Abstract
Background
Determination of clot lysis times on whole blood, diluted whole blood, plasma or plasma fraction has been used for many years to assess the overall activity of the fibrinolytic system. We designed a completely computerised semi-automatic 8-channel device for measurement and determination of fibrin clot lysis. The lysis time is evaluated by a mathematical analysis of the lysis curve and the results are expressed in minute (range: 5 to 9999). We have used this new device for Euglobulin Clot Lysis Time (ECLT) determination, which is the most common test used in laboratories to estimate plasma fibrinolytic capacity.
Results
The correlation between ECLT and manual method is very tight : R = 0,99; p < 10-6. The efficiency scores of the method are <4% in intra-assay and <7% in inter-assay. It allows to achieve the tests on hyperlipaemic samples. This new device has been easily integrated in laboratory routine and allows to achieve several ECLT every day without disturbance of laboratory workflow.
Conclusions
The routine use of this new device could be useful in various situations such as assessment in atherosclerosis and arteriosclerosis associated diseases, coagulation survey of liver transplantations, cardiovascular surgery or pharmacological research.
It has already provided highly promising results in preliminary studies on the relation between fibrinolysis and cardiovascular risk factors.
Background
The fibrinolytic system is involved in many physiological and pathophysiological processes. An increase in fibrinolytic activity may induce a bleeding tendency. More commonly, a reduced activity of the system is observed and this may contribute to the pathogenesis of thromboembolic diseases [1,2]. In earlier clinical studies, an impaired fibrinolytic function had been reported in subjects suffering from venous thrombosis [3]. Newer studies have emphasised the importance of fibrinolytic disturbances in the development of arterial disease and thrombosis [4,5]. These alterations were especially observed in patients with advanced atherosclerosis, when developing acute-coronary syndrome or diabetes mellitus [6,7]. The fibrinolytic abnormalities were also observed in hypertriglyceridemic patients before developing atherosclerosis or vascular complications [8]. Most of the tests used to measure the blood fibrinolytic potential are not suitable for routine clinical practices. Analysis of t-PA and PAI-1 considers two isolated components of the fibrinolytic system. Furthermore, these tests have to be performed in series and so cannot be done very frequently in laboratory routine for logistic and economic reasons. The Dilute Blood Clot Lysis Time is a very long and subjective procedure requiring a continuous monitoring by an observer who has to determine when the clot is lysed. The Euglobulin Clot Lysis Time (ECLT) is the most commonly used test. It seems to represent the interaction between the activity of t-PA and PAI-1 [11] and allows to measure both baseline and stimulated fibrin dissolution. Although this test is inexpensive and adapted to single tests system, it is very difficult to promote in clinical practice, since it is long, and require an observer to record the clot lysis.
We have designed a new semi-automatic method for clot lysis measurement that includes several important improvements which are: 1) the device is operator independent during the analysis of the fibrinolysis process and for the determination of the lysis time, 2) allows the analysis of samples with a broad range of lysis time, 3) allows the analysis of individual samples and is easily integrated in the laboratory routine. To answer to these points, our new system has been completely computerised. ECLT has been chosen as first application. We have compared the efficiency of ECLT with the new device to the classical manual method and to a new test from Stago, the Global Fibrinolytic Capacity (GFC). In addition, the test was also performed on high blood triglycerides level, to test the optical sensitivity and robustness of our device.
Results
Efficiency scores of the semi-automatic method and comparison with the manual method
The variability of the complete semi-automatic procedure was estimated from frozen plasma pool of the first 11 volunteers, the mean lysis time averaging 300 min. The variation coefficients for B and C points were <4% in intra-assay and < 7% in inter-assay, respectively (8 tests/day over 10 different days). The relation between manual and semi-automatic method is shown in Fig 3. For the B point the curve equation of the correlation between both methods was y = -3.04 + 0.97.x (R = 0.99, p < 10-6). The correlation appeared similar for the C point. The corresponding curve equation was y = 2.62 + 1.01.x (R = 0.99, p < 10-6).
Figure 3.
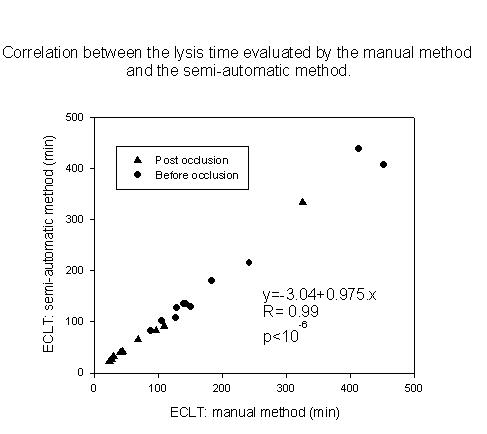
Correlation between the manual method and the semi-automatic method. ECLT by the semi-automatic method is determined by the B point. The plots represent the lysis time of the 11 volunteers before and after venous occlusion. The equation is calculated on the 24 points.
No statistically significant difference was found between manual results, B or C point, before and after venous occlusion conditions.
For the semi-automatic method, we have chosen arbitrarily the B point from the lysis curve to determine the lysis time Fig 2 and in addition, this point corresponds about to the time value of lysis assessed in manual method.
Figure 2.
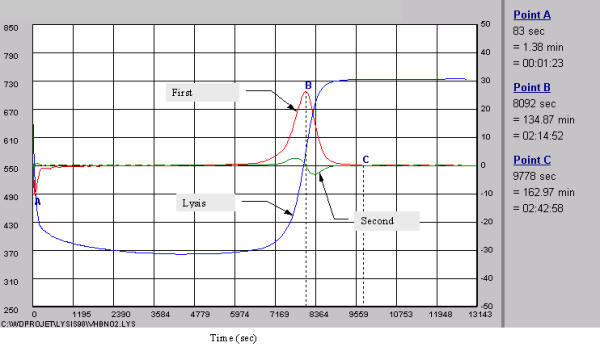
The lines represent the complete fibrinolysis process including formation, latency and dissolution of the fibrin clot, the first and second derivative. All points (A,B,C) are expressed in seconds, minutes and hours. The A point represents the peak time of fibrin clot formation, B point, the peak to fibrin clot lysis, and C point, the end of the complete fibrinolysis process.
Normal values are for men (n = 21), 208 min (range, 118–303) and for premenopausal women (n = 25), 117 min (range, 100–174) (p = 0.04).
Assay in hypertriglyceridemic condition
The increase of the blood triglycerides level induced a softening of amplitude of the signal (data not shown). This softening did not affect the determination of the lysis time by our device. However, for a direct observer, the variation in turbidity induced by clot formation and lysis in hyperlipemic condition was very difficult to detect for triglycerides above 300 mg/dl Fig 4.
Figure 4.
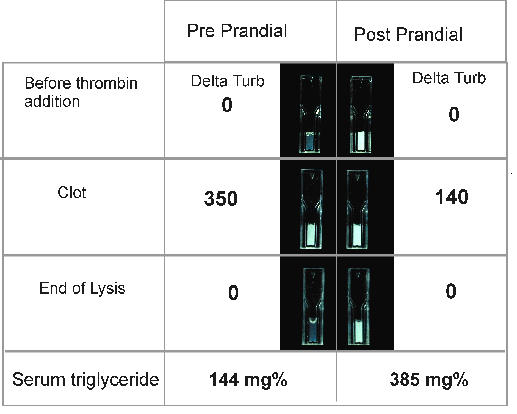
Optical evolution of the turbidity of the euglobulin fraction during the fibrinolysis process. D.Turb: Delta turbidity represents the variation of the signal compared to the start value of the process. Delta turbidity is expressed in arbitrary units. Pre/Post prandial : Total Cholesterol: 319/304 mg%, Triglycerides: 144/385 mg%.
Until 800 mg/dl of triglycerides, the semi-automatic system has always achieved an appropriate analysis of the curve.
Relation between the ECLT and Global Fibrinolytic Capacity
For basal and stimulation states, the relationship between the two tests is better described by an inverse exponential curve y = 54.31 e-0.008x, R2 = 0.71, independently of the stimulation state or the way to achieve Fig 5.
Figure 5.
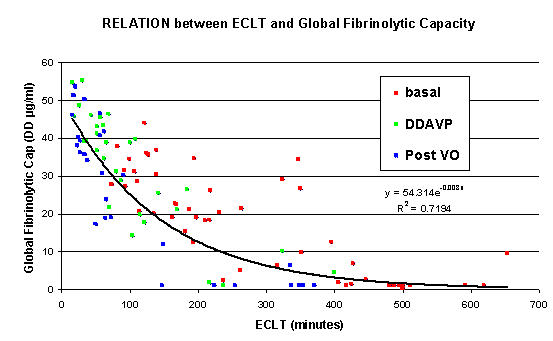
Correlation between the plasma fibrinolytic capacity evaluated by GFC and the ECLT achieved by the semi-automatic method. First group at basal (red) and post venous occlusion (blue) (n = 25). Second group with Desmopresin stimulation [n = 17, basal condition (red), DDAVP 30 min, DDAVP 60 min, DDAVP 120 min (green)].
When the ECLT was lower than 200 minutes, we observed a fair correlation between both tests (R2 = 0.55). For ECLT > 300 min, DD generation in GFC is suppressed.
Table 1 shows the results of ECLT and GFC of different groups in basal and stimulate conditions.
Table 1.
Values of ECLT and GFC in different groups.
| ECLT (min) | GFC D-Dimer (μg/ml) | |
| Group 1 (n = 25) | ||
| Basal condition | 264 (165–482) | 20.3 (1.1–26.7) |
| Post venous occlusion | 56 (26–167) | 23.7 (1.1–41.7 |
| Group 2 (n = 17) | ||
| DDAVP (basal condition) | 181 (108–249) | 20.6 (7.6–31.3) |
| DDAVP (30 min) | 35 (25–53) | 39 (30.4–46) |
| DDAVP (60 min) | 61 (45–98) | 42.13 (26–45) |
| DDAVP (120 min) | 107 (76–246) | 27 (12.1–38.7) |
| Median (25% – 75%) |
Both tests were identically sensitive to fibrinogen level, since the fibrinogen level arises, the fibrinolytic capacity decreased Fig 6.
Figure 6.

Correlation between the patient fibrinogen levels from the two groups, and the fibrinolytic capacity evaluated by Euglobulin Clot Lysis Time and Global Fibrinolytic Capacity, in basal condition (n = 42).
Discussion
Our aim was to create a device which allowed to reintroduce the fibrinolysis measurement in routine practice without disturbing the laboratory workflow, in this work we chose ECLT. An optical computerised system for the follow-up of fibrinolysis process and for determination of lysis time made the test autonomous and allowed further developments. With our device, we obtained a very tight correlation with the manual method, but this one has been made in condition unachievable in routine. The response to venous occlusion were identical for manual and semi-automatic method.
We have obtained a significant difference between normal values in men and premenopausal women in accordance to the notion that women have a better fibrinolytic capacity than men [15].
Another procedure, allowing quantitative ECLT assessment with a microtiter plate reader, was reported by Urano et al [11]. It provided reliable and reproducible data, with the lysis time determined as the half lysis point obtained by calculation of the midpoint between maximum and minimum points of turbidity. However, the turbidity of the clot was only measured every 30 minutes by the microtiter plate reader. We used a higher sampling rate of the recorder, i.e. every one minute. Our mathematical analysis theoretically allows a better accuracy since it is based on much more discrete measurements than in the system of Urano using only an unique half lysis point.
Moreover, our device allows to analyse up to 8 samples individually and to measure the lysis time over a broad range (5 minutes to 9999 minutes) strictly stabilized at 37°C. The mathematical analysis determines not only the critical points (A, B, C) of the lysis curve, but will also enable to explore the kinetics of fibrin clot degradation in the future.
Finally, this device is able to determine the lysis time in hyperlipemic condition. The GFC from Stago can also be applied on single samples. This test evaluates the fibrinolytic process by the generation of D-Dimer from an external and standardized fibrin clot. It is rather expensive due to the reagent itself and the cost of DD evaluation. At first sight, we thought that the fibrinogen level had no effect on GFC and that this test could result in a different information than ECLT. But, both tests are surprisingly, equally sensitive to patient's fibrinogen level. Fibrinogen can indirectly or directly interfere with fibrinolysis process. The first mechanism may be common to both tests, the fibrinogen level can represent chronic inflammation which may impaire fibrinolytic balance [16].
The second mechanism (direct effect) is probably different for ECLT than for GFC. For ECLT, the density of fibrin clot is in relation with fibrinogen level and this density may also modulates lysis time [17]. For GFC, a constant amount of t-PA is added in plasma sample which is placed at the surface of a standardised fibrin clot (the reaction is stopped after one hour). A previous study [18] reported that t-PA incubated with plasma in presence of a fibrin clot induced fibrinogen proteolysis. The authors concluded, that the products of t-PA-induced degradation of cross-linked fibrin could potentiate t-PA-mediated fibrinogenolysis by providing a surface for t-PA and plasminogen binding thereby promoting plasmin generation. This plasmin is a more important mediator of fibrinogenolysis than plasmin generated on the clot surface. We suppose that, a part of plasma plasminogen is less available for the standardised fibrin clot degradation and that this phenomenon can induce a decrease of D-Dimer generated during the test.
The GFC shows a weak sensitivity in hypofibrinolytic patients with no possibility to distinguish between two different samples or to measure a first slight response to a treatment. Alternative protocols should be designed to allow an evaluation of these samples. Several tests on plasma were achieved on samples providing from venous occlusion and hepatic transplantation (data not shown). The device determine easily the lysis time on plasma. However, in basal condition fibrinolysis is too slow. Different preparations of reagents are in development, to reduce lysis time, for the new applications with our device. The new apparatus is an important tool for studying fibrinolysis in-vitro, moreover, it can reintroduce lysis time in routine for a better approach of hypofibrinolysis.
It has allowed us to achieve preliminary studies on the effect of lipids level on ECLT in men and women [19] and the relations between the classical cardiovascular risk factors and ECLT [20].
Conclusion
In conclusion, we observed a good correlation between the measurement of lysis time by the manual method and the new device. This new evaluation of the ECLT is more objective. The results are highly reproducible and the test easy to perform. The reagents and disposable are not expensive (micro-cuvets, acetic acid, Owren solution and thrombin). At the moment, it is integrated in our laboratory routine practice for extended evaluation.
This ECLT test should be reintegrated in clinical routine, and could be conveniently added to the panel of estimates of biological risk factors for atherothrombosis. Moreover, this device is not limited to ECLT, it could be used with different sample preparations.
Materials and methods
Population data
The normal values in non-stimulated condition were obtained from 25 women and 21 men. Men were of 49 years old (range, 32–54) and women 44 years old (range, 31–48) (p = 0.3). Volunteers were free from any clinical signs of peripheral arterial disease, diabetes mellitus, arterial hypertension, history of cardiovascular disease, inflammatory syndrome and smoking habit. They were also free of medication and women were in premenopausal status. For correlation with the manual method, blood samples from others 11 fasting volunteers from laboratory were obtained before and after a 10 minutes venous occlusion. The relation with the Global Fibrinolytic Capacity (GFC) was established on 42 samples obtained from patients explored for thromboembolic disorders. Among these, 25 gave another sample after a 10 minutes venous occlusion. The stimulation conditions were also evaluated on patients, taken 0.5 hour, 1 or 2 hours after desmopressin infusion (therapeutic challenge) in mild haemophilia A and von Willebrand patients.
Blood sampling
For the normal values, blood samples from antecubital vein were obtained between 08:00 and 09:30 a.m. Samples were harvested in 0.13 M-citrated vacuum tubes (Venoject® Terumo) and immediately put into melting ice. After centrifugation at 4000 g for 10 min at 4°C, the plasma samples were stored at -80°C until analysis. The tests were performed either immediately or on frozen/thawed samples (-80°C). Fibrinogen level was tested on STA® apparatus according to manufacturer's instructions.
Euglobulin fraction preparation
300 μl of acetic acid (0.25%) and 3.6 ml of desionized water were added to 400 μl plasma (final pH ≅ 5.9). The sample was then put into melting ice during 20 min and centrifuged at 4000 g for 10 min at 4°C. The supernatant was discarded and the pellet was resuspended in 400 μl of Owren-Koller buffer (DIAGNOSTICA STAGO®, France). The clot formation started when 100 μl of thrombin (1.75 U/ml, DIAGNOSTICA STAGO®, France) were added.
ECLT measurement: conventional manual method
The euglobulin activated fraction was put at 37°C in a thermostatic bath and the clot was carefully examined every 5 minutes by the operator who was continously present during the measurement. Lysis was considered complete when the clot was no more apparent (end time point expressed in min).
Semi-automatic ECLT measurement
The procedure is very easy, the sample (euglobulin fraction, plasma or others) is introduced in a microcuvette and put in the apparatus, in a well. Then, the operator follows three operations to start the tests: 1- identification of sample, 2- add thrombin, 3- push on set button to start the recording of fibrinolysis process.
The new device composition was designed as Figure 1:
Figure 1.
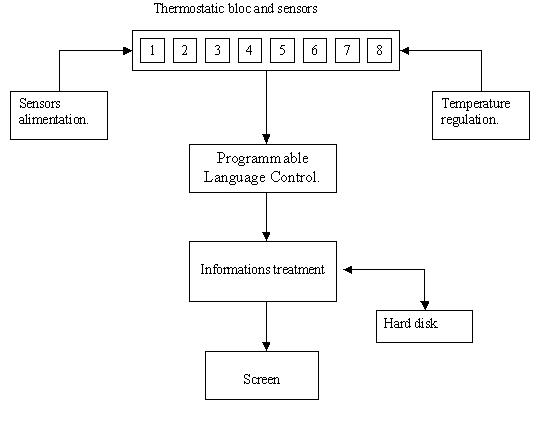
Outline of the new device for ECLT measurement.
- a thermostatic bloc (37°c) with eight photometric measure channels.
- a programmable language control (PLC).
- a computer and a software for the mathematical analysis of the fibrinolysis process.
Thermostatic bloc
the bloc is made of pure aluminium (360 × 30 × 100 mm) and is warmed by two resistances of 20 W. It is designed to insert spectrophotometric micro-cuvets (10 × 4 × 45 mm Sarstedt®) into 8 wells. Each well includes one emitter (diode: SFH 409) and one receptor (phototransistor: SFH309FA), both operating at 890 nm. When the clot is formed, the phototransistor signal decreases reflecting a Tyndall effect [12].
Programmable Language Control
ED&A V10, Antwerpen, Belgium. The sensors are connected to the PLC which transforms the analogic data (0–10 V) into digital data (12 bits). The data are transmitted to the computer by RS232.
Data analysis
The computer records every one minute the data from each channel. The software (developed with Windev® 4.1, France) generates the graph of the fibrinolytic process. At the end, the curve graph is analysed with a mathematical algorithm. The first and second derivatives are computed by a convolution matrix13. These calculations aim at determining the peak time to clot formation (A point, first derivative), the peak time to clot lysis (B point, first derivative), and the end of the complete fibrinolysis process (C point, when the first and the second derivatives are under the background ≅ 0). Later, other parameters should also be considered. The design of a lysis curve is exemplified in Figure 2. The x-axis/y-axis respectively represent time and evolution of the signal sensor. Mathematical analysis or complete procedure can be started individually for each sample and at every moment without disturbing the measurements of other samples. The final assembly was performed by EREM, (Marcinelle, Belgium) and the system is distributed since 2001 by BIOK, (Montigny-Le-Tilleul, Belgium).
The Global Fibrinolytic Capacity (Stago, France)
This test estimates the fibrinolytic activity in plasma through the measurement of generated D-Dimer from a standardized fibrin amount in presence of a constant amount of exogenous t-PA and by contact phase activators [14]. The test was performed according to manufacturer's instructions and the results were expressed as generated DD (μg/ml).
Test of hypertriglyceridemic status
To assess the blood triglycerides effect on the optical property of the euglobulin fraction, two samples were obtained from one hyperlipemic volunteer in preprandial and 2 hours postprandial states. The ECLT was examined on both samples with a photographic follow up. The total cholesterol and the triglycerides were determined by routine laboratory methods, on a Synchron LX20 (Beckman®).
Statistical analysis
SigmaStat® Jandle Scientific software was used. Non parametric statistical analysis with Spearman test for the correlations and Mann-Whitney test for the comparison between men and women were used. The relation between the GFC and the semi-automatic method was described by an exponential equation. Data are presented as median values and ranges (25%–75%). A p value < 0.05 was considered as statistically significant.
Contributor Information
K Zouaoui Boudjeltia, Email: kaveroes@hotmail.com.
Ph Cauchie, Email: philippe.cauchie@chu-charleroi.be.
Cl Remacle, Email: cremacle@ucl.ac.be.
M Guillaume, Email: michel.guillaume@chu-charleroi.be.
D Brohée, Email: dany.brohee@chu-charleroi.be.
JL Hubert, Email: erem@charline.be.
M Vanhaeverbeek, Email: michel.vanhaeverbeek@chu-charleroi.be.
References
- Björn W. The fibrinolytic enzyme system: basic principles and links to venous and arterial thrombosis. Hematol Oncol Clin. 2000;14:325–338. doi: 10.1016/s0889-8588(05)70136-2. [DOI] [PubMed] [Google Scholar]
- Booth NA. Fibrinolysis and thrombosis. Baillieres Best Pract Res Clin Haematol. 1999;12:423–433. doi: 10.1053/beha.1999.0034. [DOI] [PubMed] [Google Scholar]
- Hadjez JM, Combe S, Horellou MH, Conard J, Nguyen G, Van Dreden P, Samama M. Heterogeneous mechanisms responsible for reduced fibrinolytic capacity in patients with history of venous thrombosis. Fibrinolysis. 1994;8:87–95. [Google Scholar]
- Hiraga T, Shimada M, Tsukada T, Murase T. Hypertriglyceridemia, but not hypercholesterolemia, is associated with the alterations of fibrinolytic system. Horm Metab Res. 1996;28:603–606. doi: 10.1055/s-2007-979862. [DOI] [PubMed] [Google Scholar]
- Lowe G. Coagulation, fibrinolysis and cardiovascular disease. Fibrinolysis Proteolysis. 1999;13:91–98. [Google Scholar]
- Juhan-Vague I, Collen D. On the role of coagulation and fibrinolysis in atherosclerosis. Ann Epidemiol. 1992;4:427–438. doi: 10.1016/1047-2797(92)90092-5. [DOI] [PubMed] [Google Scholar]
- Juhan-Vague I, Valadier J, Alessi MC, Aillaud MF, Ansaldi J, Philip-Joet C, Holvoet P, Serradimigni A, Collen D. Deficient t-PA release and elevated PAI-1 in patient with spontaneous or recurrent deep venous thrombosis. Thromb Haemost. 1987;57:67–72. [PubMed] [Google Scholar]
- Ridker PM, Vaughan DE, Stampfer MJ, Manson JE, Hennekens CH. Endogenous tissue-type plasminogen activator and risk of myocardial infarction. Lancet. 1993;341:1165–1168. doi: 10.1016/0140-6736(93)90998-V. [DOI] [PubMed] [Google Scholar]
- Jespersen J, Bertina RM, Haverkate F. Ecat Assay Procedures; A manual of Laboratory Techniques. Dordrecht: Kluwer. 1992. pp. 125–129.
- Chakrabarti R, Fearnley GR. The fibrinolytic potential as a simple measure of spontaneous fibrinolysis. J Clin Path. 1962;15:228–230. doi: 10.1136/jcp.15.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano T, Sakakibara K, Rydzewski A, Urano S, Takada Y, Takada A. Relationship between euglobulin clot lysis time and the plasma levels of tissue plasminogen activator and plasminogen activator inhibitor-1. Thromb Haemost. 1990;63:82–86. [PubMed] [Google Scholar]
- Vandevorst A. Introduction à la physique, tome 2, Edition Deboeck Université. 1991. pp. 422–424.
- Goris ML, Briandet PA. A clinical and Mathematical Introduction to Computer Processing of Scintigraphic Images. Raven Press. 1983. pp. 109–119.
- Amiral J, Malmejac A, Gin H, Pannell R, Vissac A-M, Seigneur M, Scarabin P-Y, Boisseau M, Guize L, Gurewich V. Evaluation of the fibrinolytic potential on plasma: physiological and pathological variations, and associations with cardio-vascular disease risk factors. Fibrinolysis Proteolysis. 1999;13:1–10. [Google Scholar]
- Urano T, Sumiyoshi K, Nakamura M, Mori T, Takada Y, Takada A. Fluctuation of t-PA and PAI-1 antigen levels in plasma: difference of their fluctuation patterns between male and female. Thromb Res. 1990;60:133–139. doi: 10.1016/0049-3848(90)90292-k. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Intrinsic fibrinolytic capacity and systemic inflammation: novel risk factor for arterial thrombotic disease. Haemostasis. 1997;27:2–11. doi: 10.1159/000217475. [DOI] [PubMed] [Google Scholar]
- Blombäck B, Danerjee D, Carlsson K, Hamsten A, Hessel B, Procyk R, Silveira A, Zacharski L. Native fibrin gel networks and factors influencing their formation in healthy and disease. Adv Exp Med Biol. 1990;281:1–23. doi: 10.1007/978-1-4615-3806-6_1. [DOI] [PubMed] [Google Scholar]
- Weitz JI, Leslie B, Ginsberg J. Soluble fibrin degradation products potentiate tissue plasminogen Activator-induced fibrinogen proteolysis. J Clin Invest. 1991;87:1082–1090. doi: 10.1172/JCI115069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouaoui Boudjeltia K, Guillaume M, Vanhaeverbeek M, Schonne E, Cauchie P, Remacle C. Influence of the blood lipids on euglobulin clot lysis time in men and women. Atherosclerosis. 1999;144:63 supplement. [Google Scholar]
- Zouaoui Boudjeltia K, Guillaume , Kinard K, Cauchie P, Remacle C, Ducobu J, Vanhaeverbeek M, Brohee D. Effect of blood monocyte counts on plasma fibrinolytic capacity. Atherosclerosis. 2001;2:83 supplement. [Google Scholar]


