Abstract
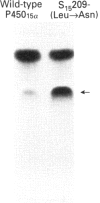
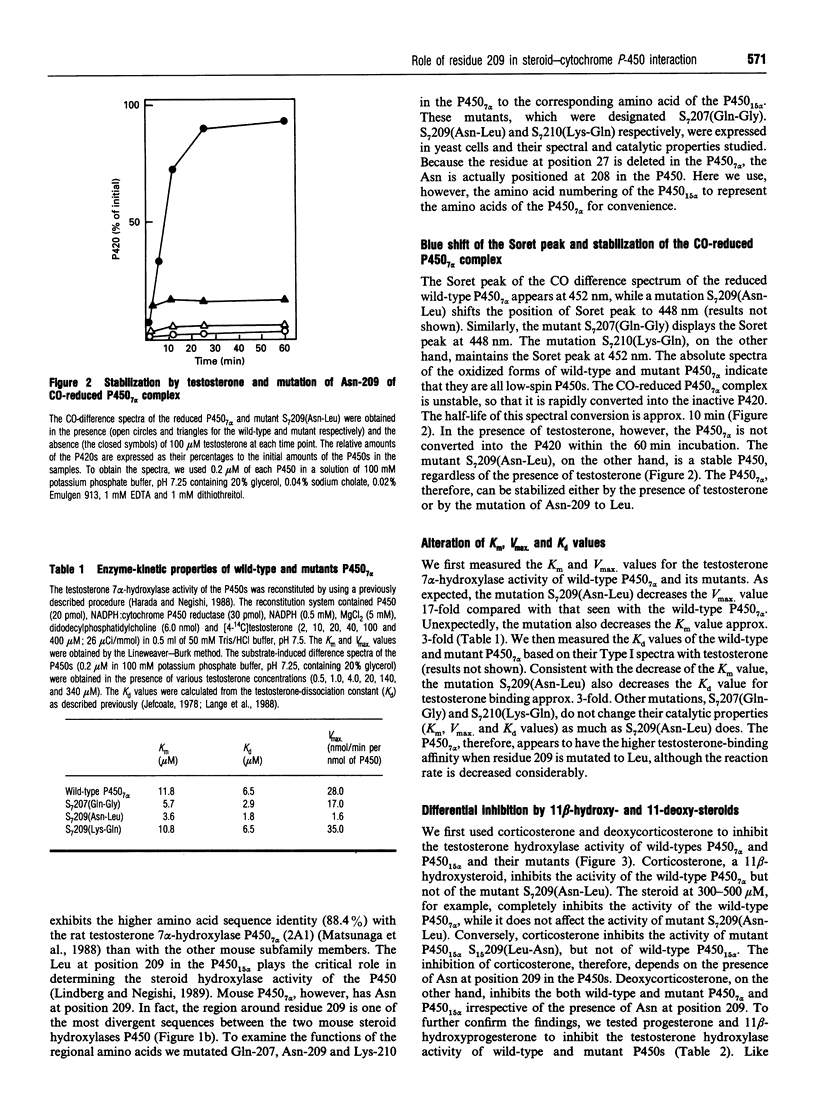
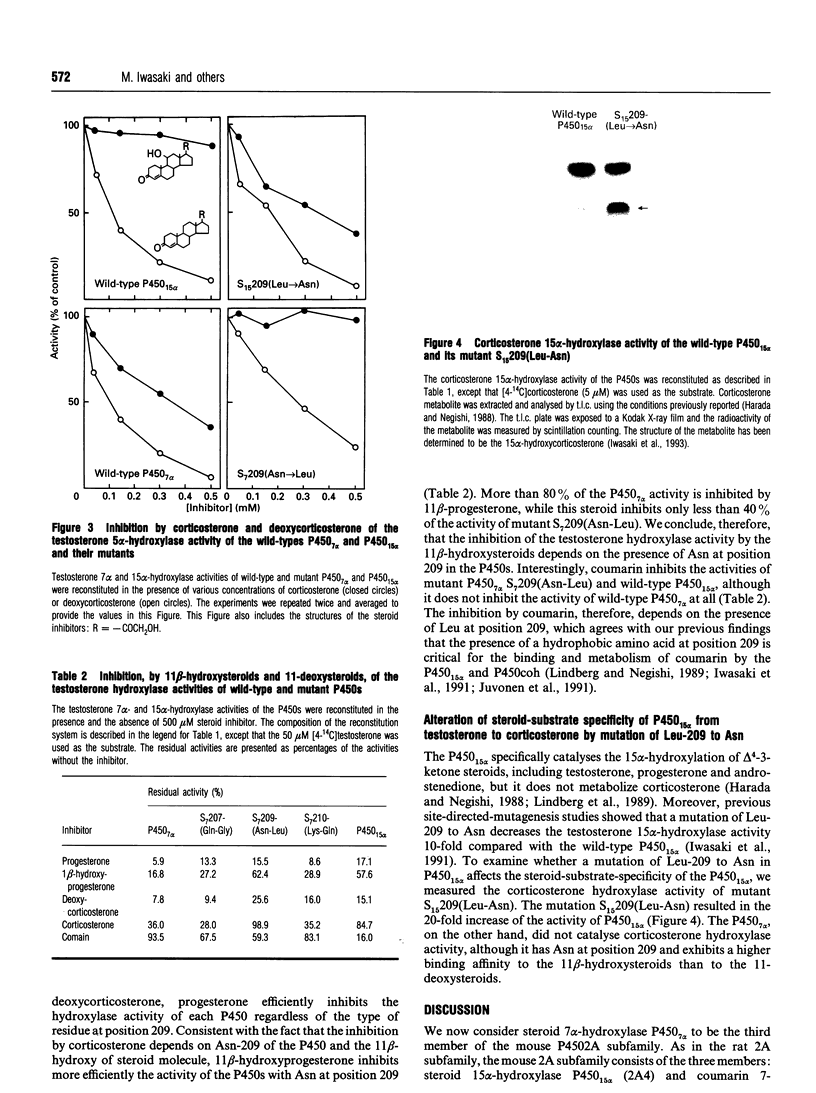
We have cloned a cDNA encoding mouse steroid 7 alpha-hydroxylase P450(7) alpha (cytochrome P-450(7) alpha) and expressed it in Saccharomyces cerevisiae. Mouse P450(7) alpha is 70% identical in its amino acid sequence with the mouse steroid 15 alpha-hydroxylase P450(15) alpha (2A4). The Leu at position 209 of P450(15) alpha is the most important residue to determine the steroid hydroxylase activity of the P450 [Lindberg and Negishi (1989) Nature (London) 339, 632-634]. The P450(7) alpha contains Asn at the position corresponding to the Leu-209 of P450(15) alpha, although both P450s hydroxylate testosterone. The CO-reduced P450(7) alpha complex is unstable, so that it is quickly converted into the inactive P420, whereas the P450(15) alpha is very stable. The P450(7) alpha, however, is stabilized either by addition of testosterone or by a mutation of Asn-209 to Leu. The mutant P450(7) alpha displays a 17-fold lower Vmax. value than the wild-type enzyme. Unexpectedly, it also has 3-fold lower Km and Kd values. Residue 209 in P450(7) alpha, therefore, appears to be located at a critical site of the haem-substrate-binding pocket. Corticosterone inhibits the testosterone 7 alpha-hydroxylase activity of the wild-type P450(7) alpha, whereas it does not inhibit the mutant P450(7) alpha. Conversely, the P450(15) alpha activity becomes inhibited by corticosterone upon the replacement of Leu-209 by Asn. In addition, this mutation increases the corticosterone 15 alpha-hydroxylase activity of P450(15) alpha at least 20-fold. Whereas the inhibition by corticosterone depends on the presence of Asn at position 209, deoxycorticosterone inhibits the activities of the P450s regardless of the type of residue at 209. The results indicate, therefore, that the identity of residue 209 determines the affinity as well as specificity of steroid binding to both P450(7) alpha and P450(15) alpha.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Domin B. A., Serabjit-Singh C. J., Philpot R. M. Quantitation of rabbit cytochrome P-450, form 2, in microsomal preparations bound directly to nitrocellulose paper using a modified peroxidase-immunostaining procedure. Anal Biochem. 1984 Feb;136(2):390–396. doi: 10.1016/0003-2697(84)90234-3. [DOI] [PubMed] [Google Scholar]
- Furuya H., Shimizu T., Hirano K., Hatano M., Fujii-Kuriyama Y., Raag R., Poulos T. L. Site-directed mutageneses of rat liver cytochrome P-450d: catalytic activities toward benzphetamine and 7-ethoxycoumarin. Biochemistry. 1989 Aug 22;28(17):6848–6857. doi: 10.1021/bi00443a011. [DOI] [PubMed] [Google Scholar]
- Gotoh O. Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J Biol Chem. 1992 Jan 5;267(1):83–90. [PubMed] [Google Scholar]
- Graham-Lorence S., Khalil M. W., Lorence M. C., Mendelson C. R., Simpson E. R. Structure-function relationships of human aromatase cytochrome P-450 using molecular modeling and site-directed mutagenesis. J Biol Chem. 1991 Jun 25;266(18):11939–11946. [PubMed] [Google Scholar]
- Harada N., Negishi M. Substrate specificities of cytochrome P-450, C-P-450(16)alpha and P-450(15)alpha, and contribution to steroid hydroxylase activities in mouse liver microsomes. Biochem Pharmacol. 1988 Dec 15;37(24):4778–4780. doi: 10.1016/0006-2952(88)90352-8. [DOI] [PubMed] [Google Scholar]
- Imai Y., Nakamura M. Point mutations at threonine-301 modify substrate specificity of rabbit liver microsomal cytochromes P-450 (laurate (omega-1)-hydroxylase and testosterone 16 alpha-hydroxylase). Biochem Biophys Res Commun. 1989 Feb 15;158(3):717–722. doi: 10.1016/0006-291x(89)92780-0. [DOI] [PubMed] [Google Scholar]
- Iwasaki M., Darden T. A., Pedersen L. G., Davis D. G., Juvonen R. O., Sueyoshi T., Negishi M. Engineering mouse P450coh to a novel corticosterone 15 alpha-hydroxylase and modeling steroid-binding orientation in the substrate pocket. J Biol Chem. 1993 Jan 15;268(2):759–762. [PubMed] [Google Scholar]
- Iwasaki M., Juvonen R., Lindberg R., Negishi M. Alteration of high and low spin equilibrium by a single mutation of amino acid 209 in mouse cytochromes P450. J Biol Chem. 1991 Feb 25;266(6):3380–3382. [PubMed] [Google Scholar]
- Jefcoate C. R. Measurement of substrate and inhibitor binding to microsomal cytochrome P-450 by optical-difference spectroscopy. Methods Enzymol. 1978;52:258–279. doi: 10.1016/s0076-6879(78)52029-6. [DOI] [PubMed] [Google Scholar]
- Juvonen R. O., Iwasaki M., Negishi M. Structural function of residue-209 in coumarin 7-hydroxylase (P450coh). Enzyme-kinetic studies and site-directed mutagenesis. J Biol Chem. 1991 Sep 5;266(25):16431–16435. [PubMed] [Google Scholar]
- Kronbach T., Johnson E. F. An inhibitory monoclonal antibody binds in close proximity to a determinant for substrate binding in cytochrome P450IIC5. J Biol Chem. 1991 Apr 5;266(10):6215–6220. [PubMed] [Google Scholar]
- Lindberg R. L., Negishi M. Alteration of mouse cytochrome P450coh substrate specificity by mutation of a single amino-acid residue. Nature. 1989 Jun 22;339(6226):632–634. doi: 10.1038/339632a0. [DOI] [PubMed] [Google Scholar]
- Lindberg R., Burkhart B., Ichikawa T., Negishi M. The structure and characterization of type I P-450(15) alpha gene as major steroid 15 alpha-hydroxylase and its comparison with type II P-450(15) alpha gene. J Biol Chem. 1989 Apr 15;264(11):6465–6471. [PubMed] [Google Scholar]
- Matsunaga E., Zeugin T., Zanger U. M., Aoyama T., Meyer U. A., Gonzalez F. J. Sequence requirements for cytochrome P-450IID1 catalytic activity. A single amino acid change (Ile380 Phe) specifically decreases Vmax of the enzyme for bufuralol but not debrisoquine hydroxylation. J Biol Chem. 1990 Oct 5;265(28):17197–17201. [PubMed] [Google Scholar]
- Matsunaga T., Nagata K., Holsztynska E. J., Lapenson D. P., Smith A., Kato R., Gelboin H. V., Waxman D. J., Gonzalez F. J. Gene conversion and differential regulation in the rat P-450 IIA gene subfamily. Purification, catalytic activity, cDNA and deduced amino acid sequence, and regulation of an adult male-specific hepatic testosterone 15 alpha-hydroxylase. J Biol Chem. 1988 Dec 5;263(34):17995–18002. [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Coon M. J., Estabrook R. W., Feyereisen R., Fujii-Kuriyama Y., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991 Jan-Feb;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- Negishi M., Lindberg R., Burkhart B., Ichikawa T., Honkakoski P., Lang M. Mouse steroid 15 alpha-hydroxylase gene family: identification of type II P-450(15)alpha as coumarin 7-hydroxylase. Biochemistry. 1989 May 16;28(10):4169–4172. doi: 10.1021/bi00436a007. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Strobel H. W. Secondary structure prediction of 52 membrane-bound cytochromes P450 shows a strong structural similarity to P450cam. Biochemistry. 1989 Jan 24;28(2):656–660. doi: 10.1021/bi00428a036. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Oeda K., Sakaki T., Ohkawa H. Expression of rat liver cytochrome P-450MC cDNA in Saccharomyces cerevisiae. DNA. 1985 Jun;4(3):203–210. doi: 10.1089/dna.1985.4.203. [DOI] [PubMed] [Google Scholar]
- Poulos T. L. Modeling of mammalian P450s on basis of P450cam X-ray structure. Methods Enzymol. 1991;206:11–30. doi: 10.1016/0076-6879(91)06073-c. [DOI] [PubMed] [Google Scholar]
- Samokyszyn V. M., Miller D. M., Reif D. W., Aust S. D. Inhibition of superoxide and ferritin-dependent lipid peroxidation by ceruloplasmin. J Biol Chem. 1989 Jan 5;264(1):21–26. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Squires E. J., Negishi M. Reciprocal regulation of sex-dependent expression of testosterone 15 alpha-hydroxylase (P-450(15 alpha)) in liver and kidney of male mice by androgen. Evidence for a single gene. J Biol Chem. 1988 Mar 25;263(9):4166–4171. [PubMed] [Google Scholar]