Abstract
Background
Neuroplasticity as a mechanism to overcome central nervous system injury resulting from different neurological diseases has gained increasing attention in recent years. However, deficiency of these repair mechanisms leads to the accumulation of neuronal damage and therefore long-term disability. To date, the mechanisms by which remyelination occurs and why the extent of remyelination differs interindividually between multiple sclerosis patients regardless of the disease course are unclear. A member of the neurotrophins family, the brain-derived neurotrophic factor (BDNF) has received particular attention in this context as it is thought to play a central role in remyelination and thus neuroplasticity, neuroprotection, and memory.
Objective
To analyse the current literature regarding BDNF in different areas of multiple sclerosis and to provide an overview of the current state of knowledge in this field.
Conclusion
To date, studies assessing the role of BDNF in patients with multiple sclerosis remain inconclusive. However, there is emerging evidence for a beneficial effect of BDNF in multiple sclerosis, as studies reporting positive effects on clinical as well as MRI characteristics outweighed studies assuming detrimental effects of BDNF. Furthermore, studies regarding the Val66Met polymorphism have not conclusively determined whether this is a protective or harmful factor in multiple sclerosis, but again most studies hypothesized a protective effect through modulation of BDNF secretion and anti-inflammatory effects with different effects in healthy controls and patients with multiple sclerosis, possibly due to the pro-inflammatory milieu in patients with multiple sclerosis. Further studies with larger cohorts and longitudinal follow-ups are needed to improve our understanding of the effects of BDNF in the central nervous system, especially in the context of multiple sclerosis.
Keywords: BDNF, brain-derived neurotrophic factor, multiple sclerosis, neuroplasticity, neuroprotection, remyelination, neuronal damage
1. Introduction
Neuroplasticity as a mechanism to overcome central nervous system (CNS) injury has gained increasing attention in the context of different neurological diseases during the last years. In the field of multiple sclerosis (MS), histopathological studies have shown that remyelination can be found in a subset of patients with inflammatory processes as well as marked neurodegeneration (1). However, deficiency of these repair mechanisms leads to accumulation of neuronal damage and therefore long-term disability (2).
To date, the mechanisms by which remyelination occurs and why the extent of remyelination differs interindividually between MS patients regardless of the disease course are unclear. However, as increasingly effective therapeutic options designed to halt inflammation and slow neurodegeneration are available, approaches that might reverse preexisting, already accumulated damage are becoming increasingly important.
1.1. General information on BDNF and its cellular effects
A member of the neurotrophins family, the brain-derived neurotrophic factor (BDNF) has received particular attention in this context. As the most abundant growth factor in the brain, BDNF is thought to be of uttermost importance for the regulation of neuronal survival and death, neuronal proliferation as well as differentiation, synaptic plasticity, and repair processes, thus playing a key role in neuroprotection and neuroplasticity (3–6).
In the CNS, BDNF is synthesized and secreted by neurons as well as activated astrocytes and microglial cells. Astrocytes are the first cells to react to inflammatory CNS damage by expressing various surface markers, neurotrophic factors, and cytokines (7), thereby attracting microglia and macrophages to the site of damage. In turn, their activation leads to further release of cytokines as well as neurotrophic factors and phagocytosis of damaged cells (8). Microglia and macrophages exist in three different phenotypes. M0 is the resting state of microglial cells, while M1 and M2 are active forms, with M1 exerting inflammatory and M2 anti-inflammatory effects (9, 10). The M2-polarization can be induced by BDNF via activation of the tropomyosin-related kinase B (TrkB) receptor, which in turn induces the synthesis of anti-inflammatory cytokines and neurotrophic factors such as BDNF (10–12).
BDNF is synthesized and released in an activity-dependent manner (8) and through the expression of TrkB receptors, macrophages and microglia are able to react to BDNF, thus BDNF release occurs in an autocrine manner (8). Therefore, damage of astrocytes exerts neuroprotective effects through recruitment of macrophages and microglia by inducing their BDNF synthesis. This is further supported, as lesions with ongoing inflammation show higher concentrations of BDNF with upregulation of TrkB receptors on damaged neurons. Therefore, signaling of infiltrating microglia and macrophages through BDNF to damaged neurons supports the resolution of inflammation, neuronal survival and regeneration (13).
In the periphery, BDNF is also synthesized and released by platelets, monocytes and macrophages as well as activated T- and B-cells (14–16). Furthermore, endothelial cells in the periphery and in the brain are also capable of secreting BDNF (17–19), which can be triggered by various stimuli such as TNF-α, a proinflammatory cytokine (20) thought to play a pivotal role in MS pathophysiology (21). As endothelial cells release BDNF and also express TrkB receptors, an autocrine effect is hypothesized. However, TNF-α can not only induce the release of BDNF from endothelial cells, but also activates microglia, thus TNF-α can induce further cytokine release, thus amplifying the neuroinflammatory process (22). Yet, besides these deleterious effects, TNF-α also exhibits neuroprotective effects, as it is capable of inducing BDNF synthesis and release of astrocytes through MEK/ERK pathway (23, 24). Furthermore, as a negative feedback mechanism, BDNF was found to be capable of reducing TNF-a levels (24). On astrocytes, two different TNF-α receptors (TNFR) activating MAP kinase as well as NF-ĸB pathways were found (23): TNFR1 mainly exerts inflammatory and pro-apoptotic processes, while TNFR2 exerts anti-inflammatory effects, proliferation of oligodendrocyte progenitor cells (OPC) and thus remyelination and neuronal survival. TNFR2 was also found to be capable of enhancing BDNF translation through activation of NF-ĸB and cyclic adenosine monophosphate (AMP)-response element binding protein (CREB) (23–25). Still, these mechanisms seem to be time dependent. Rowhani-Rad and colleagues found the early inflammatory processes in experimental autoimmune encephalomyelitis (EAE)-lesions to reduce BDNF through activation of TNFR1, with increasing TNF-a levels. Within 2 weeks after induction of the lesions, BDNF levels increased while TNF-a levels decreased through TFNR-2 activation (24).
The release of BDNF depends on the neuronal activity, though different neuropeptides and hormones can also influence the secretion. If glutamate is released from excitatory synapses, depolarization is caused by an influx of Ca2+ and Na+ via α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-D-aspartate (NMDA) and voltage-dependent Ca2+-channels. This causes calcium-dependent activation of Ca2+-calmodulin-dependent protein kinases (CaMK), protein kinase C (PKC), and mitogen-activated protein kinases (MAPKs), which in turn leads to transcription of the BDNF gene by CREB and nuclear factor ĸB (NF-ĸB). After transcription, BDNF is packaged in vesicles and transported axonally to the dendrites and presynaptic endings, where it can be released through the activation of glutamate receptors (26). A detailed description of the synthesis and release of BDNF was reviewed by Kowiański et al. (27) as well as Marosi and Mattson (26).
1.2. BDNF signaling pathways
After release, BDNF binds to its two receptors: TrkB receptor and neurotrophin receptor p75 (nt-p75). The nt-p75 receptor, as a member of the tumor necrosis factor (TNF) family, provides pro-apoptotic functions. However, because of its low affinity to BDNF, expression of ceramides, NF-ĸB, and jun-kinases leading to apoptosis only will be activated at higher concentrations (28). It is hypothesized that because of increasing BDNF secretion in inflammatory lesions with a high density of these receptors at the edge of inflammatory lesions, cell death will be initiated in case of severe neuronal damage.
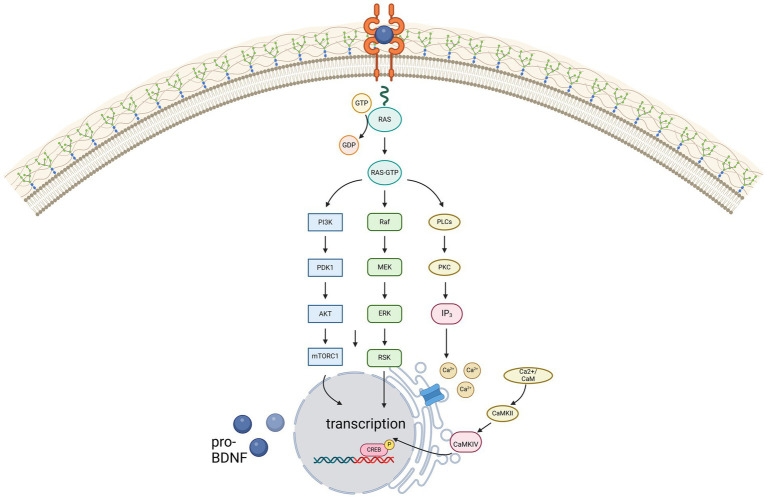
Contrarily, due to its high affinity for BDNF, TrkB is activated even at low concentrations of BDNF, thereby activating phospholipase C gamma (PLC-γ), phosphatidylinositol-3 kinase (PI3-K) and MAP kinase pathway, illustrated in Figure 1. For remyelination, the proliferation, migration, and maturation of OPC to mature oligodendrocytes forming new myelin sheaths is crucial. Among others, intrinsic signaling cascades involving mTOR activation were shown to be relevant for this process (29). mTOR – a serine/threonine kinase - binds different proteins to form two protein complexes referred to as mTORC1 and mTORC2 (30). For synaptic plasticity, particularly mTORC1, consisting of mTOR as well as two proteins called raptor and GbL, is relevant (31, 32). By activation of the ribosomal protein S6 kinase (p70S6K), mTORC1 induces translation of proteins related to synaptic formation as well as excitatory postsynaptic currents (31), outgrowth of cytoskeletal filaments, axons as well as dendrites and dendritic spines (31, 33). Furthermore, the PI3K signaling pathway controls suppression of pro-apoptotic factors as Bad, Forkhead and thus reducing Fas ligand (33). Synergistically, activation of the MEK/ERK signaling pathway induces translation of the anti-apoptotic Bcl-2 (33). mTORC1 also inhibits autophagy through inhibition of ULK1, thus exerting further neuroprotective effects (31). However, as BDNF can activate these pathways through TrkB-receptors, BDNF is hypothesized to be a key factor promoting maturation of OPCs. Wong et al. could show, that in TrkB knock-out mice myelin protein expression, myelination as well as proliferative potential of OPCs was significantly decreased, thus proving that TrkB receptors are expressed on OPCs (34).
Figure 1.
Illustration of the three main signaling pathways of BDNF, mediated via the high-affinity receptor TrkB. Created in BioRender.com.
However, the increased expression of antioxidant enzymes and improved DNA repair mechanisms also contribute to the survival of neurons. But not only the survival of neurons is supported by BDNF: by activating p21-ras, BDNF can stimulate neurite outgrowth and synaptogenesis, increase cytoskeletal dynamics, and modulate cell adhesion (23). Furthermore, the NMDA-receptor subunits NR1 and early growth response factor-3 mediated mechanisms can be increased by BDNF via CREB, being hypothesized to improve cognition by increasing Ca2+ influx in synapses involved in learning and memory (23). Therefore, BDNF plays a pivotal role in long-term potentiation. Additionally, as BDNF can increase the synthesis of the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) as well as the transcriptional factor FOXO3a, with both playing a key role in the cellular energy homeostasis, maintenance, and formation of synapses is enhanced (23, 35).
1.3. BDNF in context of MS
Because of these effects, BDNF has gained increasing attention in the context of MS, especially regarding possible effects on clinical improvement by its remyelinating mechanisms (36, 37). Interestingly, decreased (38–42) as well as increased (39, 43–46) or normal (37, 47, 48) BDNF levels were found in patients with MS (pwMS) compared to healthy controls (49). Still, most studies reported an increase of BDNF concentrations in presence of disease activity as clinical relapse (37, 39, 40, 42, 46, 50). Decreasing BDNF concentrations with disease progression may be linked to decreased BDNF secretion of lymphocytes, as a change of lymphocyte phenotype was found with disease progression (14). Still, some studies found no association of low BDNF levels with disease progression (51, 52).
To date, knowledge of the role of BDNF in the field of MS is scarce as studies have been inconclusive so far. Accordingly, this review aimed to analyse the current literature regarding BDNF in different areas of multiple sclerosis and to provide an overview of the current state of knowledge in this field.
2. BDNF-polymorphism
BDNF is encoded on chromosome 11 band p13-14 (53). Only a few genetic variants were discovered in the coding intron regions, with the Val66Met polymorphism being the best-studied variant to date. In this single nucleotide polymorphism (SNP), the amino acid valine is exchanged for methionine in codon 66 (54). Despite this polymorphism, a functional protein with an altered pro-domain is synthesized, which, however, leads to changes in protein folding and thus reduced binding to TrĸB (55), but also to changes in the interaction with various other proteins (54). It has been shown that the binding behavior of translin, a DNA-binding protein, to BDNF messenger ribonucleic acid (mRNA) is disrupted, resulting in impaired trafficking of BDNF transcripts within dendrites (56). Additionally, it is hypothesized that the SNP results in altered intracellular packing and transport (1, 55, 57). Both in vitro studies and studies in healthy volunteers have shown that the altered intracellular transport leads to an abnormal secretion, thereby reducing the activity-dependent BDNF concentration by 18-30% (1, 55, 57). Some studies could not find a reduction of peripheral BDNF levels, which is why a local influence of activity-dependent secretion by the polymorphism has been postulated (45, 58). Altered secretion of a pro-apoptotically active precursor of BDNF due to altered intracellular trafficking (55, 57, 59) and alteration of the rate at which pro-BDNF is cleaved extracellularly by enzymes into mature BDNF have also been shown to be associated with the SNP (60). However, the reduced activity-dependent secretion has also been confirmed in Val66Met knock-in mice (61).
Nonetheless, studies remain inconclusive with some showing higher (62, 63) and some showing lower (57, 64) or equal (65, 66) BDNF-serum concentrations in healthy Val66Met carriers, whereas RRMS patients with Met alleles had higher BDNF concentrations than healthy carriers (45). However, some studies did not find differences in the BDNF serum concentration between healthy controls and RRMS patients with Val66Met polymorphism (45, 52, 67, 68). Studies also showed different results about peripheral mRNA levels, on the one hand with no detectable difference between Val homozygotes and Met carriers in either healthy subjects or RRMS patients (52, 67, 68), but on the other hand higher mRNA levels in RRMS patients compared to healthy Met carriers (45, 54).
3. BDNF and cognition
BDNF is thought to play a central role in synaptic plasticity (36, 69). It is hypothesized to play an important role in the long-term potentiation and neuroplasticity of the hippocampus (70), which is essential for learning and memory (71, 72). Several pathways how BDNF could affect episodic memory are discussed. However, increased activation of NMDA and AMPA-receptors and therefore increased intracellular calcium as well as sodium and potassium concentrations due to enhancement of protein translation via TrkB is hypothesized to play a key role (72, 73).
However, studies assessing the relationship between serum BDNF concentrations and cognitive performance have been inconclusive so far.
In animal models, low BDNF concentrations resulted in cognitive deficits due to disturbed neurotransmission and neuroplasticity (5, 74–76). In pwMS, a relationship between BDNF concentrations and cognitive performance was assumed (77). It was considered that the pathological changes leading to cognitive impairment were compensated through hippocampal hyperactivation to preserve episodic memory as seen in functional MRI (78, 79), which could be driven by BDNF (55, 80–83). Some studies reported a correlation of high BDNF levels with improvement of memory (84–86) and correlation of decreased BDNF concentrations with mild cognitive impairment even in newly diagnosed pwMS with no or mild disability (36). A study analysing changes in BDNF concentration after physical activity for 1 year found increasing BDNF levels with improvement of cognitive functions (87, 88). In reverse, a lack of BDNF or a disruption of the BDNF-TrkB pathway led to loss of synapses and decreasing cognitive functions (69, 89).
Regarding the effects of the Val66Met SNP on cognition, it is hypothesized, that the SNP prevents the development of maladaptive cortical plasticity in pwMS, resulting in improved recovery after relapses compared to Val homozygotes (90). A deleterious effect of the polymorphism on the parieto-prefrontal network and hippocampal activity, however, has been demonstrated in healthy subjects, whereas the opposite was found in RRMS patients (91). Val homozygote RRMS patients showed higher activity in the episodic memory network, maybe as form of compensatory mechanisms (92). Furthermore, pwMS with Val66Met polymorphism showing altered hippocampal activity while performing an episodic memory task (81, 82) had poorer episodic memory and executive functions (49, 81, 93). Still, some studies did not find any association of the Val66Met polymorphism with cognitive impairment in pwMS (45, 60, 94).
To summarize, some studies concluded that BDNF influences cognition, rather on a global level than influencing specific cognitive domains (86, 95), while other studies did not find an association between BDNF levels and cognition. Furthermore, most studies reported no detrimental effect of the Val66Met polymorphism on cognition. Table 1 provides an overview of the above-mentioned studies investigating the relationship between BDNF and cognition.
Table 1.
Overview of the above-mentioned studies investigating the relationship between BDNF and cognition.
| Study | Participants | Parameters | Results |
|---|---|---|---|
| Castrén et al. (84) | Animal study (rats) | Hippocampal mRNA expression of BDNF (among others) before and after induction of long-term potentiation (LTP) through stimulation of the perforant pathway. | Increase of BDNF mRNA in granular neurones of the dentate gyrus of both hemispheres by unilateral stimulation. |
| Cerasa et al. (91) | 29 patients with RRMS, 32 healthy controls (HC) | Effect of Val66Met polymorphism on brain activity during spatial working memory task measured by fMRI | Increased activation of the parieto-prefrontal network and decreased activation of the ventro-medial-prefrontal cortex and hippocampus in healthy controls, no effect in RRMS-patients. |
| Ehling et al. (95) | 19 patients with RRMS (12 treated with glatiramer acetate, 7 treatment-naïve) | Serum BDNF over 24 months | No difference between patients treated with glatiramer acetate and treatment-naïve patients and no change of BDNF serum levels over time |
| Engin et al. (87) | Animal study (mice) | Effect of α5-containing GABAa receptor knockout in dentate gyrus on cognition | Reduced tonic inhibition in knockout micee resulting in similar or better performance of memory tasks Impairment in cognitive tasks requiring formation of new/overwriting old circuits |
| Falkenberg et al. (85) | Animal study (rats) | Association of BDNF with motor activity, environment and cognition | Association of higher expression of BDNF mRNA in hippocampus with improved spatial memory and enriched environment |
| Fera et al. (92) | 26 RRMS patients without cognitive impairment, 25 healthy controls | Effect of Val66Met polymorphism on hippocampal function in fMRI during episodic memory task | Higher activity parahippocampal, in left posterior hippocampus and left posterior cingulate cortex during encoding and retrieval as well as higher connectivity between hippocampus and posterior cingulate cortex during retrieval in Val homozygote RRMS patients opposite effects in healthy controls |
| Giordano et al. (90) | 100 patients with progressive MS | Effects of BDNF and NTRK2 genes on motor recovery | In Val66Met carriers greater motor improvement after rehabilitation |
| Hakansson et al. (88) | 23 healthy participants (19 fully participated) | 35 min of aerobic exercice at moderate level, cognitive training through a computerized working memory training program or mindfulness practice using an app | Increase of serum BDNF levels after aerobic training, but not after cognitive training oder mindfulness practice Positive association of serum BDNF levels and working memory function |
| Hariri et al. (81) | 64 healthy participants | Association of Val66met genotype and hippocampal activity in fMRI during declarative memory task | Decreased hippocampal activity in Val66Met carriers compared to Val66Val during encoding and retrieval processes |
| Hulst et al. (78) | 34 patients with MS without cognitive impairment, 16 patients with MS with cognitive impairment, 30 healthy controls | fMRI during episodic memory task | Bilaterally increased brain activity parahippocampal and left anterior cingulate gyrus in patients without cognitive impairment during encoding, less brain activity para−/hippocampal and in the prefrontal cortex in cognitively impaired patients, but increased activity in posterior cingulate gyrus and left precuneus No structural differences between patients with and without cognitive impairment |
| Islam et al. (70) | Animal study (mice) | Effect of BDNF on multipotent neural stem cells | Modulation of neurogenesis by mainly mediated via TrkB, a.o. by regulating neural replacement, neural loss while |
| Loprinzi (73) | Metaanalysis | 52 studies—26 animal studies, 15 studies in humans | All animal studies showed improvement of memory, 97% increase of BDNF with 8 studies showing BDNF as mediator of the positive association of training and cognition. 44% of the studies in humans found an positive association of BDNF and cognitive improvement with 40% of these assuming BDNF as mediator |
| Liguori et al. (45) | 36 inactive patients with RRMS and 37 healthy controls | Effect of Val66Met polymorphism and BDNF levels on patients with RRMS over 24 months | Higher BDNF levels in patients with RRMS compared to healthy controls, regardless immunotherapy No correlation of Val66Met polymorphism with clinical or MRI characteristics |
| Linnarsson et al. (75) | Animal study (mice) | Effect of deletion of one copy of the BDNF gene | Significant cognitive impairment |
| Ninan et al. (83) | Animal study (mice) | Properties of hippocampal CA3-CA1 synapses of BDNF Met/Met mice and matched wild-type mice | Normal glutamatergic transmission, but decreased NMDA receptor-dependent transmission and long-term potentiation in BDNF met/met mice |
| Patanella et al. (77) | 30 patients with RRMS | PBMC production of BDNF, TNF-alpha, IL10 and IL6 | Association of low BDNF levels with worse performance in divided attention and visual scanning tasks Association of high IL-6 level with low mini mental state examination scores |
| Prokopova et al. (36) | 19 treatment naïve patients with MS, 19 healthy controls | BDNF plasma level, cognitive function as well as effect of mild stress (Stroop test) on neuroendocrine activation in early phase of multiple sclerosis | Association of cognitive impairment in male patients with MS with decreased BDNF plasma levels Higher anxiety, depression and poorer performance in Stroop test in patients with MS |
| Ramasamy et al. (60) | 188 patients with MS | Effect of Val66Met polymorphism on regional grey matter in MRI | Higher grey matter volume in the cingulate of patient with MS with Val66Met polymorphism compared to Val66Val patients |
| Vigers et al. (76) | Animal study (mice) | Quantification of dendritic spines in cortical neurons in late-onset forebrain-specific BDNF knockout mice | Smaller brain volume in knockout mice compared to wild-type mice with impairment in spatial learning and increased depression |
| Yalachkov et al. (86) | 36 patients with RRMS, PPMS, CIS | Effect of BDNF on disability and cognition after relapse | Association of disability improvement with higher serum BDNF levels and cognitive improvement with higher BDNF levels in CSF at baseline with a positive correlation between serum BDNF and EDSS improvement and CSF-BDNF with z-score change in cognitive tests |
| Zivadinov et al. (1) | 209 patients with MS (108 with cognitive testing) | Effect of Val66Met polymorphism on brain morphometry and functionality measured by MRI and cognitive testing | Positive association of Val66Met polymorphism with normalized grey matter volume and negative association with T2 lesion volume only trend towards a positive association |
4. BDNF and disability
Studies assessing the effect of BDNF on disease severity, disability improvement, or worsening are scarce.
Some studies found no relationship between the BDNF concentration and the disease severity assessed by multiple sclerosis Severity Score (96). Contrarily, in a pilot study, Yalachkov and colleagues found that higher serum BDNF concentrations at baseline were associated with a disability improvement measured as an EDSS decrease of ≥0,5 12 months after relapse (86).
However, most studies focused on the relationship between the disease severity and the BDNF Val66Met polymorphism. No differences between Val66Met carriers and Val homozygotes were found regarding disease susceptibility (58, 94, 97), age at onset or disease severity (1, 49, 97, 98) assessed by EDSS (45, 94, 97, 98). Still, Val66Met carriers showed greater motor recovery after rehabilitation assessed by 6 min walk test (6-MWT) (90). Lower methylation of the BDNF gene was associated with higher gene expression and a higher risk of achieving an EDSS of 6,0 (99, 100). Only one study found susceptibility and disease outcome to be influenced by the polymorphism, especially in females (101). Furthermore, no association of the Val66Met polymorphism was found for disease duration, relapse rate, time between relapses, or time to reach EDSS milestones like 4 or 6 or secondary progression. To this end, no detrimental effect of the SNP was found in pwMS (98, 99).
However, further studies with larger sample sizes and long-term follow-up are needed to assess the effect of BDNF on disease activity and severity, as studies are scarce and results inconclusive so far. A summary of the studies assessing the relationship between BDNF and disability can be found in Table 2.
Table 2.
Overview of the above-mentioned studies investigating the relationship between BDNF and disability.
| Study | Participants | Parameters | Results |
|---|---|---|---|
| Blanco et al. (98) | 224 patients with MS patients, 177 healthy controls | Effect of Val66Met polymorphism on disease susceptibility and severity | No association of Val66Met polymorphism with disease susceptibility or severity No effect of Val66Met polymorphism on peripheral levels BDNF in a subgroup of 12 patients with MS |
| Dinacci et al. (58) | 45 patients with MS, 34 healthy controls | Association of Val66Met polymorphism with MRI characteristics | Lower grey matter volume in Val66Val patients compared to healthy controls, but not in Val66Met patients compared to healthy controls |
| Giordano et al. (90) | 100 patients with progressive MS | Effects of BDNF and NTRK2 genes on motor recovery | In Val66Met carriers greater motor improvement after rehabilitation |
| Islas-Hernandez et al. (96) | 74 patients with RRMS, 11 patients with SPMS, 88 healthy controls | Association of total Tau and BDNF with disease severity/disability | Increased tau and decreased BDNF in patients with MS Higher total tau in patients with RRMS and higher MSSS and lower EDSS scores, no differences in SPMS and patients with severe MS compared to healthy controls |
| Liguori et al. (49) | 50 patients with RRMS, 50 healthy controls | Effect of Val66Met on MRI characteristics and cognition | Lower grey matter volume in patients withVal66Met polymorphism No association of Val66Met polymorphism with cognition in patients with RRMS and healthy controls |
| Liguori et al. (45) | 36 inactive patients with RRMS and 37 healthy controls | Effect of Val66Met polymorphism and BDNF levels on patients with RRMS over 24 months | Higher BDNF levels in patients with RRMS compared to healthy controls, regardless immunotherapy No correlation of Val66Met polymorphism with clinical or MRI characteristics |
| Lindquist et al. (97) | 951 British MS patients | Association of Val66Met polymorphism with disease susceptibility and disease severity | No association of Val66Met polymorphism with disease susceptibility or clinical course |
| Mero et al. (94) | 2,149 patients with MS, 2747 helathy controls | Association of Val66Met polymorphism with disease susceptibility, demographical and clinical parameters | No association of Val66Met polymorphism with disease susceptibility, sex, age at onset, disease course and severity, cognitive impairment |
| Mirowska-Guzel et al. (101) | 230 patients with RRMS/SPMS and 177 healthy controls | Association of BDNF gene (C270T and G196A) and disease susceptibility and disease progression | Earlier disease onset in male patients with 196G/G than 196G/A genotype Higher disease susceptibility in female patients with as well as 196G/G genotype in male patients with 270C/T genotype |
| Nociti et al. (99) | 209 patients with MS | Association of Val66Met polymorphism with methylation level of the BDNF gene and disability | Association of higher disability/disease progression and hypomethylation of BDNF gene with higher BDNF levels |
| Portaccio et al. (100) | 98 patients with MS | Association of Val66Met polymorphism with disability and cognition | Higher prevalence of Val66Met polymorphism in male patients and patients with greater disability Positive association of Val66Met polymorphism with better cognitive performance and higher EDSS |
| Yalachkov et al. (86) | 36 patients with RRMS, PPMS, CIS | Effect of BDNF on disability and cognition after relapse | Association of disability improvement with higher serum BDNF levels and cognitive improvement with higher BDNF levels in CSF at baseline with a positive correlation between serum BDNF and EDSS improvement and CSF-BDNF with z-score change in cognitive tests |
| Zivadinov et al. (1) | 209 patients with MS (108 with cognitive testing) | Effect of Val66Met polymorphism on brain morphometry and functionality measured by MRI and cognitive testing | Positive association of Val66Met polymorphism with normalized grey matter volume and negative association with T2 lesion volume onyl trend towards a positive |
5. Effect of BDNF on MRI parameters
Again, more studies analysed the association of the Val66Met polymorphism to MRI characteristics in pwMS, compared to only few studies assessing the association of these to BDNF levels.
Comini-Frota and colleagues examined the correlation between BDNF and T2-lesion number in 28 patients with RRMS compared to 28 healthy controls. While they found BDNF levels correlating negatively with the number of lesions, BDNF levels did not correlate with T1 hypointense lesions or the presence of gadolinium-enhancing lesions. The authors concluded that as BDNF controls remyelinating processes, no correlation with T1 lesions as chronic lesions was found (102). However, this argumentation does not fully explain the missing correlation between BDNF and gadolinium-enhancing lesions as they display acute inflammatory lesions (102). Furthermore, another study found a negative correlation of serum BDNF levels with T1 lesion volume as well as quotient of T1 lesion volume to total lesion volume, displaying the relative contribution of T1 lesion volume to the total lesion volume, while no correlations were found for CSF BDNF level (103). In contrast, Weinstock-Guttman and colleagues found an association between higher BDNF concentrations and higher inflammation in white matter (WM), correlating with a higher WM volume. Moreover, they found that higher BDNF is associated with microscopic damage in the normal-appearing WM (NAWM) and concluded that BDNF may play a role in the early formation of inflammatory lesions or that diffuse inflammatory infiltrates without correlate on MRI are the pathological explanation for this (52). Regarding magnetization transfer ratio (MTR), the study found a negative correlation with increased BDNF, while no correlation between grey matter (GM) volume and normal appearing GM (NAGM) MTR was found with BDNF-levels (52), which might indicate that BDNF secretion only increases in inflammatory tissue (37).
However, more studies investigated the relationship between Val66Met polymorphism with MRI changes.
Though some studies reported a higher risk of GM atrophy in pwMS with Val66Met polymorphism (49, 91), most studies reported a protective effect of the SNP on brain atrophy (52, 58, 90) as well as GM atrophy (1, 52, 58, 60). Though higher GM volume was found throughout the brain in pwMS with Val66Met polymorphism, higher volume was especially found in the cingulate (1, 60), inferior frontal gyrus, parahippocampal gyrus, middle temporal gyrus, precentral gyrus, and inferior parietal lobule of the left cerebral hemisphere, in the right cerebral hemisphere, in the precuneus and middle frontal gyrus (1), consistent with regions of pronounced expression of BDNF (104, 105). As the highest atrophy related to the lesion volume in pwMS was found in the anterior cingulate (106) especially on the left hemisphere (107), a local influence of the polymorphism on BDNF secretion and thus on local inflammation has also been discussed to have a protective effect on regional brain volume in pwMS (58). Decreased cortical thickness was found in one study (108), thus a detrimental effect of the SNP was hypothesized. In another study, higher hippocampal volumes were found in pwMS with Val66Met polymorphism, compared to Val homozygotes (109). Therefore, protection of hippocampal tissue was assumed (109). Moreover, an association of the Val66Met polymorphism was found with lower T2-lesion volume (1) in comparison with Val66Val patients, though other studies did not find differences between groups in T2 or T1 lesion volume (45, 52, 108). Matching these structural imaging findings, increased functional connectivity between the hippocampus and posterior cingulate cortex was found in pwMS with Val66Met polymorphism during an episodic memory task (92). Still, two other studies reported a negative impact of the SNP polymorphism on functional activity of the brain. While Egan and colleagues showed an altered hippocampal activation (55), another study showed altered functional connectivity extending the hippocampal system with decreased functional connectivity in the ventromedial PFC and ACC-networks (91).
Overall, the study results indicate lower rates of brain atrophy and less pronounced signs of damage associated with BDNF. Regarding the Val66Met SNP, most studies brought evidence that the SNP has different effects on MRI measures in healthy controls compared to pwMS with the majority of studies assuming a protective effect of Val66Met polymorphism in pwMS. An overview of the above-mentioned studies can be found in Table 3.
Table 3.
Overview of the above-mentioned studies investigating the relationship between BDNF and MRI characteristics.
| Study | Participants | Parameters | Results |
|---|---|---|---|
| Brod et al. (103) | 13 patients with active MS without immunotherapy | Association of serum and CSF biomarkers with MRI characteristics | Association of increased CSF neuronal cell adhesion molecules (NCAM) with T1 activity and of CSF and serum NCAM with T1 lesion volume Negative association of serum BDNF and relative contribution of T1 lesion volume to the total lesion volume |
| Cerasa et al. (91) | 29 patients with RRMS 32 healthy controls (HC) |
Effect of Val66Met polymorphism on brain activity during spatial working memory task measured by fMRI | Increased activation of the parieto-prefrontal network, disengagement of the ventro-medial-prefrontal cortex and hippocampus in HCs, no effect in RRMS patients |
| Charil et al. (106) | 425 patients with early RRMS | Association of cortical thickness with other clinicoradiological characteristics | Association of cortical thickness with lesion load and disability, especially in cingulate gyrus, insula and associative cortical regions |
| Comini-Frota et al. (102) | 28 patients with MS, 28 healthy controls | Association of BDNF and MRI characteristics | Decreased BDNF levels in patients with MS with a negative association to T2/FLAIR lesion load |
| Conner et al. (104) | Animal study (adult rats) | Detailed mapping of BDNF immunoreactivity and synthesis | Synthesis of BDNF in neurons with anterograde axonal transportand storage in axonal terminals |
| Dinacci et al. (58) | 45 patients with MS, 34 healthy controls | Association of Val66Met polymorphism with MRI characteristics | Lower grey matter volume in Val66Val patients compared to healthy controls, but not in Val66Met patients compared to healthy controls |
| Dolcetti et al. (108) | 218 patients with RRMS | Association of Val66Met polymorphism and CSF levels of pro- and anti-inflammatory molecules with clinicoradiological characteristics | Association of Val66Met polymorphism and the proinflammatory molecules MCP-1, IL-8, TNF, Eotaxin, and MIP-1b and cortical atrophy at time of diagnosis, but not with clinical characteristics |
| Fera et al. (92) | 26 RRMS patients without cognitive impairment, 25 healthy controls | Effect of Val66Met polymorphism on hippocampal function in fMRI during episodic memory task | Higher activity parahippocampal, in left posterior hippocampus and left posterior cingulate cortex during encoding and retrieval as well as higher connectivity between hippocampus and posterior cingulate cortex during retrieval in Val homozygote RRMS patients opposite effects in healthy controls |
| Giordano et al. (90) | 100 patients with progressive MS | Effects of BDNF and NTRK2 genes on motor recovery | Greater motor improvement after rehabilitation in Val66Met carriers |
| Liguori et al. (49) | 50 patients with RRMS, 50 healthy controls | Effect of Val66Met on MRI characteristics and cognition | Lower grey matter volume in patients withVal66Met polymorphism No association of Val66Met polymorphism with cognition in patients with RRMS and healthy controls |
| Liguori et al. (45) | 36 inactive patients with RRMS and 37 healthy controls | Effect of Val66Met polymorphism and BDNF levels on patients with RRMS over 24 months | Higher BDNF levels in patients with RRMS compared to healthy controls, regardless immunotherapy No correlation of Val66Met polymorphism with clinical or MRI characteristics |
| Meo et al. (109) | 50 patients with MS, 15 healthy controls | Effect of Val66Met polymorphism on hippocampal subfield volumes and cognition | Patients with MS had lower volume of hippocampus-amygdala transition area, cornus ammonis (CA1), granule cell layer of dentate gyrus, CA4 and CA3 of left hippocampal head, molecular layer of the left hippocampal body; presubiculum of right hippocampal body and right fimbria Val66Met polymorphism was associated with higher volume of hippocampal tail; CA1, ML, CA3, CA4, and GCL-DG of left hippocampal head; CA1, ML, and CA3 of the left hippocampal body; left hippocampus-amygdala transition area, presubiculum of the right hippocampal headNegative association of higher lesion load with lower volume of presubiculum of right hippocampal body Positive association of left hippocampal tail volume withvisuospatial memory and left hippocampal head volume with semantic fluency |
| Prinster et al. (107) | 188 patients with MS | MRI characteristics and association with disease duration and severity | Decreased grey matter volume of left fronto-temporal cortex and precuneus, anterior cingulate gyrus, bilateral caudate nuclei, cortical grey matter of postcentral area in patients with MS |
| Ramasamy et al. (60) | 188 patients with MS | Effect of Val66Met polymorphism on regional grey matter in MRI | Higher grey matter volume in the cingulate of patient with MS with Val66Met polymorphism compared to Val66Val patients |
| Sarchielli et al. (37) | 20 patients with RRMS (7 with acute relapse), 15 patients with SPMS, 20 healthy controls | Association of BDNF production by PBMCs unstimulated and stimulated with phytohemagglutinin, anti-OKT3 AB and MPB with clinicoradiological characteristics | Patients with MS had lower volume of hippocampus-amygdala transition area, cornus ammonis (CA1), granule cell layer of dentate gyrus, CA4 and CA3 of left hippocampal head, molecular layer of the left hippocampal body; presubiculum of right hippocampal body and right fimbria Val66Met polymorphism was associated with higher volume of hippocampal tail; CA1, ML, CA3, CA4, and GCL-DG of left hippocampal head; CA1, ML, and CA3 of the left hippocampal body; left hippocampus-amygdala transition area, presubiculum of the right hippocampal head Higher BDNF in supernatants of unstimulated and stimulated PBMCs in RRMS patients during and after relapse compared to stable phaseLower BDNF in unstimulated and stimulated PBMC supernatants of patients with SPMS compared to healthy controls, especially in patients with recent EDSS worsening (≥1 point in the last 6 months) Positive association of left hippocampal tail volume withvisuospatial memory and left hippocampal head volume with semantic fluency |
| Weinstock-Guttman et al. (52) | 52 patients with relapsing MS | Association of BDNF with clinical and MRI variables | Positive association of BDNF with contrast-enhancing lesion volume and higher white matter volume as well asnegative Association of MTR of contrast-enhancing lesion volume and normal-appearing white matter with BDNF, both after stimulation with anti-CD3 and anti-CD28; Decreasing BDNF secretion with increasing disease duration; No association with Val66Met polymorphism |
| Zivadinov et al. (1) | 209 patients with MS (108 with cognitive testing) | Effect of Val66Met polymorphism on brain morphometry and functionality measured by MRI and cognitive testing | Positive association of Val66Met polymorphism with normalized grey matter volume and negative association with T2 lesion volume only trend towards a association of Val66Met polymorphism with PASAT |
6. Effect of immunotherapies on BDNF
Immunotherapies have been hypothesized to modulate the disease course not only by exerting anti-inflammatory effects but also neuroprotective and neuroregenerative mechanisms. Therefore, the correlation of immunotherapies with BDNF was investigated in several studies. So far, especially interferon-beta (IFN- β) and glatiramer acetate have been analysed.
For glatiramer acetate, higher BDNF levels were found in pwMS (39, 45, 46, 110–112). In an ex-vivo study, a significant increase of BDNF levels in supernatants of unstimulated peripheral blood mononuclear cells (PBMC) was found 6 months after initiation of glatiramer acetate treatment, supported by studies analysing glatiramer acetate specific T-cell lines (110). As a possible mechanism, a shift of pro-inflammatory T helper (TH) 1 cells to anti-inflammatory TH2 cells, which is the main therapeutic action of glatiramer acetate, is thought to promote neuroregenerative mechanisms due to BDNF induction and release (110, 113). Furthermore, besides anti-inflammatory TH2-cytokines, glatiramer acetate-specific cells were thought to express BDNF themselves (95, 114, 115). This is further supported by animal studies also providing evidence that glatiramer acetate exhibits neuroprotective effects through BDNF. In EAE, increased BDNF levels were found after treatment initiation (39, 111, 112, 116–118) as well as an augmentation of BDNF expression in histological glatiramer acetate treated EAE (116) with reduced axonal damage and decreased neurodegeneration (119). Moreover, in EAE the clinical efficacy of glatiramer acetate was limited after BDNF deletion (120). However, other studies showed no increase of BDNF levels in pwMS treated with glatiramer acetate (50, 95, 121).
In clinically stable patients with INF-β treatment, significantly increased BDNF levels were found (38, 46) compared to treatment naïve patients, sustained after 6 months as well as a trend to increased level after 1 year in RRMS, but not in SPMS-patients (43). Though patients treated with INF-ß showed higher BDNF levels in relapse-free intervals compared to healthy controls, no additional increase of BDNF was found in relapses (42). Moreover, patients treated with IFN-β not only had higher BDNF levels compared to treatment naïve patients but also in comparison to patients treated with mitoxantrone with an inverse correlation between BDNF levels and degree of disability (51). Contrary to this, some studies reported no effect of IFN-β on BDNF levels (15, 43, 45, 122). In a mixed cohort with 38 subjects treated with IFN-β and 12 with glatiramer acetate, no significant increase of BDNF levels was found after 1 year, with no differences between treatment groups (123).
Studies investigating the effect of other immunotherapies than INF-ß and glatiramer acetate are rare. However, Golan and colleagues showed a significant increase of BDNF in patients treated with fingolimod after 6 and 12 months (121), which is in line with a previous animal study, showing that the neuroprotective effect of Fingolimod is associated with the induction of the BDNF production (124). One study also could show increased BDNF levels in RRMS-patients treated with natalizumab (125). Moreover, significantly lower BDNF concentrations in patients treated with high-dose immunoglobulins were found, possibly due to interference of immunoglobulins with neuroprotective mechanisms (122). Regarding the effects of therapy of acute relapses with glucocorticoids, increased BDNF levels could be shown in EAE (95), but could not be confirmed in humans (37, 42).
To summarize, most studies hypothesized increased BDNF concentrations in patients treated with glatiramer acetate and IFN-β, while the number of studies in patients with other immunotherapies was too small to draw a conclusion. For details of the respective studies, see Table 4.
Table 4.
Overview of the above-mentioned studies investigating the relationship between BDNF and immunotherapies.
| Study | Participants | Parameters | Results |
|---|---|---|---|
| Aharoni et al. (117) | Animal study (mice) | Expression of TH2/3 cytokines and neurotrophic factors in glatiramer acetate specific T-cells | Increased levels of BDNF and IL-10 and transforming growth factor by astrocytes and microglia (among others) in glatiramer acetate treated mice |
| Aharoni et al. (116) | Animal study (mice) | Production of BDNF and other neurotrophins, as well as histopathology under glatiramer acetate treatment | Increased neurotrophins in treatment-naïve mice after EAE induction with lower concentrations over time compared to control mice Increased levels of BDNF, NT2 and NT3 in various brain regions under glatiramer acetate treatment with particularly high lesional concentration by migration of neuronal progenitors |
| Azoulay et al. (39) | 74 patients with RRMS (26 patients treated with IFN, 27 with glatiramer acetate, 21 without immunotherapy), 28 healthy controls | Measurement of BDNF (ELISA) | Lower BDNF levels in blood and CSF in patients with RRMS, increased in patients treated with glatiramer acetate Lower BDNF levels in patients with RRMS with acute relapse compared to stable patients with RRMS Only trend to higher BDNF levels in patients treated with IFN-β |
| Azoulay et al. (38) | 22 treatment-naïve patients with RR-MS, 27 IFN-β1a-treated RR-MS patients | BDNF in the supernatants of PBMCs (ELISA) | Higher BDNF levels in patients with RRMS treated with IFN-β1a, with upregulation by stimulation with anti-CD40 |
| Blanco et al. (110) | 19 treamtent-naïve patients with MS started on glatiramer acetate | Measurement of BDNF in unstimulated and stimulated (anti-CD3, anti-CD28) supernatants from PBMC by ELISA and intracellular cytokine production by flow Cytometry over 21 months | Decrease of BDNF production as well as INF-y producing total lymphocytes, CD4+ and CD8+ T cells, reduced percentage of IL2 producing CD4+ and CD8+ T-cells; increase of CD3+ and CD4+ and CD4 + CD45RA + T-cells after 6 months |
| Caggiula et al. (43) | 25 patients with RRMS, 14 patients with SPMS treated with IFN-β | Production of BDNF, NGF, GDNF, NT3, NT4 by PBMCs | Increasing BDNF levels in clinical stable patients with RRMS after 6 months of IFN-β treatment |
| Chen et al. (111) | 12 patients with MS treated with glatiramer acetate | BDNF production in different T-cell lines | Significant higher BDNF levels by resting T-cell lines Higher BDNF levels especially in glatiramer acetate stimulated T-cell lines |
| Ehling et al. (95) | 19 patients with RRMS (12 treated with glatiramer acetate, 7 treatment-naïve) | Serum BDNF over 24 months | No difference between patients treated with glatiramer acetate and treatment-naïve patients and no change of BDNF serum levels over time |
| Golan et al. (121) | 21 patients with MS | BDNF, GDNF, ß-NGF, NT-3, NT-4, FGF, EGF, VEGF (ELISA) before fingolimod treatment and after 6 and 12 months | Increaed BDNF levels after initiation of treatment with fingolimod |
| Kalinowska-Lyszczarz et al. (123) | 50 treatment naïve patients with RRMS, 39 healthy controls | BDNF levels in PBMCs lysates over one year of immunomodulation with IFN-β or glatiramer acetate (ELISA) | Lower BDNF levels in patients with MS compared to healthy controls No change of BDNF levels under immunotherapy without difference between treatment groups |
| Lee et al. (120), | Animal study (mice) | Effect of glatiramer acetate in BDNF knock-out mice | Limited clinical efficacy of glatiramer acetate after BDNF deletion in early disease phases |
| Liguori et al. (45) | 36 inactive patients with RRMS and 37 healthy controls | Effect of Val66Met polymorphism and BDNF levels on patients with RRMS over 24 months | Higher BDNF levels in patients with RRMS compared to healthy controls, regardless immunotherapy |
| Lindquist et al. (42) | 15 patients with RRMS, 1 patient with SPMS during acute relapse | Cytokine levels (TNF-a, iNOS, CNTF, BDNF) in PBMCs (flow-cytometric approach) | No effect of glucocorticoids on BDNF levels Increased BDNF levels in patients treated with IFN-β |
| Mehrpour et al. (51) | 82 patients with MS (15 treated with avonex, 13 with Rebif, 27 with Betaferon, 3 with Mitoxantrone, 2 with immunoglobulins, 22 without immunotherapies) | Serum BDNF levels (ELISA) | Higher BDNF levels in patients treated with IFN-β1b compared to patients treated with Mitoxantrone/patients without immunotherapies Negative correlation of BDNF with clinical impairment Patients with BDNF levels over 190 pg/ml indicated EDSS < 3 with 80% sensitivity |
| Petereit et al. (122) | 27 patients with RRMS, 10 healthy controls | BDNF blood level | Higher BDNF level patients with MS compared to healthy controls IFN-β and low dose immunoglobulin had no effect on BDNF levels, while high-dose immunoglobulin decreased BDNF levels |
| Sarchielli et al. (37) | 20 patients with RRMS (7 with acute relapse), 15 patients with SPMS, 20 healthy controls | Association of BDNF production by PBMCs unstimulated and stimulated with phytohemagglutinin, anti-OKT3 AB and MPB with clinicoradiological characteristics | Higher BDNF in supernatants of unstimulated and stimulated PBMCs in RRMS patients during and after relapse compared to stable phase Lower BDNF in unstimulated and stimulated PBMC supernatants of patients with SPMS compared to healthy controls, especially in patients with recent EDSS worsening (≥1 point in the last 6 months) |
| Sarchielli et al. (46) | 60 patients with RRMS (20 patients treated with IFN-β, 20 patients with glatiramer acetate, 20 patients with high dose immunoglobulins) | Cytokine and BDNF production by PBMCs over 2 years stimulated with Phytohemagglutinin, anti-OKT3 antibody, myelin basic protein | Increasing BDNF levels over 3 months in patients with MS treated with glatiramer acetate, afterwards levels remained stable but significantly higher compared to controls Decreased IFN-g, IL4, IL5, IL10 in patients treated with glatiramer acetate No effect of IFN-β1a/minimal decrease of BDNF levels under immunoglobulin treatment Increase of IL10 in patients treated with IFN-β1a |
| Shajarian et al. (15) | 45 patients with RRMS (32 treated with IFN-β, 13 treatment naïve), 45 healthy controls | Plasma BDNF and IL6 (ELISA) | Significant lower BDNF and higher IL6 in patients with MS compared to healthy controls No significant effect of IFN-β on BDNF plasma levels, but significant positive correlation of BDNF and IL-6 |
| Smith et al. (124) | Animal study (mice) | Association of brain volume in 7 T MRI with BDNF levels in blood, cerebrospinal fluid | Progressive brain volume loss despite clinical stability Less atrophy of cerebellum and striatum in mice treated early with fingolimod due to increased BDNF levels No influence of teriflunomide on atrophy rates |
| Vacaras et al. (50) | 19 patients with RRMS treated with glatiramer acetate, 11 patients with RRMS without immunotherapy, 12 healthy controls | BDNF in plasma, TrkB activation over 1 year | Reduced BDNF plasma level in patients with MS, no influence of glatiramer acetate after one year of sustained therapy |
| Vacaras et al. (125) | 22 patients with RRMS (11 treatment naïve, 11 treated with natalizumab), 9 patients with SPMS, 20 healthy controls | BDNF in plasma (ELISA) | Higher BDNF levels in patients with MS treated with natalizumab |
| Ziemssen et al. (118) | Patient with MS treated with glatiramer acetate, Healthy control | Glatiramer acetate specific T-cell lines, BDNF reverse-transcription PCR and two different BDNF measurement methods | BDNF production of all glatiramer acetate specific TH1, TH2 and TH0 after stimulation |
| Ziemssen et al. (112) | Patients with MS treated with glatiramer acetate, healthy controls | Glatiramer acetate specific T-cell lines, BDNF reverse-transcription PCR and two different BDNF measurement methods | BDNF production of all glatiramer acetate specific TH1, Th2 and Tho cell lines after stimulation |
7. Effect of autologous hematopoietic stem cell transplantation on BDNF
Autologous hematopoietic stem cell transplantation (HSCT) has received increasing attention in the field of MS therapy in the past few years. Although studies could show a sustained suppression of inflammation and therefore disease activity, the exact mechanisms of action remain unclear (126). Muraro and colleagues showed a significant increase of naïve CD4-positive T-cells two years after HSCT, suggesting the suppression of inflammatory activity in pwMS after HSCT does not depend on persisting suppression of lymphocytes, but qualitative immunological changes (126). Consequently, an increasing number of studies with follow-up data from large registries have shown positive effects on disease activity with stabilization (127), disease severity as measured by EDSS either with stabilization or even improvement (127), fatigue and quality of life (128). Positive effects of HSCT have also been demonstrated on paraclinical markers of disease activity such as new or enlarging T2 lesions or contrast-enhancing lesions (127). However, mostly patients with active RRMS or SPMS benefit from HSCT (129). In a study comparing immunotherapies and HSCT in 110 patients with RRMS, non-myeloablative HSCT resulted in prolonged time to disease progression (130).
Our literature research revealed only one study examining the effect of HSCT on BDNF levels in serum and CSF (127). In 14 pwMS, serum BDNF levels at baseline measurement were significantly higher compared to controls with a significant decrease over 12 months, almost at the level of healthy controls. Furthermore, a significant decrease of BDNF levels was also found in CSF, though CSF BDNF levels were only accessible in 9 patients. Possibly, the decreasing BDNF levels were due to the sustained suppression of CD4+ T-cells. However, only a positive correlation was found between CSF BDNF level and the T2 lesion load at baseline and at 12 month follow-up. Though one could assume a possible detrimental effect of decreased BDNF, no neurological deterioration nor correlations between BDNF and atrophy measurement were found. Blanco and colleagues provided evidence that BDNF levels decreased due to HSCT, possibly due to a shift of pro-inflammatory TH1 to anti-inflammatory TH2-cells, supporting the hypothesis that HSCT does not only has immunosuppressive but also immunomodulatory effects (127).
As HSCT becomes more and more relevant in the treatment of MS, it is likely that the number of studies analysing the immunomodulatory effects and its relationship with BDNF will increase over the next few years. Table 5 summarises the studies mentioned above.
Table 5.
Overview of the above-mentioned studies investigating the relationship between BDNF and HSCT.
| Study | Participants/Inclusion criteria | Results |
|---|---|---|
| Muraro et al. (126) | 7 patients with MS with progression of 1.5/1 point/s in EDSS (when EDSS ≤6/≥6) in the last year before HSCT (2 years follow up) | Significant increase of naïve CD4+ cells with recent thymic origin and decrease of memory T-cells with a broader spectrum of clonal diversity and renewal of clone-specific characteristics after HSCT |
| Blanco et al. (127) | 14 patients with MS treated with HSCT (3 years follow up) | Significant decrease of serum BDNF levels over 12 months after HSCT correlation of CSF-BDNF levels with T2 lesion load before and 12 months after HSCT |
| Giedraitiene et al. (128) | 18 patients with highly active MS treated with AHSCT (2 years follow up) | Improvement of physical functioning, vitality, fatigue, pain, decrease of EDSS with positive impact on health related Quality of life |
| Mancardi et al. (129) | 74 patients with MS treated with AHSCT (median of 48.3 months follow up) | 66% patients remained stable or improved after 5 years 2 patients died |
| Burt et al. (130) | 55 patients with MS treated with HSCT and 55 patients with MS treated with immunotherapies (5 years follow up) | Disease progression in 3 patients treated with HSCT and 34 patients treated with immunotherapies EDSS improvement in patients treated with HSCT, increase of EDSS in patients treated with immunotherapies |
8. Influence of physical activity and exercise training on BDNF
In recent years, there has been increasing evidence that physical activity as well as exercise has positive effects on pwMS, exerting neuroprotective effects and thus slowing neurodegeneration and disease progression (131). It is hypothesized that BDNF – as well as other neurotrophins – are secreted in dependency of neuronal activity (132–134) and due to certain factors transmitted in the bloodstream from peripheral organs as skeletal muscles (135–137). Hence, muscle activity leads to increasing neurotrophin levels in the blood, ultimately migrating in the central and peripheral nervous system (135, 137). As a result, there have been numerous studies linking neuroprotective effects of exercise to neurotrophic factors such as BDNF (138, 139). In case of running, increasing intracellular Ca2+ concentrations and induction of CaMK via CREB were found to induce BDNF synthesis (23, 140). Still, it is controversial whether peripheral BDNF can pass the blood–brain barrier to induce effects in the CNS. Hakansson and colleagues found an immediate increase of serum BDNF levels after 35 min of physical activity, while participants doing cognitive training or mindfulness practice for 35 min showed no increases (88). A study assessing the effect of resistance training on BDNF within two hours after the training found no within and between-group differences before and no within-group differences after the 24 weeks long training intervention (141). Moreover, four other studies failed to assess within or between-group differences in BDNF serum level (142–145), while two studies only assessed within group differences with an increase of BDNF level during the exercise intervention (146, 147). However, the majority of studies assessed between-group differences favouring the exercise intervention (148–153). In a meta-analysis assessing the effects of exercise training on neurotrophic factors, the results for different neurotrophins (NGF, CNTF, IGF-1, NT3-5, GDNF, PDGF as well as VEGF) remained inconclusive, but for BDNF a significant increase after training intervention was found (154). The main problem making the interpretation of these results difficult is the different handling of the samples and the usage of different analysing kits with different sensitivity and detection ranges as well as the different training interventions, the frequency of the training as well as the duration of the intervention. Though studies in healthy controls indicated that the modality of the training as well as the intensity and duration are crucial for the positive effects of training on increasing BDNF levels (155–162), a study in pwMS found no difference of the immediate BDNF increases after continuous moderate or high-intensity interval aerobic training (163). However, aerobic exercise seems to be one of the most potent training forms regarding the effect on neurotrophic factors (164), which was confirmed for BDNF by a recent meta-analysis (154). While studies evaluating the effect of exercise on the expression of neurotrophic factors and the link to neuroprotection in humans have not been conclusive so far (154), animal models already brought strong evidence for exercise-induced increase of BDNF (138, 139, 165–168). Moreover, effects of physical training on cognitive functions mediated by BDNF were analysed in several studies. Hakansson and colleagues found a positive effect on working memory function and peripheral BDNF levels after physical activity (88). Loprinzi and colleagues furthermore reviewed studies focusing on exercise effects on memory and the role of BDNF (73). Most studies found increasing serum BDNF levels after exercise, with 8 studies also blocking BDNF pharmacologically to evaluate exercise effects on memory. When BDNF was blocked, no effects of exercise on memory could be found. Without blocking BDNF, almost half of the studies found a positive effect of exercise on memory (73).
Interestingly, only a few studies assessed the association of training with other characteristics of disease activity as MRI parameters. Savšek and colleagues found protective effects of 12 week aerobic training on atrophy of some substructures as well as a decreasing active lesion volume and count, but without effect on total brain volume, grey matter atrophy, and T2 lesion volume and count as well as cortical lesion count, accompanied with increases of BDNF serum levels in the exercise group (152).
All in all, there is emerging evidence that physical activity increases serum BDNF levels with positive effects on cognition and MRI characteristics. However, the endurance of these increases as well as the effects on physical disability remains subject of further studies. Table 6 provides an overview of the studies assessing the relationship between BDNF and training.
Table 6.
Overview of the above-mentioned studies investigating the relationship between BDNF and training.
| Study | Participants | Parameters | Results |
|---|---|---|---|
| Abbaspoor et al. (142) | 20 female patients with MS | Combined functional training or control group for 8 weeks, 3 days per week; measurement of BDNF and IGF1 | No difference for BDNF, IGF1 increased in training group |
| Banitalebi et al. (148) | 94 female pwMS | 12 weeks (3sessions/week) supervised multimodal exercise program | Increase of BDNF, NT3, NT4/5 levels |
| Begliuomini et al. (132) | 34 male healthy controls | BDNF serum levels (ELISA) every 4 h, 3 times per month | Circadian variations of BDNF with cortisol |
| Birken et al. (143) | 42 patients with progressive MS | 9 weeks endurance exercise training; BDNF, Irisin and Il-6 measurement | Increase of BDNF 30 min after bicycling; no changes after 22 sessions of training and no prolonged effects on Irisin and Il-6 |
| Diechmann et al. (154) | Metaanalysis (337 studies, 14 RCTs) | Effect of exercise on neurotrophin levels | 9 studies found between group differences for BDNF and 6 out of 12 studies favouring exercise |
| Dinoff et al. (157) | Metaanalysis (29 studies) | BDNF before and after exercise interventions for at least 2 weeks | BDNF higher after intervention; significant in aerobic, but not resistance training |
| Dinoff et al. (156) | Metaanalysis (55 studies) | Effect of single-session training on BDNF levels | Single-session training increased BDNF levels in healthy controlsLonger duration of exercises was associated with greater increases of BDNF in males but not in females with higher increases in plasma than in serum |
| Eftekhari und Etemadifar (149) | 30 female pwMS | Pilates 3 times per week for 8 weeks vs control group; BDNF and Il-10 measurement | No changes in Il-10, but increase of BDNF in exercise group |
| Ferris et al. (158) | 15 healthy controls | 2 days 30-min endurance training on bike | No significant change in BDNF correlation of BDNF with lactate cognition improved after all exercise conditions, but did not correlate with BDNF changes |
| Hakansson et al. (88) | 23 healthy controls | 35 minutes of aerobic exercice at moderate level, cognitive training through a computerized working memory training program or mindfulness practice using an app | Increase of serum BDNF levels after aerobic training, but not after cognitive training oder mindfulness practicePositive association of serum BDNF levels and working memory function |
| Jørgensen et al. (141) | 30 patients with RRMS | 24 weeks of resistance training; measurement of BDNF and S1P levels | No changes within or between groups |
| Khademosharie et al. (150) | 24 pwMS | 12 weeks resistance and endurance training, 3 sessions per week | No changes in Il-10, butincrease of BDNF in exercise group |
| Loprinzi (73) | Metaanalysis (52 studies: 26 animal studies, 15 in humans) | Association of cognition and BDNF | All animal studies showed improvement of memory, 97% increase of BDNF with 8 studies showing BDNF as mediator of the positive association of training and cognition.44% of the studies in humans found a positive association of BDNF and cognitive improvement with 40% of these assuming BDNF as mediator |
| Mandolesi et al. (166) | Animal study (mice) | Running wheel | Positive effect on axonal damage and loss of myelin associated proteins |
| Mokhtarzade et al. (151) | 63 patients with RRMS | Cycling, 3 times a week for 8 weeks, measurement of s100b, NSE, IL10, TNF-a and neurotrophic factors | BDNF and platelet derived factor increased |
| Naghibzadeh et al. (146) | 26 patients with RRMS | 4 groups (aquatic exercise, Swedish massage, aquatic exercise + Swedish massage, control group), 3 sessions per week, 30 min each for 8 weeks, measurement of BDNF 48 h before first and after last sessions | BDNF and NGF increased in aquatic exercise alone as well as in combination with Swedish massage while Il-6 decreased |
| Okzul et al. (147) | 36 pw MS, 18 healthy controls | 8 weeks combined exercise training; measurement of SOCS1, SOCS4, BDNF | Increase of BDNF after 8 weeks of exercise training |
| Reycraft et al. (160) | 8 male healthy controls | 4 weeks of exercise (moderate intensity continuous training; vigorous-intensity continuous training; sprint interval training; no exercise); BDNF before/ immediately after/ 30 minutes after/90 minutes after exercise | BDNF increase depends on exercise intensity |
| Savsek et al. (152) | 28 pwMS | 12 weeks aerobic exercise twice a week, control group; BDNF measurement | Only weak increase of BDNF but with positive effect on brain atrophy |
| Schmolesky et al. (161) | 45 healthy male controls | Six exercise conditions based on different intensities on cycle ergometers; BDNF measurement | Increase of sBDNF by 32%, but without effect of exercise intensity or duration on the amount of BDNF increase |
| Schulz et al. (144) | 28 pwMS (immune-endocrine measurements), 39 patients (coordination measurements) | Aerobic training at 60% of VO2max for 30 min; measurement of cortisol, adrenocortico-relasing-hormone, epinephrine, norepinephrine, IL6, sIL6r, BDNF, NGF | No effects on endocrine and immune parameters or neurotrophic factors, but improvement of Quality of Life and coordinative functions |
| Souza et al. (167) | Animal study (mice) | strength training, endurance and exercise training | Improvement of clinical symptoms with decrease of proinflammatory cytokines (IFN-γ, IL-17, IL-1β), IL-6, MCP-1, TNF-α, and increase of CD25 and IL-10 levels |
| Szuhany et al. (162) | Metaanalysis (29 studies) | BDNF measurement after single-session vs. regular session exercise vs. resting levels following a program of regular exercise | Moderate effect of single-session exercise on BDNF with greater effect after regular exercise; significant effect of sex with less increase of BDNF in women |
| Vaynman et al. (140) | Animal study (rats) | 4 groups (sedentary with control injection, exercise with contral injection; sedentary with KN-62 injection, exercise with KN-62 injection), voluntary exercise paradigm with running wheel | CAMKII blocked the exercise-induced increase of BDNF and the transcription activator CREB |
| Waschbisch et al. (145) | 83 patients with RRMS | Groups based on subjective report on physical activity; Measurement of T-/B-/NK-cells, Monocytes, regulatory T-Cells; BDNF, Vitamin D, Spiroergomety and TCD | No association of training with BDNF |
| Wens et al. (153) | 22 pwMS, 19 healthy controls | BDNF measurement after exercise program over 24 weeks (5 times per 2 weeks) or control group | Significant increase of BDNF levels in exercise group |
| Wrann et al. (137) | Animal study (mice) | 30 days of endurance training; measurement of Fndc5 gene expression and BDNF (among others) | Increase of BDNF expression by FNDC5, a muscle protein induced by exercise |
| Xie et al. (168) | Animal study (mice) | Swimming at moderate to high intensity for 6 weeks | No influence on clinical symptoms but reduced infiltration of inflammatory cells and decreased demyelination of spinal cord, increase of IL-10 and TGF-β, as well as BDNF |
| Zimmer et al. (163) | 60 pwMS | High intensity aerobic exercise for 3 weeks vs. standard exercise programm; BICAMS, BDNF measurement | Improvement of verbal memory, but no increase of BDNF levels |
9. Discussion and conclusion
With increasingly effective therapeutic options to halt inflammation, mechanisms to reverse preexisting, already accumulated damage are becoming increasingly important in context of neuroinflammatory diseases as MS. We reviewed the current literature regarding BDNF, which is thought to play a central role in remyelination and thus neuroplasticity, neuroprotection, and memory, in different areas of MS.
In terms of cognition, studies assuming a positive impact of BDNF on cognitive functions outweigh studies assuming a detrimental effect, although it remains unclear whether the effect is limited on different cognitive domains or whether BDNF improves cognitive functions on a more global level. In this context, especially longitudinal, prospective studies are needed, which also include other so-called “hidden symptoms” of MS such as depression and fatigue.
With regard to the effect of BDNF on disability, especially the improvement of neurological deficits, there is even less evidence, although a positive influence of BDNF on the deficits is also to be favoured here.
However, most of the studies analysed the effect of BDNF on different clinicoradiological characteristics in patients with relapsing disease course or in mixed cohorts mainly consisting of patients with relapsing disease course, therefore, drawing conclusions on the effects of BDNF in patients with progressive disease is difficult. Still, as it is hypothesized that in patients with progressive disease neuroregenerative mechanisms are failing, one could assume that BDNF mainly plays a role in relapsing disease. To date, it is unclear why these mechanisms fail in patients with progressive disease. An exhaustion of BDNF because of compensatory increased synthesis and release in the early years of the disease could be an explanation but needs to be evaluated in further studies with a larger proportion of patients with progressive disease course compared to patients with relapsing disease course.
Furthermore, studies regarding the Val66Met polymorphism have not conclusively determined whether the SNP is a protective or harmful factor in pwMS, but the majority of studies hypothesize a protective effect through modulation of BDNF secretion and anti-inflammatory effects (60). Interestingly, the polymorphism, detectable in up to 72% of the population and thus in a large proportion of healthy subjects (54), seems to have different effects in healthy subjects and pwMS (1, 100). In conclusion, the pro-inflammatory milieu in which BDNF acts in pwMS seems to be essential for these different effects (1, 92, 100). Increased de-methylation of BDNF in an inflammatory milieu could lead to increased secretion and thus neuroprotection. However, Noiciti and colleagues assumed a detrimental effect of increased BDNF secretion as it may result in depletion of the functional reserves and thus faster accumulation of long-term disability (99).
In the author’s opinion, one of the main problems here is the lack of a standardized method for measurement of BDNF. BDNF can be measured in serum as well as normal and platelet-poor plasma. However, as no correlations between serum and plasma BDNF concentrations could be found (169), it has to be assumed that the material used has a relevant influence on the study results. In plasma, BDNF is mainly stored in platelets, therefore, only low concentrations of free BDNF can be assessed. In serum, BDNF is released from platelets due to coagulation. However, in most studies serum was used for measurement of peripheral BDNF concentrations (170). But besides the different material that can be used for measurement of BDNF levels, different methods have been described (170). Mainly, different techniques using immunoassays were used and validated (171), with most studies using enzyme linked immunosorbent assay (ELISA), and to a lesser extent western blot techniques (172). However, since 2013 a single molecule array (SIMOA) technology being able to detect fluid biomarkers on a single-molecule level (171) has been introduced and is increasingly used in studies.
However, despite the inconclusive results and technical difficulties, the author thinks that there is emerging evidence for a neuroprotective and neuroregenerative effect of BDNF in MS, as studies reporting a protective effect outweighed studies assuming detrimental effects of BDNF. BDNF could therefore be a promising biomarker in the field of MS. Still, there is an urgent need for longitudinal prospective studies to improve our understanding of the effects of BDNF in the CNS, especially in context of MS.
Most studies have already shown that BDNF concentrations increase during relapse, although the extent of the increase and thus presumably the neuroregenerative capacity and corresponding recovery from relapses appears to vary between individuals. In consequence, patients with insufficient neuroregenerative capacity could be identified at an early stage and primarily be put on high efficacy therapies. However, understanding which pwMS are at risk of incomplete recovery from relapses by measuring BDNF levels and analysing the association with different clinicoradiological characteristics would significantly improve the clinical management of MS patients.
It has already been shown for glatiramer acetate or IFN-β that BDNF concentrations increase after initiation of these immunotherapies, for glatiramer acetate possibly due to a switch from pro-inflammatory TH1 to anti-inflammatory TH2 cells. Therefore, further studies comparing the effect of baseline therapies vs. high efficacy therapies on BDNF concentrations are needed. The role of BDNF as a potential therapeutic agent to improve pre-existing neurological deficits should also be analysed in further studies. Nagahara and Tuszynski already reviewed the use of BDNF in different neurological diseases as Alzheimer’s and Parkinson disease as well as spinal cord injuries (173). However, to our best knowledge, similar studies in MS are lacking. Still, as positive results in studies evaluating the effect of BDNF therapy on spinal cord injuries promoting axonal regrowth were found, BDNF could also be a promising approach in multiple sclerosis reversing the demyelination in inflammatory CNS lesions.
Author contributions
MM: Writing – original draft, Writing – review & editing.
Glossary
Glossary
- AMP
adenosine monophosphate
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BDNF
brain derived neurotrophic factor
- CaMK
Ca2+-calmodulin-dependent protein kinases
- CNS
central nervous system
- CREB
cAMP response element-binding protein
- CSF
cerebrospinal fluid
- EAE
experimental autoimmune encephalomyelitis
- EDSS
expanded disability status scale
- ERK
extracellular-signal regulated kinases
- GM
grey matter
- HSCT
hematopoietic stem cell transplantation
- IFN-β
interferon-β
- IP3
inositol triphosphate
- MAPK
mitogen-activated protein kinase
- MS
multiple sclerosis
- mRNA
messenger ribonucleic acid
- MTR
magnetization transfer ratio
- NAGM
normal appearing grey matter
- NAWM
normal appearing white matter
- NF-ĸB
nuclear factor ĸB
- NMDA
N-methyl-D-aspartate
- nt-p75
neurotrophin receptor p75
- OPC
oligodendrocyte progenitor cell
- PBMC
peripheral blood mononuclear cells
- PKC
protein kinase C
- PGC-1α
proliferator-activated receptor gamma coactivator 1-alpha
- PLC-γ
phospholipase C gamma
- PI3-K
phosphatidylinositol-3 kinase
- pwMS
patients with MS
- RRMS
relapsing remitting MS
- SNP
single nucleotide polymorphism
- SPMS
secondary progressive MS
- TH-cells
T-helper cells
- TNF
tumor necrosis factor
- TNFR
tumor necrosis factor receptor
- Trĸβ
tropomyosin-related kinase β
- WM
white matter
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Zivadinov R, Weinstock-Guttman B, Benedict R, Tamaño-Blanco M, Hussein S, Abdelrahman N, et al. Preservation of gray matter volume in multiple sclerosis patients with the met allele of the rs6265 (Val66Met) SNP of brain-derived neurotrophic factor. Hum Mol Genet. (2007) 16:2659–68. doi: 10.1093/hmg/ddm189 [DOI] [PubMed] [Google Scholar]
- 2.KhorshidAhmad T, Acosta C, Cortes C, Lakowski TM, Gangadaran S, Namaka M. Transcriptional regulation of brain-derived neurotrophic factor (BDNF) by methyl CpG binding protein 2 (MeCP2): a novel mechanism for re-myelination and/or myelin repair involved in the treatment of multiple sclerosis (MS). Mol Neurobiol. (2016) 53:1092–107. doi: 10.1007/s12035-014-9074-1 [DOI] [PubMed] [Google Scholar]
- 3.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. (2003) 4:299–309. doi: 10.1038/nrn1078 [DOI] [PubMed] [Google Scholar]
- 4.Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond). (2006) 110:167–73. doi: 10.1042/CS20050163 [DOI] [PubMed] [Google Scholar]
- 5.Lu B, Nagappan G, Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. (2014) 220:223–50. doi: 10.1007/978-3-642-45106-5_9 [DOI] [PubMed] [Google Scholar]
- 6.Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, et al. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci. (2000) 20:6888–97. doi: 10.1523/JNEUROSCI.20-18-06888.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Feng G-D, Liang Z, Vitale A, Jiao X-Y, Ju G, et al. In vitro beneficial activation of microglial cells by mechanically-injured astrocytes enhances the synthesis and secretion of BDNF through p38MAPK. Neurochem Int. (2012) 61:175–86. doi: 10.1016/j.neuint.2012.04.020 [DOI] [PubMed] [Google Scholar]
- 8.Asami T, Ito T, Fukumitsu H, Nomoto H, Furukawa Y, Furukawa S. Autocrine activation of cultured macrophages by brain-derived neurotrophic factor. Biochem Biophys Res Commun. (2006) 344:941–7. doi: 10.1016/j.bbrc.2006.03.228 [DOI] [PubMed] [Google Scholar]
- 9.Charlton T, Prowse N, McFee A, Heiratifar N, Fortin T, Paquette C, et al. Brain-derived neurotrophic factor (BDNF) has direct anti-inflammatory effects on microglia. Front Cell Neurosci. (2023) 17:1188672. doi: 10.3389/fncel.2023.1188672, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miao H, Li R, Han C, Lu X, Zhang H. Minocycline promotes posthemorrhagic neurogenesis via M2 microglia polarization via upregulation of the TrkB/BDNF pathway in rats. J Neurophysiol. (2018) 120:1307–17. doi: 10.1152/jn.00234.2018 [DOI] [PubMed] [Google Scholar]
- 11.Miron VE, Boyd A, Zhao J-W, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. (2013) 16:1211–8. doi: 10.1038/nn.3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu H-C, Huang H-B, Tseng H, Hsien-Yu A, Lu M-C. Brain-derived neurotrophic factor suppressed proinflammatory cytokines secretion and enhanced MicroRNA(miR)-3168 expression in macrophages. Int J Mol Sci. (2022) 23:570. doi: 10.3390/ijms23010570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stadelmann C, Kerschensteiner M, Misgeld T, Brück W, Hohlfeld R, Lassmann H. BDNF and gp145trkB in multiple sclerosis brain lesions: neuroprotective interactions between immune and neuronal cells? Brain. (2002) 125:75–85. doi: 10.1093/brain/awf015 [DOI] [PubMed] [Google Scholar]
- 14.Ksiazek-Winiarek DJ, Szpakowski P, Glabinski A. Neural plasticity in multiple sclerosis: the functional and molecular background. Neural Plast. (2015) 2015:307175. doi: 10.1155/2015/307175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shajarian M, Alsahebfosoul F, Etemadifar M. The effect of IFN-β treatment on plasma levels of BDNF and IL-6 in relapsing-remitting multiple sclerosis patients. Neuroimmunomodulation. (2021) 28:150–7. doi: 10.1159/000515595 [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto H, Gurney ME. Human platelets contain brain-derived neurotrophic factor. J Neurosci. (1990) 10:3469–78. doi: 10.1523/JNEUROSCI.10-11-03469.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cefis M, Chaney R, Quirié A, Santini C, Marie C, Garnier P, et al. Endothelial cells are an important source of BDNF in rat skeletal muscle. Sci Rep. (2022) 12:311. doi: 10.1038/s41598-021-03740-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo S, Kim W, Lok J, Lee S-R, Besancon E, Luo B-H, et al. Neuroprotection via matrix-trophic coupling between cerebral endothelial cells and neurons. Proc Natl Acad Sci USA. (2008) 105:7582–7. doi: 10.1073/pnas.0801105105, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. (1999) 13:450–64. doi: 10.1006/mcne.1999.0762 [DOI] [PubMed] [Google Scholar]
- 20.Bayas A, Hummel V, Kallmann BA, Karch C, Toyka KV, Rieckmann P. Human cerebral endothelial cells are a potential source for bioactive BDNF. Cytokine. (2002) 19:55–8. doi: 10.1006/cyto.2002.0892 [DOI] [PubMed] [Google Scholar]
- 21.Fresegna D, Bullitta S, Musella A, Rizzo F, de Vito F, Guadalupi L, et al. Re-examining the role of TNF in MS pathogenesis and therapy. Cells. (2020) 9:e2290. doi: 10.3390/cells9102290, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brum C, Stertz L, Borba E, Rumi D, Kapczinski F, Camozzato A. Association of serum brain-derived neurotrophic factor (BDNF) and tumor necrosis factor-alpha (TNF-α) with diagnosis of delirium in oncology inpatients. Revista brasileira de psiquiatria. (2015) 37:197–202. doi: 10.1590/1516-4446-2014-1450 [DOI] [PubMed] [Google Scholar]
- 23.Saha RN, Liu X, Pahan K. Up-regulation of BDNF in astrocytes by TNF-alpha: a case for the neuroprotective role of cytokine. J. Neuroimmune Pharmacol. (2006) 1:212–22. doi: 10.1007/s11481-006-9020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowhani-Rad Soodeh, Taherianfard M. (2019): Crosstalk between BDNF and TNFα in brain versus serum of the cuprizone-induced multiple sclerosis in C57BL/6 mice. Physiology and Pharmacology. Online verfügbar unter, Available at: https://www.semanticscholar.org/paper/Crosstalk-between-BDNF-and-TNF%CE%B1-in-brain-versus-of-Rowhani-Rad-Taherianfard/31d392be49773c998d1d1d2bdf1ede062c982c20
- 25.Bałkowiec-Iskra E, Vermehren-Schmaedick A, Balkowiec A. Tumor necrosis factor-α increases brain-derived neurotrophic factor expression in trigeminal ganglion neurons in an activity-dependent manner. Neuroscience. (2011) 180:322–33. doi: 10.1016/j.neuroscience.2011.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. (2014) 25:89–98. doi: 10.1016/j.tem.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: a key factor with multipotent impact on brain Signaling and synaptic plasticity. Cell Mol Neurobiol. (2018) 38:579–93. doi: 10.1007/s10571-017-0510-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casaccia-Bonnefil P, Gu C, Khursigara G, Chao MV. p75 neurotrophin receptor as a modulator of survival and death decisions. Microsc Res Tech. (1999) 45:217–24. doi: [DOI] [PubMed] [Google Scholar]
- 29.Ma D, Zhang H, Yin L, Xu H, Wu L, Shaji R, et al. Human iPSC-derived endothelial cells promote CNS remyelination via BDNF and mTORC1 pathway. Glia. (2024) 72:133–55. doi: 10.1002/glia.24466 [DOI] [PubMed] [Google Scholar]
- 30.Chen A, Xiong L-J, Tong Y, Mao M. Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol Med Rep. (2013) 8:1011–6. doi: 10.3892/mmr.2013.1628 [DOI] [PubMed] [Google Scholar]
- 31.An X, Yao X, Li B, Yang W, Cui R, Zhao G, et al. Role of BDNF-mTORC1 Signaling pathway in female depression. Neural Plast. (2021) 2021:6619515. doi: 10.1155/2021/6619515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao T, Qi Y, Li Y, Xu K. PI3 kinase regulation of neural regeneration and muscle hypertrophy after spinal cord injury. Mol Biol Rep. (2012) 39:3541–7. doi: 10.1007/s11033-011-1127-1 [DOI] [PubMed] [Google Scholar]
- 33.Akram R, Anwar H, Javed M, Rasul A, Imran A, Malik SA, et al. Axonal regeneration: underlying molecular mechanisms and potential therapeutic targets. Biomedicines. (2022) 10:123186. doi: 10.3390/biomedicines10123186, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong AW, Xiao J, Kemper D, Kilpatrick TJ, Murray SS. Oligodendroglial expression of TrkB independently regulates myelination and progenitor cell proliferation. J Neurosci. (2013) 33:4947–57. doi: 10.1523/JNEUROSCI.3990-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng A, Wan R, Yang J-L, Kamimura N, Son T, Ouyang X, et al. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat Commun. (2012) 3:1250. doi: 10.1038/ncomms2238, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prokopova B, Hlavacova N, Vlcek M, Penesova A, Grunnerova L, Garafova A, et al. Early cognitive impairment along with decreased stress-induced BDNF in male and female patients with newly diagnosed multiple sclerosis. J Neuroimmunol. (2017) 302:34–40. doi: 10.1016/j.jneuroim.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 37.Sarchielli P, Greco L, Stipa A, Floridi A, Gallai V. Brain-derived neurotrophic factor in patients with multiple sclerosis. J Neuroimmunol. (2002) 132:180–8. doi: 10.1016/s0165-5728(02)00319-3 [DOI] [PubMed] [Google Scholar]
- 38.Azoulay D, Mausner-Fainberg K, Urshansky N, Fahoum F, Karni A. Interferon-beta therapy up-regulates BDNF secretion from PBMCs of MS patients through a CD40-dependent mechanism. J Neuroimmunol. (2009) 211:114–9. doi: 10.1016/j.jneuroim.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 39.Azoulay D, Vachapova V, Shihman B, Miler A, Karni A. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: reversal by glatiramer acetate. J Neuroimmunol. (2005) 167:215–8. doi: 10.1016/j.jneuroim.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 40.Frota ER, Rodrigues DH, Donadi EA, Brum DG, Maciel DRK, Teixeira AL. Increased plasma levels of brain derived neurotrophic factor (BDNF) after multiple sclerosis relapse. Neurosci Lett. (2009) 460:130–2. doi: 10.1016/j.neulet.2009.05.057, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Lalive PH, Kantengwa S, Benkhoucha M, Juillard C, Chofflon M. Interferon-beta induces brain-derived neurotrophic factor in peripheral blood mononuclear cells of multiple sclerosis patients. J Neuroimmunol. (2008) 197:147–51. doi: 10.1016/j.jneuroim.2008.04.033 [DOI] [PubMed] [Google Scholar]
- 42.Lindquist S, Hassinger S, Lindquist JA, Sailer M. The balance of pro-inflammatory and trophic factors in multiple sclerosis patients: effects of acute relapse and immunomodulatory treatment. Multiple Sclerosis. (2011) 17:851–66. doi: 10.1177/1352458511399797 [DOI] [PubMed] [Google Scholar]
- 43.Caggiula M, Batocchi A, Frisullo G, Angelucci F, Patanella AK, Sancricca C, et al. Neurotrophic factors in relapsing remitting and secondary progressive multiple sclerosis patients during interferon beta therapy. Clin. Immunol. (2006) 118:77–82. doi: 10.1016/j.clim.2005.09.005, PMID: [DOI] [PubMed] [Google Scholar]
- 44.Gielen A, Khademi M, Muhallab S, Olsson T, Piehl F. Increased brain-derived neurotrophic factor expression in white blood cells of relapsing-remitting multiple sclerosis patients. Scand J Immunol. (2003) 57:493–7. doi: 10.1046/j.1365-3083.2003.01260.x [DOI] [PubMed] [Google Scholar]
- 45.Liguori M, Fera F, Patitucci A, Manna I, Condino F, Valentino P, et al. A longitudinal observation of brain-derived neurotrophic factor mRNA levels in patients with relapsing-remitting multiple sclerosis. Brain Res. (2009) 1256:123–8. doi: 10.1016/j.brainres.2008.11.047 [DOI] [PubMed] [Google Scholar]
- 46.Sarchielli P, Zaffaroni M, Floridi A, Greco L, Candeliere A, Mattioni A, et al. Production of brain-derived neurotrophic factor by mononuclear cells of patients with multiple sclerosis treated with glatiramer acetate, interferon-beta 1a, and high doses of immunoglobulins. Multiple Sclerosis. (2007) 13:313–31. doi: 10.1177/1352458506070146 [DOI] [PubMed] [Google Scholar]
- 47.Damasceno A, Damasceno B, Cendes F, Damasceno A, Moraes AS, Farias A, et al. Serum BDNF levels are not reliable correlates of neurodegeneration in MS patients. Mult Scler Relat Disord. (2015) 4:65–6. doi: 10.1016/j.msard.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 48.Naegelin Y, Saeuberli K, Schaedelin S, Dingsdale H, Magon S, Baranzini S, et al. Levels of brain-derived neurotrophic factor in patients with multiple sclerosis. Ann. Clin. Trans. Neurol. (2020) 7:2251–61. doi: 10.1002/acn3.51215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liguori M, Fera F, Gioia MC, Valentino P, Manna I, Condino F, et al. Investigating the role of brain-derived neurotrophic factor in relapsing-remitting multiple sclerosis. Genes Brain Behav. (2007) 6:177–83. doi: 10.1111/j.1601-183X.2006.00245.x [DOI] [PubMed] [Google Scholar]
- 50.Vacaras V, Major ZZ, Muresanu DF, Krausz TL, Marginean I, Buzoianu DA. Effect of glatiramer acetate on peripheral blood brain-derived neurotrophic factor and phosphorylated TrkB levels in relapsing-remitting multiple sclerosis. CNS Neurol Disord Drug Targets. (2014) 13:647–51. doi: 10.2174/1871527313666140618110049 [DOI] [PubMed] [Google Scholar]
- 51.Mehrpour M, Akhoundi FH, Delgosha M, Keyvani H, Motamed MR, Sheibani B, et al. Increased serum brain-derived neurotrophic factor in multiple sclerosis patients on interferon-β and its impact on functional abilities. Neurologist. (2015) 20:57–60. doi: 10.1097/NRL.0000000000000053 [DOI] [PubMed] [Google Scholar]
- 52.Weinstock-Guttman B, Zivadinov R, Tamaño-Blanco M, Abdelrahman N, Badgett D, Durfee J, et al. Immune cell BDNF secretion is associated with white matter volume in multiple sclerosis. J Neuroimmunol. (2007) 188:167–74. doi: 10.1016/j.jneuroim.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 53.Maisonpierre PC, le Beau MM, Espinosa R, Ip NY, Belluscio L, de la Monte SM, et al. Human and rat brain-derived neurotrophic factor and neurotrophin-3: gene structures, distributions, and chromosomal localizations. Genomics. (1991) 10:558–68. doi: 10.1016/0888-7543(91)90436-i, PMID: [DOI] [PubMed] [Google Scholar]
- 54.Shen T, You Y, Joseph C, Mirzaei M, Klistorner A, Graham SL, et al. BDNF polymorphism: a review of its diagnostic and clinical relevance in neurodegenerative disorders. Aging Dis. (2018) 9:523–36. doi: 10.14336/AD.2017.0717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. (2003) 112:257–69. doi: 10.1016/s0092-8674(03)00035-7 [DOI] [PubMed] [Google Scholar]
- 56.Chiaruttini C, Vicario A, Li Z, Baj G, Braiuca P, Wu Y, et al. Dendritic trafficking of BDNF mRNA is mediated by translin and blocked by the G196A (Val66Met) mutation. Proc Natl Acad Sci USA. (2009) 106:16481–6. doi: 10.1073/pnas.0902833106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Z-Y, Patel PD, Sant G, Meng C-X, Teng KK, Hempstead BL, et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. (2004) 24:4401–11. doi: 10.1523/JNEUROSCI.0348-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dinacci D, Tessitore A, Russo A, de Bonis ML, Lavorgna L, Picconi O, et al. BDNF Val66Met polymorphism and brain volumes in multiple sclerosis. Neurol. Sci. (2011) 32:117–23. doi: 10.1007/s10072-010-0433-z, PMID: [DOI] [PubMed] [Google Scholar]
- 59.Hamamcioglu K, Reder AT. Interferon-beta regulates cytokines and BDNF: greater effect in relapsing than in progressive multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England). (2007) 13:459–70. doi: 10.1177/1352458506069672 [DOI] [PubMed] [Google Scholar]
- 60.Ramasamy DP, Ramanathan M, Cox JL, Antulov R, Weinstock-Guttman B, Bergsland N, et al. Effect of Met66 allele of the BDNF rs6265 SNP on regional gray matter volumes in patients with multiple sclerosis: a voxel-based morphometry study. Pathophysiology. (2011) 18:53–60. doi: 10.1016/j.pathophys.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 61.Chen Z-Y, Jing D, Bath KG, Ieraci A, Khan T, Siao C-J, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. (2006) 314:140–3. doi: 10.1126/science.1129663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lang UE, Hellweg R, Sander T, Gallinat J. The met allele of the BDNF Val66Met polymorphism is associated with increased BDNF serum concentrations. Mol Psychiatry. (2009) 14:120–2. doi: 10.1038/mp.2008.80 [DOI] [PubMed] [Google Scholar]
- 63.Minelli A, Zanardini R, Bonvicini C, Sartori R, Pedrini L, Gennarelli M, et al. BDNF serum levels, but not BDNF Val66Met genotype, are correlated with personality traits in healthy subjects. Eur Arch Psychiatry Clin Neurosci. (2011) 261:323–9. doi: 10.1007/s00406-011-0189-3 [DOI] [PubMed] [Google Scholar]
- 64.Ozan E, Okur H, Eker C, Eker OD, Gönül A, Akarsu N. The effect of depression, BDNF gene val66met polymorphism and gender on serum BDNF levels. Brain Res Bull. (2010) 81:61–5. doi: 10.1016/j.brainresbull.2009.06.022 [DOI] [PubMed] [Google Scholar]
- 65.Yoshimura R, Kishi T, Suzuki A, Umene-Nakano W, Ikenouchi-Sugita A, Hori H, et al. The brain-derived neurotrophic factor (BDNF) polymorphism Val66Met is associated with neither serum BDNF level nor response to selective serotonin reuptake inhibitors in depressed Japanese patients. Prog Neuro-Psychopharmacol Biol Psychiatry. (2011) 35:1022–5. doi: 10.1016/j.pnpbp.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 66.Zhou Z, Lu T, Xu G, Yue X, Zhu W, Ma M, et al. Decreased serum brain-derived neurotrophic factor (BDNF) is associated with post-stroke depression but not with BDNF gene Val66Met polymorphism. Clin Chem Lab Med. (2011) 49:185–9. doi: 10.1515/CCLM.2011.039 [DOI] [PubMed] [Google Scholar]
- 67.Hashimoto T, Lewis DA. BDNF Val66Met polymorphism and GAD67 mRNA expression in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. (2006) 163:534–7. doi: 10.1176/appi.ajp.163.3.534 [DOI] [PubMed] [Google Scholar]
- 68.Tramontina J, Frey BN, Andreazza AC, Zandona M, Santin A, Kapczinski F. Val66met polymorphism and serum brain-derived neurotrophic factor levels in bipolar disorder. Mol Psychiatry. (2007) 12:230–1. doi: 10.1038/sj.mp.4001941 [DOI] [PubMed] [Google Scholar]
- 69.Amidfar M, de Oliveira J, Kucharska E, Budni J, Kim Y-K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. (2020) 257:118020. doi: 10.1016/j.lfs.2020.118020, PMID: [DOI] [PubMed] [Google Scholar]
- 70.Islam O, Loo TX, Heese K. Brain-derived neurotrophic factor (BDNF) has proliferative effects on neural stem cells through the truncated TRK-B receptor, MAP kinase, AKT, and STAT-3 signaling pathways. Curr Neurovasc Res. (2009) 6:42–53. doi: 10.2174/156720209787466028 [DOI] [PubMed] [Google Scholar]
- 71.Leal G., Bramham C. R., Duarte C. B. (2017): Chapter 8—BDNF and hippocampal synaptic plasticity. Litwack Gerald. (Ed.), Vitamins and hormones: Neurotrophins, Academic Press, 153–195 [DOI] [PubMed] [Google Scholar]
- 72.Seidler K, Barrow M. Intermittent fasting and cognitive performance - targeting BDNF as potential strategy to optimise brain health. Front Neuroendocrinol. (2022) 65:100971. doi: 10.1016/j.yfrne.2021.100971 [DOI] [PubMed] [Google Scholar]
- 73.Loprinzi PD. Does brain-derived neurotrophic factor mediate the effects of exercise on memory? Phys Sportsmed. (2019) 47:395–405. doi: 10.1080/00913847.2019.1610255 [DOI] [PubMed] [Google Scholar]
- 74.Dincheva I, Lynch NB, Lee FS. The role of BDNF in the development of fear learning. Depress Anxiety. (2016) 33:907–16. doi: 10.1002/da.22497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Linnarsson S, Björklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. (1997) 9:2581–7. doi: 10.1111/j.1460-9568.1997.tb01687.x [DOI] [PubMed] [Google Scholar]
- 76.Vigers AJ, Amin DS, Talley-Farnham T, Gorski JA, Xu B, Jones KR. Sustained expression of brain-derived neurotrophic factor is required for maintenance of dendritic spines and normal behavior. Neuroscience. (2012) 212:1–18. doi: 10.1016/j.neuroscience.2012.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patanella AK, Zinno M, Quaranta D, Nociti V, Frisullo G, Gainotti G, et al. Correlations between peripheral blood mononuclear cell production of BDNF, TNF-alpha, IL-6, IL-10 and cognitive performances in multiple sclerosis patients. J Neurosci Res. (2010) 88:1106–12. doi: 10.1002/jnr.22276 [DOI] [PubMed] [Google Scholar]
- 78.Hulst HE, Schoonheim MM, Roosendaal SD, Popescu V, Schweren LJS, van der Werf YD, et al. Functional adaptive changes within the hippocampal memory system of patients with multiple sclerosis. Hum Brain Mapp. (2012) 33:2268–80. doi: 10.1002/hbm.21359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kern KC, Ekstrom AD, Suthana NA, Giesser BS, Montag M, Arshanapalli A, et al. Fornix damage limits verbal memory functional compensation in multiple sclerosis. NeuroImage. (2012) 59:2932–40. doi: 10.1016/j.neuroimage.2011.09.071 [DOI] [PubMed] [Google Scholar]
- 80.Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. (2009) 29:12764–7. doi: 10.1523/JNEUROSCI.3566-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. (2003) 23:6690–4. doi: 10.1523/JNEUROSCI.23-17-06690.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu B. BDNF and activity-dependent synaptic modulation. Learn Memory. (2003) 10:86–98. doi: 10.1101/lm.54603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, et al. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J Neurosci. (2010) 30:8866–70. doi: 10.1523/JNEUROSCI.1405-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castrén E, Pitkänen M, Sirviö J, Parsadanian A, Lindholm D, Thoenen H, et al. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. Neuroreport. (1993) 4:895–8. doi: 10.1097/00001756-199307000-00014 [DOI] [PubMed] [Google Scholar]
- 85.Falkenberg T, Mohammed AK, Henriksson B, Persson H, Winblad B, Lindefors N. Increased expression of brain-derived neurotrophic factor mRNA in rat hippocampus is associated with improved spatial memory and enriched environment. Neurosci Lett. (1992) 138:153–6. doi: 10.1016/0304-3940(92)90494-r [DOI] [PubMed] [Google Scholar]
- 86.Yalachkov Y, Anschütz V, Maiworm M, Jakob J, Schaller-Paule MA, Schäfer JH, et al. Serum and cerebrospinal fluid BDNF concentrations are associated with neurological and cognitive improvement in multiple sclerosis: a pilot study. Mult Scler Relat Disord. (2023) 71:104567. doi: 10.1016/j.msard.2023.104567 [DOI] [PubMed] [Google Scholar]
- 87.Engin E., Żarnowska E., Sigal M., Keist R., Zeller A., Pearce R., et al. , (2013): Alpha5-containing GABAA receptors in dentate gyrus enable cognitive flexibility. FASEB J. Online verfügbar unter, Available at: https://www.semanticscholar.org/paper/Alpha5%E2%80%90containing-GABAA-receptors-in-dentate-gyrus-Engin-%C5%BBarnowska/2ee0614f381d68b821e698e92403e16659a7ead6
- 88.Håkansson K, Ledreux A, Daffner K, Terjestam Y, Bergman P, Carlsson R, et al. BDNF responses in healthy older persons to 35 minutes of physical exercise, cognitive training, and mindfulness: associations with working memory function. J Alzheimer’s Disease. (2017) 55:645–57. doi: 10.3233/JAD-160593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song J-H, Yu J-T, Tan L. Brain-derived neurotrophic factor in Alzheimer’s disease: risk, mechanisms, and therapy. Mol Neurobiol. (2015) 52:1477–93. doi: 10.1007/s12035-014-8958-4 [DOI] [PubMed] [Google Scholar]
- 90.Giordano A, Clarelli F, Cannizzaro M, Mascia E, Santoro S, Sorosina M, et al. BDNF Val66Met polymorphism is associated with motor recovery after rehabilitation in progressive multiple sclerosis patients. Front Neurol. (2022) 13:790360. doi: 10.3389/fneur.2022.790360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cerasa A, Tongiorgi E, Fera F, Gioia MC, Valentino P, Liguori M, et al. The effects of BDNF Val66Met polymorphism on brain function in controls and patients with multiple sclerosis: an imaging genetic study. Behav Brain Res. (2010) 207:377–86. doi: 10.1016/j.bbr.2009.10.022 [DOI] [PubMed] [Google Scholar]
- 92.Fera F, Passamonti L, Cerasa A, Gioia M, Liguori M, Manna I, et al. The BDNF Val66Met polymorphism has opposite effects on memory circuits of multiple sclerosis patients and controls. PLoS One. (2013) 8:e61063. doi: 10.1371/journal.pone.0061063, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kambeitz JP, Bhattacharyya S, Kambeitz-Ilankovic LM, Valli I, Collier DA, McGuire P. Effect of BDNF val(66)met polymorphism on declarative memory and its neural substrate: a meta-analysis. Neurosci Biobehav Rev. (2012) 36:2165–77. doi: 10.1016/j.neubiorev.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 94.Mero I-L, Smestad C, Lie BA, Lorentzen ÅR, Sandvik L, Landrø NI, et al. Polymorphisms of the BDNF gene show neither association with multiple sclerosis susceptibility nor clinical course. J Neuroimmunol. (2012) 244:107–10. doi: 10.1016/j.jneuroim.2012.01.011 [DOI] [PubMed] [Google Scholar]
- 95.Ehling R, di Pauli F, Lackner P, Rainer C, Kraus V, Hegen H, et al. Impact of glatiramer acetate on paraclinical markers of neuroprotection in multiple sclerosis: a prospective observational clinical trial. J Neuroimmunol. (2015) 287:98–105. doi: 10.1016/j.jneuroim.2015.08.004, PMID: [DOI] [PubMed] [Google Scholar]
- 96.Islas-Hernandez A, Aguilar-Talamantes H, Bertado-Cortes B, Mejia-delCastillo G d J, Carrera-Pineda R, Cuevas-Garcia CF, et al. BDNF and tau as biomarkers of severity in multiple sclerosis. Biomark Med. (2018) 12:717–26. doi: 10.2217/bmm-2017-0374, PMID: [DOI] [PubMed] [Google Scholar]
- 97.Lindquist S, Schott BH, Ban M, Compston DAS, Sawcer S, Sailer M. The BDNF-Val66Met polymorphism: implications for susceptibility to multiple sclerosis and severity of disease. J Neuroimmunol. (2005) 167:183–5. doi: 10.1016/j.jneuroim.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 98.Blanco Y, Gómez-Choco M, Arostegui JL, Casanova B, Martínez-Rodríguez JE, Boscá I, et al. No association of the Val66Met polymorphism of brain-derived neurotrophic factor (BDNF) to multiple sclerosis. Neurosci Lett. (2006a) 396:217–9. doi: 10.1016/j.neulet.2005.11.032 [DOI] [PubMed] [Google Scholar]
- 99.Nociti V, Santoro M, Quaranta D, Losavio F, de Fino C, Giordano R, et al. BDNF rs6265 polymorphism methylation in multiple sclerosis: a possible marker of disease progression. PLoS One. (2018) 13:e0206140. doi: 10.1371/journal.pone.0206140, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Portaccio E, Bellinvia A, Prestipino E, Nacmias B, Bagnoli S, Razzolini L, et al. The brain-derived neurotrophic factor Val66Met polymorphism can protect against cognitive impairment in multiple sclerosis. Front Neurol. (2021) 12:645220. doi: 10.3389/fneur.2021.645220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mirowska-Guzel D, Mach A, Gromadzka G, Czlonkowski A, Czlonkowska A. BDNF A196G and C270T gene polymorphisms and susceptibility to multiple sclerosis in the polish population. Gender differences. J Neuroimmunol. (2008) 193:170. doi: 10.1016/j.jneuroim.2007.10.013 [DOI] [PubMed] [Google Scholar]
- 102.Comini-Frota ER, Rodrigues DH, Miranda EC, Brum DG, Kaimen-Maciel DR, Donadi EA, et al. Serum levels of brain-derived neurotrophic factor correlate with the number of T2 MRI lesions in multiple sclerosis. Braz. J. Med. Biol. Res. (2012) 45:68–71. doi: 10.1590/s0100-879x2011007500165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brod SA, Lincoln JA, Nelson F. Myelinating proteins in MS are linked to volumetric brain MRI changes. J. Neuroimaging. (2019) 29:400–5. doi: 10.1111/jon.12605 [DOI] [PubMed] [Google Scholar]
- 104.Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. (1997) 17:e2295. doi: 10.1523/JNEUROSCI.17-07-02295.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zachary C, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. (2004) 24:4250–8. doi: 10.1523/JNEUROSCI.3920-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Charil A, Dagher A, Lerch JP, Zijdenbos AP, Worsley KJ, Evans AC. Focal cortical atrophy in multiple sclerosis: relation to lesion load and disability. NeuroImage. (2007) 34:e6. doi: 10.1016/j.neuroimage.2006.10.006 [DOI] [PubMed] [Google Scholar]
- 107.Prinster A, Quarantelli M, Orefice G, Lanzillo R, Brunetti A, Mollica C, et al. Grey matter loss in relapsing–remitting multiple sclerosis: a voxel-based morphometry study. NeuroImage. (2006) 29:859–67. doi: 10.1016/j.neuroimage.2005.08.034 [DOI] [PubMed] [Google Scholar]
- 108.Dolcetti E, Bruno A, Azzolini F, Gilio L, Moscatelli A, de Vito F, et al. The BDNF Val66Met polymorphism (rs6265) modulates inflammation and neurodegeneration in the early phases of multiple sclerosis. Genes. (2022) 13. doi: 10.3390/genes13020332, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Meo E, Portaccio E, Prestipino E, Nacmias B, Bagnoli S, Razzolini L, et al. Effect of BDNF Val66Met polymorphism on hippocampal subfields in multiple sclerosis patients. Mol Psychiatry. (2022) 27:1010–9. doi: 10.1038/s41380-021-01345-1, PMID: [DOI] [PubMed] [Google Scholar]
- 110.Blanco Y, Moral EA, Costa M, Gómez-Choco M, Torres-Peraza JF, Alonso-Magdalena L, et al. Effect of glatiramer acetate (Copaxone) on the immunophenotypic and cytokine profile and BDNF production in multiple sclerosis: a longitudinal study. Neurosci Lett. (2006b) 406:270–5. doi: 10.1016/j.neulet.2006.07.043 [DOI] [PubMed] [Google Scholar]
- 111.Chen M, Valenzuela R, Dhib-Jalbut S. Glatiramer acetate-reactive T cells produce brain-derived neurotrophic factor. J Neurol Sci. (2003) 215:37–44. doi: 10.1016/s0022-510x(03)00177-1, PMID: [DOI] [PubMed] [Google Scholar]
- 112.Ziemssen T, Kümpfel T, Schneider H, Klinkert WEF, Neuhaus O, Hohlfeld R. Secretion of brain-derived neurotrophic factor by glatiramer acetate-reactive T-helper cell lines: implications for multiple sclerosis therapy. J Neurol Sci. (2005) 233:109. doi: 10.1016/j.jns.2005.03.010 [DOI] [PubMed] [Google Scholar]
- 113.Ruggieri M, Avolio C, Livrea P, Trojano M. Glatiramer acetate in multiple sclerosis: a review. CNS Drug Rev. (2007) 13:178–91. doi: 10.1111/j.1527-3458.2007.00010.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arnon R, Aharoni R. Mechanism of action of glatiramer acetate in multiple sclerosis and its potential for the development of new applications. Proc Natl Acad Sci USA. (2004) 101:14593–8. doi: 10.1073/pnas.0404887101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ziemssen T, Schrempf W. Glatiramer acetate: mechanisms of action in multiple sclerosis. Int Rev Neurobiol. (2007) 79:537–70. doi: 10.1016/S0074-7742(07)79024-4 [DOI] [PubMed] [Google Scholar]
- 116.Aharoni R, Eilam R, Domev H, Labunskay G, Sela M, Arnon R. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc Natl Acad Sci USA. (2005) 102:19045–50. doi: 10.1073/pnas.0509438102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aharoni R, Kayhan B, Eilam R, Sela M, Arnon R. Glatiramer acetate-specific T cells in the brain express T helper 2/3 cytokines and brain-derived neurotrophic factor in situ. Proc Natl Acad Sci USA. (2003) 100:14157–62. doi: 10.1073/pnas.2336171100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ziemssen T, Kümpfel T, Klinkert WEF, Neuhaus O, Hohlfeld R. Glatiramer acetate-specific T-helper 1- and 2-type cell lines produce BDNF: implications for multiple sclerosis therapy. Brain-derived neurotrophic factor. Brain. (2002) 125:2381–91. doi: 10.1093/brain/awf252 [DOI] [PubMed] [Google Scholar]
- 119.Gilgun-Sherki Y, Panet H, Holdengreber V, Mosberg-Galili R, Offen D. Axonal damage is reduced following glatiramer acetate treatment in C57/bl mice with chronic-induced experimental autoimmune encephalomyelitis. Neurosci Res. (2003) 47:201–7. doi: 10.1016/s0168-0102(03)00217-7 [DOI] [PubMed] [Google Scholar]
- 120.Lee D-H, Geyer E, Flach A-C, Jung K, Gold R, Flügel A, et al. Central nervous system rather than immune cell-derived BDNF mediates axonal protective effects early in autoimmune demyelination. Acta Neuropathol. (2012) 123:247–58. doi: 10.1007/s00401-011-0890-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Golan M, Mausner-Fainberg K, Ibrahim B, Benhamou M, Wilf-Yarkoni A, Kolb H, et al. Fingolimod increases brain-derived neurotrophic factor level secretion from circulating T cells of patients with multiple sclerosis. CNS Drugs. (2019) 33:1229–37. doi: 10.1007/s40263-019-00675-7 [DOI] [PubMed] [Google Scholar]
- 122.Petereit HF, Lindemann H, Schoppe S. Effect of immunomodulatory drugs on in vitro production of brain-derived neurotrophic factor. Multiple Sclerosis. (2003) 9:16–20. doi: 10.1191/1352458503ms869oa [DOI] [PubMed] [Google Scholar]
- 123.Kalinowska-Łyszczarz A, Pawlak MA, Wyciszkiewicz A, Osztynowicz K, Kozubski W, Michalak S. Immune-cell BDNF expression in treatment-naïve relapsing-remitting multiple sclerosis patients and following one year of immunomodulation therapy. Neurol Neurochir Pol. (2018) 52:483–9. doi: 10.1016/j.pjnns.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 124.Smith PA, Schmid C, Zurbruegg S, Jivkov M, Doelemeyer A, Theil D, et al. Fingolimod inhibits brain atrophy and promotes brain-derived neurotrophic factor in an animal model of multiple sclerosis. J Neuroimmunol. (2018) 318:103–13. doi: 10.1016/j.jneuroim.2018.02.016 [DOI] [PubMed] [Google Scholar]
- 125.Văcăraş V, Major Z, Buzoianu AD, Zsigmond A, Dana BA. Brain-derived neurotrophic factor levels under chronic natalizumab treatment in multiple sclerosis. A preliminary report. Neurol Neurochir Pol. (2017) 51:221–6. doi: 10.1016/j.pjnns.2017.03.002, PMID: [DOI] [PubMed] [Google Scholar]
- 126.Muraro PA, Douek DC, Packer A, Chung K, Guenaga FJ, Cassiani-Ingoni R, et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. (2005) 201:805–16. doi: 10.1084/jem.20041679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Blanco Y, Saiz A, Costa M, Torres-Peraza JF, Carreras E, Alberch J, et al. Evolution of brain-derived neurotrophic factor levels after autologous hematopietic stem cell transplantation in multiple sclerosis. Neurosci Lett. (2005) 380:122–6. doi: 10.1016/j.neulet.2005.01.032 [DOI] [PubMed] [Google Scholar]
- 128.Giedraitiene N, Gasciauskaite G, Kaubrys G. Impact of autologous HSCT on the quality of life and fatigue in patients with relapsing multiple sclerosis. Sci Rep. (2022) 12:15404. doi: 10.1038/s41598-022-19748-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mancardi GL, Sormani MP, Di Gioia M, Vuolo L, Gualandi F, Amato MP, et al. Autologous haematopoietic stem cell transplantation with an intermediate intensity conditioning regimen in multiple sclerosis: the Italian multi-Centre experience. Multiple Sclerosis. (2012) 18:835–42. doi: 10.1177/1352458511429320 [DOI] [PubMed] [Google Scholar]
- 130.Burt RK, Balabanov R, Burman J, Sharrack B, Snowden JA, Oliveira MC, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA. (2019) 321:165–74. doi: 10.1001/jama.2018.18743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dalgas U, Langeskov-Christensen M, Stenager E, Riemenschneider M, Hvid LG. Exercise as medicine in multiple sclerosis—time for a paradigm shift: preventive, symptomatic, and disease-modifying aspects and perspectives. Curr Neurol Neurosci Rep. (2019) 19:88. doi: 10.1007/s11910-019-1002-3 [DOI] [PubMed] [Google Scholar]
- 132.Begliuomini S, Lenzi E, Ninni F, Casarosa E, Merlini S, Pluchino N, et al. Plasma brain-derived neurotrophic factor daily variations in men: correlation with cortisol circadian rhythm. J Endocrinol. (2008) 197:429–35. doi: 10.1677/JOE-07-0376 [DOI] [PubMed] [Google Scholar]
- 133.Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem. (2008) 89:312–23. doi: 10.1016/j.nlm.2007.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thoenen H. Neurotrophins and neuronal plasticity. Science. (1995) 270:593–8. doi: 10.1126/science.270.5236.593 [DOI] [PubMed] [Google Scholar]
- 135.Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol. (2019) 15:383–92. doi: 10.1038/s41574-019-0174-x [DOI] [PubMed] [Google Scholar]
- 136.Sakuma K, Yamaguchi A. The recent understanding of the neurotrophin’s role in skeletal muscle adaptation. J Biomed Biotechnol. (2011) 2011:201696. doi: 10.1155/2011/201696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, et al. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. (2013) 18:649–59. doi: 10.1016/j.cmet.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gentile A, Musella A, de Vito F, Rizzo FR, Fresegna D, Bullitta S, et al. Immunomodulatory effects of exercise in experimental multiple sclerosis. Front Immunol. (2019) 10:2197. doi: 10.3389/fimmu.2019.02197, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guo L, Lozinski B, Yong VW. Exercise in multiple sclerosis and its models: focus on the central nervous system outcomes. J Neurosci Res. (2020) 98:509–23. doi: 10.1002/jnr.24524, PMID: [DOI] [PubMed] [Google Scholar]
- 140.Vaynman S, Ying Z, Gomez-Pinilla F. The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience. (2007) 144:825–33. doi: 10.1016/j.neuroscience.2006.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jørgensen MLK, Kjølhede T, Dalgas U, Hvid LG. Plasma brain-derived neurotrophic factor (BDNF) and sphingosine-1-phosphat (S1P) are NOT the main mediators of neuroprotection induced by resistance training in persons with multiple sclerosis-a randomized controlled trial. Mult Scler Relat Disord. (2019) 31:106–11. doi: 10.1016/j.msard.2019.03.029 [DOI] [PubMed] [Google Scholar]
- 142.Abbaspoor E, Zolfaghari M, Ahmadi B, Khodaei K. The effect of combined functional training on BDNF, IGF-1, and their association with health-related fitness in the multiple sclerosis women. J. Growth Hormone Res. Soc. Int. IGF Res. Soc. (2020) 52:101320. doi: 10.1016/j.ghir.2020.101320 [DOI] [PubMed] [Google Scholar]
- 143.Briken S, Rosenkranz S, Keminer O, Patra S, Ketels G, Heesen C, et al. Effects of exercise on Irisin, BDNF and IL-6 serum levels in patients with progressive multiple sclerosis. J Neuroimmunol. (2016) 299:53–8. doi: 10.1016/j.jneuroim.2016.08.007, PMID: [DOI] [PubMed] [Google Scholar]
- 144.Schulz K-H, Gold SM, Witte J, Bartsch K, Lang UE, Hellweg R, et al. Impact of aerobic training on immune-endocrine parameters, neurotrophic factors, quality of life and coordinative function in multiple sclerosis. J Neurol Sci. (2004) 225:11–8. doi: 10.1016/j.jns.2004.06.009 [DOI] [PubMed] [Google Scholar]
- 145.Waschbisch A, Wenny I, Tallner A, Schwab S, Pfeifer K, Mäurer M. Physical activity in multiple sclerosis: a comparative study of vitamin D, brain-derived neurotrophic factor and regulatory T cell populations. Eur Neurol. (2012) 68:122–8. doi: 10.1159/000337904 [DOI] [PubMed] [Google Scholar]
- 146.Naghibzadeh A, Mohammadi G, Darlington PJ, Rezaei Namjoo F, Rashidlamir A. Aquatic exercise with Swedish massage increases neurotrophic factors and decreases interleukin-6 (IL-6) in relapsing remitting multiple sclerosis. Bio Exerc. (2019) 15:171–86. [Google Scholar]
- 147.Ozkul C, Guclu-Gunduz A, Irkec C, Fidan I, Aydin Y, Ozkan T, et al. Effect of combined exercise training on serum brain-derived neurotrophic factor, suppressors of cytokine signaling 1 and 3 in patients with multiple sclerosis. J Neuroimmunol. (2018) 316:121–9. doi: 10.1016/j.jneuroim.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 148.Banitalebi E, Ghahfarrokhi M, Negaresh R, Kazemi A, Faramarzi M, Motl RW, et al. Exercise improves neurotrophins in multiple sclerosis independent of disability status. Mult Scler Relat Disord. (2020) 43:102143. doi: 10.1016/j.msard.2020.102143, PMID: [DOI] [PubMed] [Google Scholar]
- 149.Eftekhari E, Etemadifar M. Interleukin-10 and brain-derived neurotrophic factor responses to the mat Pilates training in women with multiple sclerosis. Sci Med. (2018) 28:31668. doi: 10.15448/1980-6108.2018.4.31668 [DOI] [Google Scholar]
- 150.Khademosharie M, Tadibi V, Behpoor N, Hamedinia MR. The effect of 12-weeks concurent training on the serum levels NGF, BDNF, and VDBP in women with multiple sclerosis (2018) 17:77–86. doi: 10.22631/ijaep.v7i1.228, [DOI] [Google Scholar]
- 151.Mokhtarzade M, Motl R, Negaresh R, Zimmer P, Khodadoost M, Baker JS, et al. Exercise-induced changes in neurotrophic factors and markers of blood-brain barrier permeability are moderated by weight status in multiple sclerosis. Neuropeptides. (2018) 70:93–100. doi: 10.1016/j.npep.2018.05.010 [DOI] [PubMed] [Google Scholar]
- 152.Savšek L, Stergar T, Strojnik V, Ihan A, Koren A, Špiclin Ž, et al. Impact of aerobic exercise on clinical and magnetic resonance imaging biomarkers in persons with multiple sclerosis: an exploratory randomized controlled trial. J Rehabil Med. (2021) 53:jrm00178. doi: 10.2340/16501977-2814, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wens I, Keytsman C, Deckx N, Cools N, Dalgas U, Eijnde BO. Brain derived neurotrophic factor in multiple sclerosis: effect of 24 weeks endurance and resistance training. Eur J Neurol. (2016) 23:1028–35. doi: 10.1111/ene.12976 [DOI] [PubMed] [Google Scholar]
- 154.Diechmann MD, Campbell E, Coulter E, Paul L, Dalgas U, Hvid LG. Effects of exercise training on neurotrophic factors and subsequent neuroprotection in persons with multiple sclerosis-a systematic review and meta-analysis. Brain Sci. (2021) 11:111499. doi: 10.3390/brainsci11111499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Church DD, Hoffman JR, Mangine GT, Jajtner AR, Townsend JR, Beyer KS, et al. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J. Appl. Physiol. (2016) 121:123–8. doi: 10.1152/japplphysiol.00233.2016 [DOI] [PubMed] [Google Scholar]
- 156.Dinoff A, Herrmann N, Swardfager W, Lanctôt KL. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. Eur J Neurosci. (2017) 46:1635–46. doi: 10.1111/ejn.13603 [DOI] [PubMed] [Google Scholar]
- 157.Dinoff A, Herrmann N, Swardfager W, Liu CS, Sherman C, Chan S, et al. The effect of exercise training on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF): a meta-analysis. PLoS One. (2016) 11:e0163037. doi: 10.1371/journal.pone.0163037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ferris LT, Williams JS, Shen C-L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. (2007) 39:728–34. doi: 10.1249/mss.0b013e31802f04c7 [DOI] [PubMed] [Google Scholar]
- 159.Lippi G, Mattiuzzi C, Sanchis-Gomar F. Updated overview on interplay between physical exercise, neurotrophins, and cognitive function in humans. J Sport Health Sci. (2020) 9:74–81. doi: 10.1016/j.jshs.2019.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Reycraft JT, Islam H, Townsend LK, Hayward GC, Hazell TJ, Macpherson REK. Exercise intensity and recovery on circulating brain-derived neurotrophic factor. Med Sci Sports Exerc. (2020) 52:1210–7. doi: 10.1249/MSS.0000000000002242 [DOI] [PubMed] [Google Scholar]
- 161.Schmolesky MT, Webb DL, Hansen RA. The effects of aerobic exercise intensity and duration on levels of brain-derived neurotrophic factor in healthy men. J. Sports Sci. Med. (2013) 12:502–11. [PMC free article] [PubMed] [Google Scholar]
- 162.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. (2015) 60:56–64. doi: 10.1016/j.jpsychires.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zimmer P, Bloch W, Schenk A, Oberste M, Riedel S, Kool J, et al. High-intensity interval exercise improves cognitive performance and reduces matrix metalloproteinases-2 serum levels in persons with multiple sclerosis: a randomized controlled trial. Multiple Sclerosis. (2018) 24:1635–44. doi: 10.1177/1352458517728342 [DOI] [PubMed] [Google Scholar]
- 164.El-Sayes J, Harasym D, Turco CV, Locke MB, Nelson AJ. Exercise-induced neuroplasticity: a mechanistic model and prospects for promoting plasticity. Neuroscientist. (2019) 25:65–85. doi: 10.1177/1073858418771538 [DOI] [PubMed] [Google Scholar]
- 165.Bonfiglio T, Olivero G, Vergassola M, Di Cesare Mannelli L, Pacini A, Iannuzzi F, et al. Environmental training is beneficial to clinical symptoms and cortical presynaptic defects in mice suffering from experimental autoimmune encephalomyelitis. Neuropharmacology. (2019) 145:75–86. doi: 10.1016/j.neuropharm.2018.01.026 [DOI] [PubMed] [Google Scholar]
- 166.Mandolesi G, Bullitta S, Fresegna D, de Vito F, Rizzo FR, Musella A, et al. Voluntary running wheel attenuates motor deterioration and brain damage in cuprizone-induced demyelination. Neurobiol Dis. (2019) 129:102–17. doi: 10.1016/j.nbd.2019.05.010, PMID: [DOI] [PubMed] [Google Scholar]
- 167.Souza PS, Gonçalves ED, Pedroso GS, Farias HR, Junqueira SC, Marcon R, et al. Physical exercise attenuates experimental autoimmune encephalomyelitis by inhibiting peripheral immune response and blood-brain barrier disruption. Mol Neurobiol. (2017) 54:4723–37. doi: 10.1007/s12035-016-0014-0 [DOI] [PubMed] [Google Scholar]
- 168.Xie Y, Li Z, Wang Y, Xue X, Ma W, Zhang Y, et al. Effects of moderate- versus high- intensity swimming training on inflammatory and CD4+ T cell subset profiles in experimental autoimmune encephalomyelitis mice. J Neuroimmunol. (2019) 328:60–7. doi: 10.1016/j.jneuroim.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 169.Tsuchimine S, Sugawara N, Ishioka M, Yasui-Furukori N. Preanalysis storage conditions influence the measurement of brain-derived neurotrophic factor levels in peripheral blood. Neuropsychobiology. (2014) 69:83–8. doi: 10.1159/000358061 [DOI] [PubMed] [Google Scholar]
- 170.Gejl A, Enevold C, Bugge A, Andersen MS, Nielsen CH, Andersen LB. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci Rep. (2019) 9:9655. doi: 10.1038/s41598-019-45976-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, Campbell TG, et al. The Simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J. Laborat. Autom. (2016) 21:533–47. doi: 10.1177/2211068215589580 [DOI] [PubMed] [Google Scholar]
- 172.Naegelin Y, Dingsdale H, Säuberli K, Schädelin S, Kappos L, Barde Y-A. Measuring and validating the levels of brain-derived neurotrophic factor in human serum. eNeuro. (2018) 5:e419. doi: 10.1523/ENEURO.0419-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. (2011) 10:209–19. doi: 10.1038/nrd3366 [DOI] [PubMed] [Google Scholar]