradicated neither postoperative complications nor cancer recurrence.
A surgical break of the bowel wall disrupts the gut barrier which undergoes repair in the days and weeks following surgery. This repair process is paramount to the prevention of postoperative infectious complications like anastomotic leak (AL) [8]. We and others have shown in the last few years that gut microbiota influences the healing process of the bowel and the restoration of the gut barrier after surgery [8–10]. Based on our recent work, we believe that weakened gut barrier function, namely due to poor healing after surgery, leads to persistent systemic low-grade inflammation and a higher risk of local and systemic cancer recurrence [8, 9, 11]. Improvement of the gut barrier function in patients with CRC is under the control of both macroscopic and microscopic factors.
MACROSCOPIC FACTORS
The most severe form of a disrupted gut barrier is an overt leak of fecal material from the bowel, which usually occurs at the surgery site and specifically the anastomosis site. Colorectal AL is a major and unpredictable complication. It is expected to occur in approximately 3 to 19% of patients undergoing colorectal resection [12, 13]. This wide range depends on many factors, including the type of surgery being performed, technical factors and patient comorbidities [13–15]. These risk factors may be influenced by the gut microbiota to various degrees [10].
Macroscopically, there is consensus among surgeons that a gastrointestinal anastomosis should be free of tension and torsion [16, 17]. This premise could not be validated by epidemiological human studies due to ethical reasons, as an anastomosis cannot be mechanically left under tension or torsion after surgery. Nonetheless, it is logical to assume that mechanical stress could lead to anastomotic disruption simply by inducing rupture of the sutures or staple lines. It is common practice however to verify these mechanical aspects in the operating room, making their impact on the pathogenesis of AL probably low.
MICROSCOPIC FACTORS
Epithelial proliferation
The inner layer of the colonic wall is in contact with the microbial communities in the lumen. It is estimated that 70% of the bacteria in the body reside in the colon and rectum [18]. It is therefore safe to assume that the healing process after an invasive surgical procedure would be influenced by the abundant gut microbiota in the bowel lumen and would be dependent on the pathogenic potential and biological functions of the bacteria involved [8, 9].
The healing of the epithelial layer is a frequently assessed parameter in animal models of bowel injury [19, 20]. Epithelial healing is usually studied in conditions where the epithelial lining is the most affected, as is the case for inflammatory bowel disease (IBD) and other inflammatory conditions. In a surgical context, even if the epithelial layer is not what provides tensile strength to the anastomosis, it constitutes an important barrier against gut pathogens, and its sealing may be expected to protect the deeper submucosal matrix against deleterious inflammatory stimuli [21].
In germ-free mice, the inoculation with a complex microbiota induces maturation of the epithelium along with increased proliferation [22]. In our previous work, we have shown for the first time that healing after colonic surgery is severely impaired in germ-free mice [8]. Epithelial regeneration requires nutritional substrates, and more specifically microbiota-derived substrates. Short-chain fatty acids (SCFAs) are among the major nutritional substrates for the gut epithelial lining [23]. They are known to promote epithelial integrity and gut barrier function [24, 25]. SCFAs are bacterial metabolites that are produced by colonic bacteria upon the fermentation of dietary fibers [26]. These fibers escape digestion in the small bowel and are therefore metabolized mostly by bacteria in the cecum and the proximal colon, a process that culminates in the production of SCFAs [27]. Butyrate, propionate and acetate are the main SCFAs produced in the large bowel [26].
Among these metabolites, butyrate is a major energy source for epithelial cells [28]. It promotes the proliferation of colonocytes and is expected to contribute therefore to the regeneration of the injured mucosa [23, 29]. In addition to promoting epithelial proliferation, butyrate was shown to enhance the gut barrier function by promoting the development of interepithelial tight junctions [30, 31]. The reinforcement of the gut barrier function by butyrate was also mediated by an enhancement of the mucus layer, specifically by an upregulation of MUC-2, the major mucin produced by epithelial cells [32]. These beneficial properties protect against systemic invasion by luminal pathogens.
This key bacterial metabolite is therefore expected to be of potential interest in colorectal surgery. The effects of butyrate have been assessed in the context of colonic anastomotic healing in murine models [33–35]. These experiments suggested that this SCFA improves anastomotic healing and the tensile strength of the anastomosis, but the findings were inconsistent [33–35]. From a practical perspective, oral administration of butyrate after surgery would expose the gut to this metabolite for only a short period of time, without considering the significant distance that separates the mouth from the colon and rectum. Another option would be intrarectal administration, but this approach might not be well received by the surgical community, as enemas may pose an undesirable mechanical stress on a fresh and fragile colorectal anastomosis. The alternative in this case would be to promote the production of this metabolite by the gut microbiota to consistently increase its luminal levels in the gut, in addition to ensuring a continuous mucosal exposure.
We previously conducted animal experiments in which we exploited the benefits of butyrate and other SCFAs in the healing of colonic anastomoses in mice [21]. Due to the limitations of direct administration of butyrate, our strategy was to modulate the colonic microbiota toward a butyrogenic profile that produces higher levels of SCFAs. The approach we tested was a supplementation with fermentable fibers, specifically oligosaccharides. Mice therefore received dietary supplementation with inulin or galacto-oligosaccharides (GOS) for 2 weeks before undergoing a colonic anastomosis under general anesthesia. The supplementation continued after surgery until sacrifice on postoperative day (POD) 6. Mice supplemented with inulin and GOS displayed increased levels of SCFAs in the gut and improved macroscopic healing of the anastomosis [21]. With respect to what was mentioned above, our experiment showed that the increased levels of butyrate, propionate and acetate coincided with an improved microscopic healing of the anastomosis. When assessed by a pathologist blinded to the intervention group, the wound site in mice supplemented with oligosaccharides was found to exhibit higher reepithelization and continuity of the mucosal layer. Since butyrate is known to promote epithelial proliferation, we assessed this parameter by quantifying the Ki-67 proliferation marker at the wound site by immunohistochemistry. Interestingly, this marker was found to be increased in mice supplemented with the inulin prebiotic. These findings support the role of these SCFAs, and of the microbiota, in the healing of the colonic anastomosis and restoration of the epithelial layer and therefore of the gut barrier after a surgical injury.
Considering the above, it is important to assess the effect of microbiota-mediated epithelial proliferation in the specific context of cancer. Patients with CRC constitute a large proportion of patients undergoing colorectal resections with anastomosis. It is therefore important to ensure that higher bacterial-derived butyrate levels will not increase the proliferation of cancer cells and promote cancer progression. This consideration is valid, but the short period of supplementation with such oligosaccharides will most probably not affect cancer progression and the overall oncological prognosis. Most importantly, while butyrate is known to promote the proliferation of normal colonocytes, it is known to inhibit that of cancer cells, a process that is best known as the “butyrate paradox” [36]. We have previously published a review article on this specific and yet complex question [23]. Briefly, in physiological circumstances, colonocytes metabolize butyrate via mitochondrial oxidation [23, 37]. In neoplastic cells, specifically colorectal adenocarcinoma cells, the cellular metabolism shifts toward aerobic glycolysis, which induces an accumulation of unused butyrate [23, 36, 38]. Butyrate exerts a histone deacetylase (HDAC) inhibitor function, which prevents the modulation of chromatin toward a pro-carcinogenic profile by inhibiting proto-oncogenes and activating tumor suppressor genes [23, 39–41]. In short, in patients with CRC, targeting the microbiota to improve epithelial proliferation through SCFAs may be beneficial not only against anastomotic complications but also against cancer progression.
Submucosal recovery
The submucosa is believed to confer tensile strength and solidity to the anastomosis, as it harbors a high concentration of connective tissue and collagen [14, 21]. It may be considered therefore as the cornerstone of anastomotic healing. This layer is not frequently assessed in injury models as these usually concentrate on mucosal injuries and do not routinely involve a radical transection of the colonic wall. The overwhelming body of evidence on the relation between the gut microbiota and anastomotic leak focuses on the preservation of collagen content at the anastomotic site.
Many bacterial species have been shown to activate colonic collagenases. Among these, the most famous one is Enterococcus faecalis [42]. E. faecalis is a Gram-positive commensal that is often incriminated in cases of nosocomial infections, including urinary tract infections, wound infections, endocarditis and bacteremia [43]. The team of Dr. John Alverdy at the University of Chicago has shown that some strains of E. faecalis have the capacity to activate matrix metalloproteinase 9 (MMP-9), a collagenolytic enzyme, which induces a degradation of anastomotic collagen and AL [42, 44]. Other bacterial species have been shown by the same team to activate intestinal collagenases as well, contributing to the pathophysiology of AL. Among these, Pseudomonas aeruginosa, Serratia marcescens and Bacillus subtilis were shown to induce a degradation of the extracellular matrix at the anastomotic site, thus preventing proper wound healing [45, 46]. Collagenase-producing bacteria are not present in all patients with anastomotic complications [47], suggesting that the healing process and the implication of the microbiota are more complex and involve other factors.
In our previous work, we have shown that fecal microbiota transplantation (FMT) using preoperative fecal samples from patients with AL induced poor anastomotic healing in mice when compared to FMT from patients with optimal healing [8, 9]. In the mice transplanted with feces from leaky patients, the anastomosis was shown to harbor lower collagen concentrations, as assessed by the concentration of hydroxyproline. Higher collagenase activity was identified as well in these mice, but the incriminated collagenolytic enzyme was MMP-2 with little to no involvement of MMP-9 [8, 21]. This sheds light on the complexity of the microbiota effect on colonic healing after surgery, and the necessity to determine reliable biomarkers to properly assess the risk of leak before surgery. We specifically showed that strains of Parabacteroides goldsteinii and Alistipes onderdonkii resist the preoperative bowel preparation and directly modulate the inflammatory reaction in the bowel mucosa after surgery, leading sometimes to destructive rather than reparative inflammation [8].
Bacterial metabolites like SCFAs may preserve the collagen layer and strengthen the submucosa after a surgical injury. In our experiment with oligosaccharides [21], mice fed the inulin and GOS-supplemented diets were shown to harbor higher collagen content at the anastomotic site. This was thought to be due to the ability of butyrate to inhibit collagenase expression. Previous work has suggested that butyrate can inhibit matrix metalloproteinases in joint cartilage [48], but this effect was not clearly demonstrated in the gut. Our data showed that butyrate, but also propionate and acetate, were associated with higher levels of hydroxyproline at the anastomosis, and with lower levels of collagenolytic activity at the wound site as well [21].
Ischemia and epithelial oxygenation
The perfusion of colorectal anastomoses is considered a vital factor in the healing process after surgery, as blood supply provides the necessary oxygenation, nutrients and molecules required to maintain tissue viability and to promote repair mechanisms [49, 50]. This led to the implementation in surgical practice of strategies to assess colonic perfusion when this parameter is suspected to be compromised [51, 52]. Suboptimal perfusion of the anastomosis has been further shown to impair the healing process after surgery in mice [53], reinforcing the importance of this factor in the pathogenesis of anastomotic leak in surgical practice.
Perfusion is believed to be directly related to the integrity of the vessels that irrigate the anastomosed bowel segment. This integrity could be due to technical operative factors such as the level of ligation, or to the health of the patient’s vessels, namely in the case of chronic vascular disease [54]. There is no evidence of a potential role of the gut microbiota in these rather mechanical factors. Besides, there is no indication of a potential mechanism by which gut bacteria may influence vascular supply. However, gut bacteria may modulate epithelial oxygenation microscopically [55, 56].
Higher epithelial oxygenation was linked to colonic inflammation, carcinogenesis, and the expansion of deleterious species like Escherichia coli [57]. The maintenance of a hypoxic environment was reported to promote crosstalk between the microbiota and the host, nutrient absorption, and the maintenance of the barrier function [55, 58]. These beneficial effects may be mediated by the hypoxia-induced factor (HIF) [59]. In the context of colonic anastomoses, healing seems to be improved by hyperoxia according to a systematic review, and this improvement was inversely correlated with the abundance of gut anaerobes, which were reported to be increased by hypoxia and associated with poor healing [60]. The findings of these different reports are not in agreement, especially given that anaerobes are reported in other manuscripts as being beneficial for gut homeostasis [61, 62]. This warrants further work on how hypoxia and hyperoxia interact with the microbiota and influence the gut barrier function, and how this balance regulates healing in the specific context of invasive colorectal surgery.
Inflammatory stimuli
The major mechanism that our previous work unveiled is that microbiota-mediated low-grade inflammatory signals directly affect repair mechanisms and the restoration of the gut barrier after surgery [8, 9]. In the wound healing process, there is an initial inflammatory phase characterized by the infiltration of polymorphonuclears (PMNs) at the injury site and the release of pro-inflammatory cytokines and chemokines including tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), IL-6 and monocyte chemoattractant protein 1 (MCP-1) [63]. During this repair process, there is a polarization of macrophages from the pro-inflammatory M1 to an M2 phenotype, which induces an anti-inflammatory response to promote regeneration and healing [63, 64]. During this transition, TNF-α decreases and the production of transforming growth factor beta (TGF-β) increases [65, 66]. This cytokine promotes fibroblastic activity and the accumulation of components of the extracellular matrix at the wound site, including collagen and fibronectin [67, 68]. In this phase, M1 macrophages transition toward an M2 phenotype, which promotes regeneration and remodeling of the wound site [69–71]. When the transition from the inflammatory phase to the proliferation phase is dysregulated, excessive inflammatory signals persist and prevent the wound from healing properly [69, 72]. Impaired healing leads to chronic wounds, which drive chronic inflammation, and which promotes the growth of bacteria at the wound site that become resistant to local bactericidal processes [69, 72, 73]. Bacterial growth at the wound site drives local inflammation, leading to a vicious circle where the continuous production of reactive oxygen species (ROS) and cytokines leads to the production of destructive enzymes and sustained inflammation, which prevents the healing process from evolving [74, 75]. TNF-α is believed to be an important driver of chronic inflammation in chronic non-healing wounds [76], and its suppression with anti-TNF agents was shown to help resume the normal healing process [77, 78].
This dysregulation and polarization toward a pro-inflammatory profile is also a hallmark of chronic intestinal inflammation, namely Crohn’s disease and ulcerative colitis [79–81]. Inflammation is known to correlate with the activation of MMPs, and inflammatory cytokines are believed to be activators of these collagenases [82–84]. Environmental factors that contribute to a pro-inflammatory state are therefore expected to prevent wound healing and to generate a chronic injury in the bowel, therefore weakening the gut barrier.
Gut bacteria are potent regulators of intestinal inflammation. Several species are known to activate the inflammatory cascade while others may harbor anti-inflammatory properties. Among pro-inflammatory microbes, members of the Enterobacteriaceae family have been shown to induce intestinal inflammation, including Escherichia coli, Salmonella, Citrobacter rodentium [85], and others like Helicobacter hepaticus [86, 87]. Gut bacteria may also have anti-inflammatory properties that can be mediated by beneficial metabolites such as the SCFAs presented earlier [88, 89]. Other species may induce an anti-inflammatory response either by inhibiting the production of pro-inflammatory cytokines or by promoting the production of anti-inflammatory molecules [90]. These bacteria include Bifidobacteria, Lactobacilli, Bacteroides spp. and Parabacteroides spp. [90–92]. By modulating the inflammatory response, gut bacteria may exacerbate or alleviate acute inflammation at the anastomotic site and consequently modulate the healing process of the gut barrier. In murine models of colonic anastomosis, the modulation of peritoneal inflammation was shown to influence the healing at the surgical site, and the isolation of the anastomosis in rats with peritonitis was further shown to prevent the deleterious effects of excessive peritoneal inflammation on anastomotic healing [93, 94].
In our work, we found that a subclinical pro-inflammatory state in the colonic mucosa of patients with CRC leads to poor restoration of the gut barrier after surgery [8, 9]. This pro-inflammatory environment was shown to be mediated by the gut microbiota, as the transfer of patient microbiota to mice induces the same increase in many pro-inflammatory cytokines in the mice colonic tissue [8, 9]. We found that several bacterial strains modulate the levels of inflammatory cytokines both in the mucosa and in the intraluminal environment, leading sometimes to a heightened level of systemic inflammation before surgery as demonstrated by higher circulating levels of white blood cells (WBCs) [8, 9]. We also showed that mild suppression of WBCs systemically, specifically monocytes and neutrophils, improved anastomotic healing and prevented bacterial translocation, suggesting that a controlled rather than a chaotic inflammatory reaction after surgery is highly beneficial to restore the gut barrier [8].
Interestingly, we found that the modulation of local and systemic inflammation by specific bacterial strains, specifically P. goldsteinii, was modulated by the peroxisome proliferator-activated receptor-gamma (PPAR-γ), which is activated by butyrate [11]. This pathway is particularly of interest in CRC as it reinforces the gut barrier and may further inhibit cancer cell proliferation and tumor progression [23]. Its activation by gut microbiota or bacterial metabolites not only improves surgical healing but also promotes favorable oncological outcomes [11].
GUT BARRIER RECOVERY AND COLORECTAL CANCER
In our most recent work, we shed light on how the consolidation of the gut barrier using prebiotics prevents not only local but also systemic dissemination of cancer cells, influencing therefore disease-free and overall survival [11].
We found in this work using long-term clinical data that patients with poor postoperative healing and AL experience more local and distant cancer recurrence, and lower overall survival [11]. Based on these findings, we aimed at evaluating whether a weaker gut barrier at the bowel anastomosis site may allow residual cancer cells to escape the bowel lumen, and found that this was the case. We therefore tested whether PPAR-γ-stimulating compounds may prevent cancer cell proliferation, dissemination and implantation in extracolonic tissue. We assessed dietary supplementation with inulin, which promotes butyrate production, and 5-aminosalicylate (5-ASA), a direct PPAR-γ activator. We found that both supplements reinforced the gut barrier function, prevented the escape of cancer cells and diminished tumor burden [11].
Most importantly, we found that a stronger gut barrier prevented the escape of enteric bacteria out of the gut, postoperative sepsis, and the translocation of cancer-promoting bacterial lipopolysaccharides (LPS) into the circulation [11]. This led to a less proinflammatory and potentially less procarcinogenic systemic environment and was associated with a lower fixation and proliferation of cancer cells in the liver [11].
FUTURE DIRECTIONS
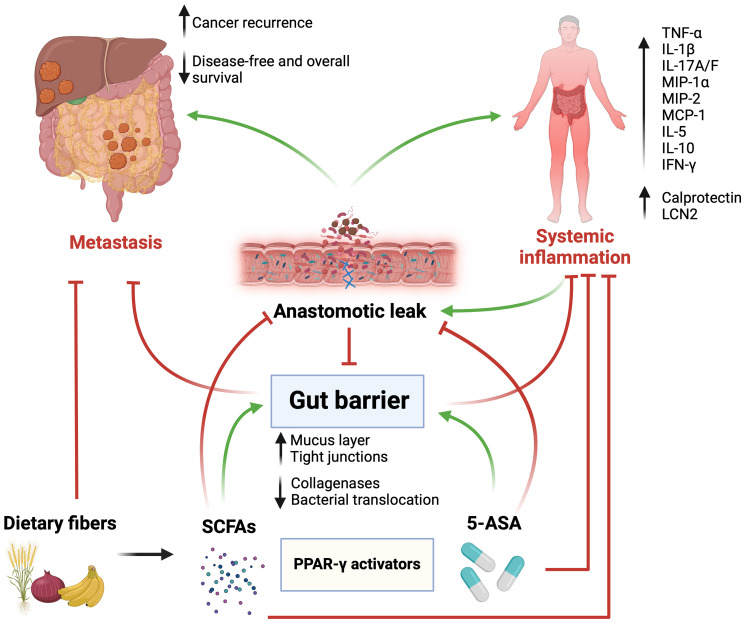
These findings pave the way toward dietary supplements that may stimulate PPAR-γ in an effort to consolidate the gut barrier function and prevent the systemic spread of procarcinogenic factors through a weakened colorectal mucosal layer. Such supplements may include fermentable dietary fibers and 5-ASA. The latter is a commonly used medication in patients with inflammatory bowel disease, and may therefore act as an anti-inflammatory agent to alleviate low-grade inflammation in patients at risk of experiencing poor anastomotic healing [95]. Other anti-inflammatory agents include biological ones, namely anti-TNF-α monoclonal antibodies [96, 97]. While this approach does not necessarily affect PPAR-γ, it was shown in our previous work to improve anastomotic healing in mice [8]. Nonetheless, such agents harbor non-negligible side effect profiles and costs [98–100]. Furthermore, even if excessive TNF-α may be deleterious in the tissue repair process, it seems to drive some vital functions in tissue remodeling. These include the activation of the inflammatory response at the wound site, and the subsequent modulation of cytoskeleton elements and keratinocytes that promote regeneration and tissue repair [101] (Figure 1).
Figure 1. Graphical abstract.
Surgery and anastomotic leak (AL) weaken the gut barrier function, which promotes the dissemination and implantation of colorectal cancer cells in the peritoneal cavity and distant organs. A disrupted gut barrier leads to intestinal and systemic inflammation that promote carcinogenesis and decrease survival. Activation of peroxisome proliferator-activated receptor-gamma (PPAR-γ) by 5-aminosalicylate (5-ASA) and short-chain fatty acids, derived from bacterial fermentation of dietary fibers, reinforces the gut barrier, prevents AL, decreases inflammation, and ultimately improves oncological outcomes. Abbreviations: TNF-α: tumor necrosis factor alpha; IL1β: interleukin 1 beta; IL17A/F: interleukin 17 A/F; MIP-1α: macrophage inflammatory protein 1 alpha; MIP-2: macrophage inflammatory protein 2; MCP-1: monocyte chemoattractant protein 1; IL-5: interleukin 5; IL-10: interleukin 10; IFN-γ: interferon gamma; LCN2: lipocalin 2. Green arrows indicate promotion/stimulation, red arrows indicate inhibition/prevention. Created with https://www.biorender.com/.
Finally, reinforcing the gut barrier may alleviate systemic inflammation in patients with CRC and prevent the emergence of an oncogenic environment both locally in the bowel and systemically. This may in turn prevent the escape of cancer cells and their implantation in the peritoneal cavity or in distant organs. Ultimately, we showed that gut barrier integrity not only protects against postoperative sepsis and surgical complications, but is a pivotal factor in the prevention of local and systemic cancer recurrence, and perhaps in the response to systemic therapy. Future clinical studies are now urgently required to assess whether barrier-reinforcing agents improve outcomes in patients with CRC.
ACKNOWLEDGMENTS AND FUNDING
MMS was supported by the Cancer Research Society (grant FRN-159775), Canadian Institutes of Health Research (grant PJT-175181), and New Frontiers in Research Fund (Exploration grant NFRFE-2020-00991 to MMS and CR). RH is the recipient of scholarships from the Fonds de recherche du Québec – Santé (FRQS) and Ministère de la Santé et des Services sociaux (MSSS) (Resident Physician Health Research Career Training Program) and Canadian Institutes of Health Research (Graduate Scholarships program). We thank Claire McCartney for her help in editing the manuscript.
AUTHOR CONTRIBUTIONS
RH and MMS drafted the manuscript; RH, CR, and MMS edited and revised the manuscript.
CONFLICTS OF INTEREST
Authors have no conflicts of interest to declare.
REFERENCES
- 1. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017; 66:683–91. 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020; 70:145–64. 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 3. Spaander MCW, Zauber AG, Syngal S, Blaser MJ, Sung JJ, You YN, Kuipers EJ. Young-onset colorectal cancer. Nat Rev Dis Primers. 2023; 9:21. 10.1038/s41572-023-00432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cross AJ, Wooldrage K, Robbins EC, Kralj-Hans I, MacRae E, Piggott C, Stenson I, Prendergast A, Patel B, Pack K, Howe R, Swart N, Snowball J, et al. Faecal immunochemical tests (FIT) versus colonoscopy for surveillance after screening and polypectomy: a diagnostic accuracy and cost-effectiveness study. Gut. 2019; 68:1642–52. 10.1136/gutjnl-2018-317297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vilsan J, Maddineni SA, Ahsan N, Mathew M, Chilakuri N, Yadav N, Munoz EJ, Nadeem MA, Abbas K, Razzaq W, Abdin ZU, Ahmed M. Open, Laparoscopic, and Robotic Approaches to Treat Colorectal Cancer: A Comprehensive Review of Literature. Cureus. 2023; 15:e38956. 10.7759/cureus.38956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Battersby CLF, Battersby NJ, Slade DAJ, Soop M, Walsh CJ. Preoperative mechanical and oral antibiotic bowel preparation to reduce infectious complications of colorectal surgery - the need for updated guidelines. J Hosp Infect. 2019; 101:295–99. 10.1016/j.jhin.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 7. Ripollés-Melchor J, Ramírez-Rodríguez JM, Casans-Francés R, Aldecoa C, Abad-Motos A, Logroño-Egea M, García-Erce JA, Camps-Cervantes Á, Ferrando-Ortolá C, Suarez de la Rica A, Cuellar-Martínez A, Marmaña-Mezquita S, Abad-Gurumeta A, et al. , and POWER Study Investigators Group for the Spanish Perioperative Audit and Research Network (REDGERM). Association Between Use of Enhanced Recovery After Surgery Protocol and Postoperative Complications in Colorectal Surgery: The Postoperative Outcomes Within Enhanced Recovery After Surgery Protocol (POWER) Study. JAMA Surg. 2019; 154:725–36. 10.1001/jamasurg.2019.0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hajjar R, Gonzalez E, Fragoso G, Oliero M, Alaoui AA, Calvé A, Vennin Rendos H, Djediai S, Cuisiniere T, Laplante P, Gerkins C, Ajayi AS, Diop K, et al. Gut microbiota influence anastomotic healing in colorectal cancer surgery through modulation of mucosal proinflammatory cytokines. Gut. 2023; 72:1143–54. 10.1136/gutjnl-2022-328389. [DOI] [PubMed] [Google Scholar]
- 9. Hajjar R, Fragoso G, Oliero M, Alaoui AA, Calvé A, Vennin Rendos H, Cuisiniere T, Taleb N, Thérien S, Dagbert F, Loungnarath R, Sebajang H, Schwenter F, et al. Basal levels of microbiota-driven subclinical inflammation are associated with anastomotic leak in patients with colorectal cancer. Gut. 2024; 73:1031–33. 10.1136/gutjnl-2023-329929. [DOI] [PubMed] [Google Scholar]
- 10. Hajjar R, Santos MM, Dagbert F, Richard CS. Current evidence on the relation between gut microbiota and intestinal anastomotic leak in colorectal surgery. Am J Surg. 2019; 218:1000–7. 10.1016/j.amjsurg.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 11. Hajjar R, Oliero M, Fragoso G, Ajayi AS, Alaoui AA, Vennin Rendos H, Calvé A, Cuisiniere T, Gerkins C, Thérien S, Taleb N, Dagbert F, Sebajang H, et al. Modulating Gut Microbiota Prevents Anastomotic Leak to Reduce Local Implantation and Dissemination of Colorectal Cancer Cells after Surgery. Clin Cancer Res. 2024; 30:616–28. 10.1158/1078-0432.CCR-23-1601. [DOI] [PubMed] [Google Scholar]
- 12. Saur NM, Paulson EC. Operative Management of Anastomotic Leaks after Colorectal Surgery. Clin Colon Rectal Surg. 2019; 32:190–95. 10.1055/s-0038-1677025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parthasarathy M, Greensmith M, Bowers D, Groot-Wassink T. Risk factors for anastomotic leakage after colorectal resection: a retrospective analysis of 17 518 patients. Colorectal Dis. 2017; 19:288–98. 10.1111/codi.13476. [DOI] [PubMed] [Google Scholar]
- 14. Bosmans JW, Jongen AC, Bouvy ND, Derikx JP. Colorectal anastomotic healing: why the biological processes that lead to anastomotic leakage should be revealed prior to conducting intervention studies. BMC Gastroenterol. 2015; 15:180. 10.1186/s12876-015-0410-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Daams F, Luyer M, Lange JF. Colorectal anastomotic leakage: aspects of prevention, detection and treatment. World J Gastroenterol. 2013; 19:2293–97. 10.3748/wjg.v19.i15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Docherty JG, McGregor JR, Akyol AM, Murray GD, Galloway DJ. Comparison of manually constructed and stapled anastomoses in colorectal surgery. West of Scotland and Highland Anastomosis Study Group. Ann Surg. 1995; 221:176–84. 10.1097/00000658-199502000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wallace B, Schuepbach F, Gaukel S, Marwan AI, Staerkle RF, Vuille-Dit-Bille RN. Evidence according to Cochrane Systematic Reviews on Alterable Risk Factors for Anastomotic Leakage in Colorectal Surgery. Gastroenterol Res Pract. 2020; 2020:9057963. 10.1155/2020/9057963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hillman ET, Lu H, Yao T, Nakatsu CH. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ. 2017; 32:300–13. 10.1264/jsme2.ME17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iizuka M, Konno S. Wound healing of intestinal epithelial cells. World J Gastroenterol. 2011; 17:2161–71. 10.3748/wjg.v17.i17.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuhn KA, Manieri NA, Liu TC, Stappenbeck TS. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS One. 2014; 9:e114195. 10.1371/journal.pone.0114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hajjar R, Oliero M, Cuisiniere T, Fragoso G, Calvé A, Djediai S, Annabi B, Richard CS, Santos MM. Improvement of colonic healing and surgical recovery with perioperative supplementation of inulin and galacto-oligosaccharides. Clin Nutr. 2021; 40:3842–51. 10.1016/j.clnu.2021.04.032. [DOI] [PubMed] [Google Scholar]
- 22. Cherbuy C, Honvo-Houeto E, Bruneau A, Bridonneau C, Mayeur C, Duée PH, Langella P, Thomas M. Microbiota matures colonic epithelium through a coordinated induction of cell cycle-related proteins in gnotobiotic rat. Am J Physiol Gastrointest Liver Physiol. 2010; 299:G348–57. 10.1152/ajpgi.00384.2009. [DOI] [PubMed] [Google Scholar]
- 23. Hajjar R, Richard CS, Santos MM. The role of butyrate in surgical and oncological outcomes in colorectal cancer. Am J Physiol Gastrointest Liver Physiol. 2021; 320:G601–608. 10.1152/ajpgi.00316.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goverse G, Molenaar R, Macia L, Tan J, Erkelens MN, Konijn T, Knippenberg M, Cook EC, Hanekamp D, Veldhoen M, Hartog A, Roeselers G, Mackay CR, Mebius RE. Diet-Derived Short Chain Fatty Acids Stimulate Intestinal Epithelial Cells To Induce Mucosal Tolerogenic Dendritic Cells. J Immunol. 2017; 198:2172–81. 10.4049/jimmunol.1600165. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr. 2008; 100:297–305. 10.1017/S0007114508888733. [DOI] [PubMed] [Google Scholar]
- 26. Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne). 2020; 11:25. 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016; 165:1332–45. 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 28. Aguirre M, Jonkers DM, Troost FJ, Roeselers G, Venema K. In vitro characterization of the impact of different substrates on metabolite production, energy extraction and composition of gut microbiota from lean and obese subjects . PLoS One. 2014; 9:e113864. 10.1371/journal.pone.0113864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salvi PS, Cowles RA. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells. 2021; 10:1775. 10.3390/cells10071775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009; 139:1619–25. 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paradis T, Bègue H, Basmaciyan L, Dalle F, Bon F. Tight Junctions as a Key for Pathogens Invasion in Intestinal Epithelial Cells. Int J Mol Sci. 2021; 22:2506. 10.3390/ijms22052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Willemsen LE, Koetsier MA, van Deventer SJ, van Tol EA. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut. 2003; 52:1442–47. 10.1136/gut.52.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bosmans JW, Jongen AC, Boonen BT, van Rijn S, Scognamiglio F, Stucchi L, Gijbels MJ, Marsich E, Bouvy ND. Comparison of three different application routes of butyrate to improve colonic anastomotic strength in rats. Int J Colorectal Dis. 2017; 32:305–13. 10.1007/s00384-016-2718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bloemen JG, Schreinemacher MH, de Bruine AP, Buurman WA, Bouvy ND, Dejong CH. Butyrate enemas improve intestinal anastomotic strength in a rat model. Dis Colon Rectum. 2010; 53:1069–75. 10.1007/DCR.0b013e3181d881b7. [DOI] [PubMed] [Google Scholar]
- 35. Mathew AJ, Wann VC, Abraham DT, Jacob PM, Selvan BS, Ramakrishna BS, Nair AN. The effect of butyrate on the healing of colonic anastomoses in rats. J Invest Surg. 2010; 23:101–104. 10.3109/08941930903469367. [DOI] [PubMed] [Google Scholar]
- 36. Burgess DJ. Metabolism: Warburg behind the butyrate paradox? Nat Rev Cancer. 2012; 12:798. 10.1038/nrc3401. [DOI] [PubMed] [Google Scholar]
- 37. Rose S, Bennuri SC, Murray KF, Buie T, Winter H, Frye RE. Mitochondrial dysfunction in the gastrointestinal mucosa of children with autism: A blinded case-control study. PLoS One. 2017; 12:e0186377. 10.1371/journal.pone.0186377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Q, Cao L, Tian Y, Zhang P, Ding C, Lu W, Jia C, Shao C, Liu W, Wang D, Ye H, Hao H. Butyrate Suppresses the Proliferation of Colorectal Cancer Cells via Targeting Pyruvate Kinase M2 and Metabolic Reprogramming. Mol Cell Proteomics. 2018; 17:1531–45. 10.1074/mcp.RA118.000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ropero S, Esteller M. The role of histone deacetylases (HDACs) in human cancer. Mol Oncol. 2007; 1:19–25. 10.1016/j.molonc.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Han A, Bennett N, Ahmed B, Whelan J, Donohoe DR. Butyrate decreases its own oxidation in colorectal cancer cells through inhibition of histone deacetylases. Oncotarget. 2018; 9:27280–92. 10.18632/oncotarget.25546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kazemi Sefat NA, Mohammadi MM, Hadjati J, Talebi S, Ajami M, Daneshvar H. Sodium Butyrate as a Histone Deacetylase Inhibitor Affects Toll-Like Receptor 4 Expression in Colorectal Cancer Cell Lines. Immunol Invest. 2019; 48:759–69. 10.1080/08820139.2019.1595643. [DOI] [PubMed] [Google Scholar]
- 42. Shogan BD, Belogortseva N, Luong PM, Zaborin A, Lax S, Bethel C, Ward M, Muldoon JP, Singer M, An G, Umanskiy K, Konda V, Shakhsheer B, et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med. 2015; 7:286ra68. 10.1126/scitranslmed.3010658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kau AL, Martin SM, Lyon W, Hayes E, Caparon MG, Hultgren SJ. Enterococcus faecalis tropism for the kidneys in the urinary tract of C57BL/6J mice. Infect Immun. 2005; 73:2461–68. 10.1128/IAI.73.4.2461-2468.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jacobson RA, Wienholts K, Williamson AJ, Gaines S, Hyoju S, van Goor H, Zaborin A, Shogan BD, Zaborina O, Alverdy JC. Enterococcus faecalis exploits the human fibrinolytic system to drive excess collagenolysis: implications in gut healing and identification of druggable targets . Am J Physiol Gastrointest Liver Physiol. 2020; 318:G1–9. 10.1152/ajpgi.00236.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hyoju SK, Klabbers RE, Aaron M, Krezalek MA, Zaborin A, Wiegerinck M, Hyman NH, Zaborina O, Van Goor H, Alverdy JC. Oral Polyphosphate Suppresses Bacterial Collagenase Production and Prevents Anastomotic Leak Due to Serratia marcescens and Pseudomonas aeruginosa. Ann Surg. 2018; 267:1112–18. 10.1097/SLA.0000000000002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Praagh JB, Luo JN, Zaborina O, Alverdy JC. Involvement of the Commensal Organism Bacillus subtilis in the Pathogenesis of Anastomotic Leak. Surg Infect (Larchmt). 2020; 21:865–70. 10.1089/sur.2019.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anderson DI, Keskey R, Ackerman MT, Zaborina O, Hyman N, Alverdy JC, Shogan BD. Enterococcus faecalis Is Associated with Anastomotic Leak in Patients Undergoing Colorectal Surgery . Surg Infect (Larchmt). 2021; 22:1047–51. 10.1089/sur.2021.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bo W, Zhou J, Wang K. Sodium butyrate abolishes the degradation of type II collagen in human chondrocytes. Biomed Pharmacother. 2018; 102:1099–104. 10.1016/j.biopha.2018.03.062. [DOI] [PubMed] [Google Scholar]
- 49. Trencheva K, Morrissey KP, Wells M, Mancuso CA, Lee SW, Sonoda T, Michelassi F, Charlson ME, Milsom JW. Identifying important predictors for anastomotic leak after colon and rectal resection: prospective study on 616 patients. Ann Surg. 2013; 257:108–13. 10.1097/SLA.0b013e318262a6cd. [DOI] [PubMed] [Google Scholar]
- 50. Phillips B. Reducing gastrointestinal anastomotic leak rates: review of challenges and solutions. Open Access Surgery. 2016; 9:5–14. 10.2147/oas.S54936. [DOI] [Google Scholar]
- 51. Ryu HS, Lim SB, Choi ET, Song I, Lee JL, Kim CW, Yoon YS, Park IJ, Yu CS, Kim JC. Intraoperative perfusion assessment of the proximal colon by a visual grading system for safe anastomosis after resection in left-sided colorectal cancer patients. Sci Rep. 2021; 11:2746. 10.1038/s41598-021-82486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bornstein JE, Munger JA, Deliz JR, Mui A, Chen CS, Kim S, Khaitov S, Chessin DB, Ferguson TB, Bauer JJ. Assessment of Bowel End Perfusion After Mesenteric Division: Eye Versus SPY. J Surg Res. 2018; 232:179–85. 10.1016/j.jss.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 53. Pommergaard HC, Achiam MP, Burcharth J, Rosenberg J. Impaired blood supply in the colonic anastomosis in mice compromises healing. Int Surg. 2015; 100:70–76. 10.9738/INTSURG-D-13-00191.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McDermott FD, Heeney A, Kelly ME, Steele RJ, Carlson GL, Winter DC. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg. 2015; 102:462–79. 10.1002/bjs.9697. [DOI] [PubMed] [Google Scholar]
- 55. Singhal R, Shah YM. Oxygen battle in the gut: Hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J Biol Chem. 2020; 295:10493–505. 10.1074/jbc.REV120.011188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano RN, Lewis JD, Li H, Thom SR, Bushman FD, Vinogradov SA, Wu GD. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014; 147:1055–63.e8. 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cevallos SA, Lee JY, Tiffany CR, Byndloss AJ, Johnston L, Byndloss MX, Bäumler AJ. Increased Epithelial Oxygenation Links Colitis to an Expansion of Tumorigenic Bacteria. mBio. 2019; 10:e02244-19. 10.1128/mBio.02244-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Muenchau S, Deutsch R, de Castro IJ, Hielscher T, Heber N, Niesler B, Lusic M, Stanifer ML, Boulant S. Hypoxic Environment Promotes Barrier Formation in Human Intestinal Epithelial Cells through Regulation of MicroRNA 320a Expression. Mol Cell Biol. 2019; 39:e00553-18. 10.1128/MCB.00553-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim YI, Yi EJ, Kim YD, Lee AR, Chung J, Ha HC, Cho JM, Kim SR, Ko HJ, Cheon JH, Hong YR, Chang SY. Local Stabilization of Hypoxia-Inducible Factor-1α Controls Intestinal Inflammation via Enhanced Gut Barrier Function and Immune Regulation. Front Immunol. 2020; 11:609689. 10.3389/fimmu.2020.609689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Makanyengo SO, Carroll GM, Goggins BJ, Smith SR, Pockney PG, Keely S. Systematic Review on the Influence of Tissue Oxygenation on Gut Microbiota and Anastomotic Healing. J Surg Res. 2020; 249:186–96. 10.1016/j.jss.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 61. Kanauchi O, Matsumoto Y, Matsumura M, Fukuoka M, Bamba T. The beneficial effects of microflora, especially obligate anaerobes, and their products on the colonic environment in inflammatory bowel disease. Curr Pharm Des. 2005; 11:1047–53. 10.2174/1381612053381675. [DOI] [PubMed] [Google Scholar]
- 62. Maier E, Anderson RC, Roy NC. Understanding how commensal obligate anaerobic bacteria regulate immune functions in the large intestine. Nutrients. 2014; 7:45–73. 10.3390/nu7010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kotwal GJ, Chien S. Macrophage Differentiation in Normal and Accelerated Wound Healing. Results Probl Cell Differ. 2017; 62:353–64. 10.1007/978-3-319-54090-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Khashim Z, Daying D, Hong DY, Ringler JA, Herting S, Jakaitis D, Maitland D, Kallmes DF, Kadirvel R. The Distribution and Role of M1 and M2 Macrophages in Aneurysm Healing after Platinum Coil Embolization. AJNR Am J Neuroradiol. 2020; 41:1657–62. 10.3174/ajnr.A6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, Roy S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015; 185:2596–606. 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liarte S, Bernabé-García Á, Nicolás FJ. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells. 2020; 9:306. 10.3390/cells9020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Juhl P, Bondesen S, Hawkins CL, Karsdal MA, Bay-Jensen AC, Davies MJ, Siebuhr AS. Dermal fibroblasts have different extracellular matrix profiles induced by TGF-β, PDGF and IL-6 in a model for skin fibrosis. Sci Rep. 2020; 10:17300. 10.1038/s41598-020-74179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Maroni D, Davis JS. Transforming growth factor Beta 1 stimulates profibrotic activities of luteal fibroblasts in cows. Biol Reprod. 2012; 87:127. 10.1095/biolreprod.112.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Landén NX, Li D, Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016; 73:3861–85. 10.1007/s00018-016-2268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rigamonti E, Zordan P, Sciorati C, Rovere-Querini P, Brunelli S. Macrophage plasticity in skeletal muscle repair. Biomed Res Int. 2014; 2014:560629. 10.1155/2014/560629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front Physiol. 2018; 9:419. 10.3389/fphys.2018.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Verdolino DV, Thomason HA, Fotticchia A, Cartmell S. Wound dressings: curbing inflammation in chronic wound healing. Emerg Top Life Sci. 2021; 5:523–37. 10.1042/ETLS20200346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound Healing: A Cellular Perspective. Physiol Rev. 2019; 99:665–706. 10.1152/physrev.00067.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in Chronic Wounds. Int J Mol Sci. 2016; 17:2085. 10.3390/ijms17122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nouvong A, Ambrus AM, Zhang ER, Hultman L, Coller HA. Reactive oxygen species and bacterial biofilms in diabetic wound healing. Physiol Genomics. 2016; 48:889–96. 10.1152/physiolgenomics.00066.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Xiao T, Yan Z, Xiao S, Xia Y. Proinflammatory cytokines regulate epidermal stem cells in wound epithelialization. Stem Cell Res Ther. 2020; 11:232. 10.1186/s13287-020-01755-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cowin AJ, Hatzirodos N, Rigden J, Fitridge R, Belford DA. Etanercept decreases tumor necrosis factor-alpha activity in chronic wound fluid. Wound Repair Regen. 2006; 14:421–26. 10.1111/j.1743-6109.2006.00141.x. [DOI] [PubMed] [Google Scholar]
- 78. Streit M, Beleznay Z, Braathen LR. Topical application of the tumour necrosis factor-alpha antibody infliximab improves healing of chronic wounds. Int Wound J. 2006; 3:171–79. 10.1111/j.1742-481X.2006.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Das P, Rampal R, Udinia S, Kumar T, Pilli S, Wari N, Ahmed IK, Kedia S, Gupta SD, Kumar D, Ahuja V. Selective M1 macrophage polarization in granuloma-positive and granuloma-negative Crohn’s disease, in comparison to intestinal tuberculosis. Intest Res. 2018; 16:426–35. 10.5217/ir.2018.16.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhu W, Yu J, Nie Y, Shi X, Liu Y, Li F, Zhang XL. Disequilibrium of M1 and M2 macrophages correlates with the development of experimental inflammatory bowel diseases. Immunol Invest. 2014; 43:638–52. 10.3109/08820139.2014.909456. [DOI] [PubMed] [Google Scholar]
- 81. Han X, Ding S, Jiang H, Liu G. Roles of Macrophages in the Development and Treatment of Gut Inflammation. Front Cell Dev Biol. 2021; 9:625423. 10.3389/fcell.2021.625423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999; 117:814–22. 10.1016/s0016-5085(99)70339-2. [DOI] [PubMed] [Google Scholar]
- 83. Roomi MW, Kalinovsky T, Rath M, Niedzwiecki A. Cytokines, inducers and inhibitors modulate MMP-2 and MMP-9 secretion by human Fanconi anemia immortalized fibroblasts. Oncol Rep. 2017; 37:1842–48. 10.3892/or.2017.5368. [DOI] [PubMed] [Google Scholar]
- 84. Gao CQ, Sawicki G, Suarez-Pinzon WL, Csont T, Wozniak M, Ferdinandy P, Schulz R. Matrix metalloproteinase-2 mediates cytokine-induced myocardial contractile dysfunction. Cardiovasc Res. 2003; 57:426–33. 10.1016/s0008-6363(02)00719-8. [DOI] [PubMed] [Google Scholar]
- 85. Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017; 10:18–26. 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cao X. Intestinal inflammation induced by oral bacteria. Science. 2017; 358:308–9. 10.1126/science.aap9298. [DOI] [PubMed] [Google Scholar]
- 87. Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011; 4:22–30. 10.1038/mi.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nakkarach A, Foo HL, Song AA, Mutalib NEA, Nitisinprasert S, Withayagiat U. Anti-cancer and anti-inflammatory effects elicited by short chain fatty acids produced by Escherichia coli isolated from healthy human gut microbiota. Microb Cell Fact. 2021; 20:36. 10.1186/s12934-020-01477-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019; 10:277. 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front Immunol. 2021; 12:578386. 10.3389/fimmu.2021.578386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jenab A, Roghanian R, Emtiazi G. Bacterial Natural Compounds with Anti-Inflammatory and Immunomodulatory Properties (Mini Review). Drug Des Devel Ther. 2020; 14:3787–801. 10.2147/DDDT.S261283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lai HC, Lin TL, Chen TW, Kuo YL, Chang CJ, Wu TR, Shu CC, Tsai YH, Swift S, Lu CC. Gut microbiota modulates COPD pathogenesis: role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut. 2022; 71:309–21. 10.1136/gutjnl-2020-322599. [DOI] [PubMed] [Google Scholar]
- 93. Wu Z, Boersema GS, Kroese LF, Taha D, Vennix S, Bastiaansen-Jenniskens YM, Lam KH, Kleinrensink GJ, Jeekel J, Peppelenbosch M, Lange JF. Reducing colorectal anastomotic leakage with tissue adhesive in experimental inflammatory bowel disease. Inflamm Bowel Dis. 2015; 21:1038–46. 10.1097/MIB.0000000000000336. [DOI] [PubMed] [Google Scholar]
- 94. Ghiselli R, Lucarini G, Ortenzi M, Salvolini E, Saccomanno S, Orlando F, Provinciali M, Casciani F, Guerrieri M. Anastomotic healing in a rat model of peritonitis after non-steroidal anti-inflammatory drug administration. Eur J Histochem. 2020; 64:3085. 10.4081/ejh.2020.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tursi A, Joseph RE, Streck P. Expanding applications: the potential usage of 5-aminosalicylic acid in diverticular disease. Dig Dis Sci. 2011; 56:3112–21. 10.1007/s10620-011-1731-x. [DOI] [PubMed] [Google Scholar]
- 96. Cui G, Fan Q, Li Z, Goll R, Florholmen J. Evaluation of anti-TNF therapeutic response in patients with inflammatory bowel disease: Current and novel biomarkers. EBioMedicine. 2021; 66:103329. 10.1016/j.ebiom.2021.103329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fausel R, Afzali A. Biologics in the management of ulcerative colitis - comparative safety and efficacy of TNF-α antagonists. Ther Clin Risk Manag. 2015; 11:63–73. 10.2147/TCRM.S55506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li J, Zhang Z, Wu X, Zhou J, Meng D, Zhu P. Risk of Adverse Events After Anti-TNF Treatment for Inflammatory Rheumatological Disease. A Meta-Analysis. Front Pharmacol. 2021; 12:746396. 10.3389/fphar.2021.746396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schabert VF, Watson C, Joseph GJ, Iversen P, Burudpakdee C, Harrison DJ. Costs of tumor necrosis factor blockers per treated patient using real-world drug data in a managed care population. J Manag Care Pharm. 2013; 19:621–30. 10.18553/jmcp.2013.19.8.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR, Yang SH. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int J Mol Sci. 2021; 22:2719. 10.3390/ijms22052719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Banno T, Gazel A, Blumenberg M. Effects of tumor necrosis factor-alpha (TNF alpha) in epidermal keratinocytes revealed using global transcriptional profiling. J Biol Chem. 2004; 279:32633–42. 10.1074/jbc.M400642200. [DOI] [PubMed] [Google Scholar]