Abstract
We report a site-selective ipso-nitration of aryl germanes in the presence of boronic esters, silanes, halogens, and additional functionalities. The protocol is characterized by operational simplicity, proceeds at room temperature, and is enabled by [Ru(bpy)3](PF6)2/blue light photocatalysis. Owing to the exquisite robustness of the [Ge] functionality, nitrations of alternative functional handles in the presence of the germane are also feasible, as showcased herein.
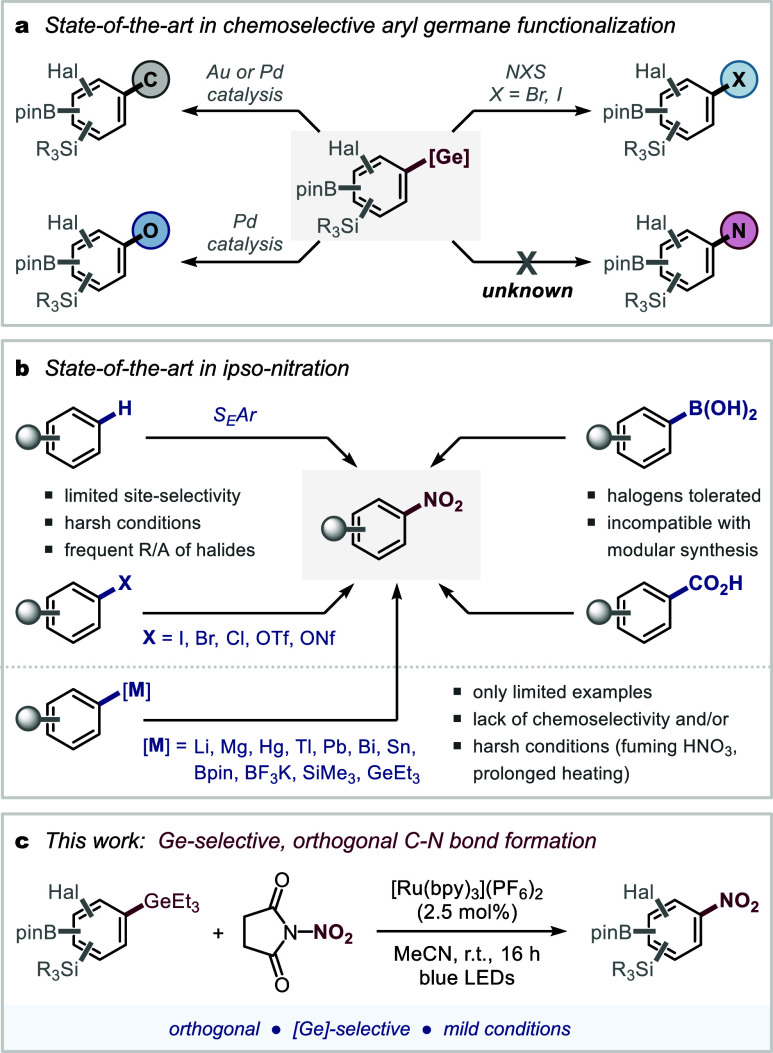
Oorganogermanes have historically displayed limited reactivity and applications in synthesis and catalysis.1,2 However, recent developments demonstrated that under electrophilic reactivity modes via Pd or Au catalysis or electrophilic aromatic substitution the Ge functionality becomes the most reactive, allowing for preferential and Ge-selective arylation,3 alkynylation,4 halogenation,5 and C–O bond formation6 while tolerating more established coupling partners such as halides, silanes, boronic acid derivatives (Bpin) and additional functionalities (Figure 1a). Moreover, organogermanes are highly robust and nontoxic reagents, as well as straightforward to synthesize and handle.7,8
Figure 1.
State of art in site-selective C–Ge functionalization, C–H nitration, and ipso-nitration of arenes and this work.26
However, C–N bond formation of organogermanes in the presence of alternative coupling handles, such as Bpin or silanes, is still unprecedented (for any kind of N-derivative and oxidation state).9 This report discloses the first site-selective ipso-nitration of aryl germanes.
Owing to their industrial and commercial applications,10 nitroarenes are an important class of compounds in organic chemistry and play a vital role in the synthesis of various pharmaceuticals,11 agrochemicals, plastics, explosives, dyes, or polymers.12 They are also key functionalities in synthesis as cross-coupling partners13 or precursors (e.g., to anilines14 and heterocycles13a,15).
While synthetic access to nitroarenes can in principle be accomplished via direct C–H nitration through electrophilic aromatic substitution,16 radical-based processes,17 or metal catalysis,18 the control of chemo- and site-selectivity remains challenging for such processes. The ipso-functionalization of suitable molecular handles is hence a valuable complementary approach, especially in the context of modular and programmable syntheses via the sequential decoration of building blocks that contain multiple handles. The current methodological repertoire19 involves the ipso-nitration of aryl (pseudo)halides,20 carboxylates,21 boronic acids,22 amines,23 or organometallic species (ArMgX or ArLi), see Figure 1b.24 Demonstration of site-selective ipso-nitration in the presence of multiple alternative molecular handles is limited, however, and has only been shown toward aryl halides.25
However, in the context of modular synthesis campaigns, site-selective functionalization would be of utmost value. Clearly, the extent of modularity will depend on the number of tolerated molecular handles and in this context, these handles should ideally be bench-stable, easy to handle and readily purifyable, which rules out several of the currently available handles for ipso-nitration.
We envisioned that development of an ipso-nitration of a new “handle”, i.e., ArGeR3, would therefore be enabling, especially if the protocol for the first time also tolerates multiple alternative bench-stable coupling handles, such as Bpin, silanes, and halogens.
We initially set out to investigate electrophilic nitration conditions and explored conditions known to form nitronium ions (NO2+) in situ, involving N-nitrosaccharin in HFIP.16b While we observed highly efficient and mild nitration of para-t-Bu phenylgermane to 1 in a 95% yield at room temperature, our further investigations revealed that extensions to intramolecular competitions of aryl germanes containing also SiMe3 and Bpin was not feasible under these conditions due to the incompatibility of these functionalities with the employed reaction conditions.27,28
We therefore explored alternative conditions. Inspired by Katayev and co-workers’ recent developments of the photoassisted nitration of boronic acids,29 we set out to examine the feasibility of nitrating an aryl germane employing N-nitrosuccinimide as a NO2 source and [Ru(bpy)3](PF6)2 as a photocatalyst under blue-light irradiation. This protocol proved to be effective and resulted in the desired product (1) in an 84% yield at room temperature (Scheme 1a). Our further studies revealed that the addition of a tetrafluoroborate anion (AgBF4 or NaBF4) was also beneficial and strongly favored the nitration of electron-deficient germanes (2) (see the Supporting Information).30
Scheme 1. Site-Selectivity and Orthogonality of Aryl Germane ipso-Nitration.

Without the addition of NaBF4.
Reaction time of 7 h.
Quantified by 1H NMR against an internal standard.
9% of nitration at silane.
Reaction conditions: aryl germane (0.3 mmol, 1.0 equiv), N-nitrosuccinimide (0.6 mmol, 2.0 equiv), [Ru(bpy)3](PF6)2 (0.0075 mmol, 2.5 mol %), NaBF4 (0.45 mmol, 1.5 equiv), and MeCN (1.2 mL). Yields of isolated products are given.
Pleasingly, site-selective nitration of [Ge] in the presence of other functional handles proved to be effective under these conditions (Scheme 1a). We observed the exclusive nitration of the Ge-site over C–Br and C–Cl (3–8), Bpin (9 and 10), and SiMe3 (11 and 12). Notably, nitration of germylated clofibrate was also achieved in an excellent yield (8). While the yield of the isolated material was moderate in some of these cases, we detected only the product resulting from germane nitration. It was the only product visible by GC-MS and TLC analyses and hence straightforwardly isolated.
Beyond these intramolecular competitions, we also observed high Ge-selectivity in intermolecular competitions with Ar-SiMe3 (13) and Ar-Bpin (14) under these reaction conditions (Scheme 1b). Exclusive functionalization of ArGeEt3 in a 98% yield took place in the presence of Ar-Bpin (14), which remained fully untouched in the process. In the case of Ar-SiMe3 (13), in addition to nitration of the germane (in 81%), a small amount of of nitration at the silane (9%) was also observed, contrary to the intramolecular competitions.
Intrigued by the superiority of the Ge functionality under these conditions, we turned to computational studies. Previous studies on photocatalytic nitration of olefins and arenes using N-nitrosuccinimide indicated the likely involvement of nitryl radicals.22,29 In line with this, when we attempted reactions in the presence of several radical quenchers such as 1,4-dinitrobenzene, diphenylethylene, styrene, benzoquinone, and diallyl ether, the nitration was fully suppressed (see the Supporting Information).
Our computational studies at CPCM (MeCN) M06-2X/def2-TZVP//B3LYP-D3BJ/6-31++G(d,p) (LANL2DZ for Ru) level of theory31,32 indicate that the nitration at [Ge] via a radical process has an activation free energy barrier of 23.6 kcal/mol and proceeds via formation of a σ-complex (I, Scheme 2). It is calculated to be substantially favored over nitration at Bpin (by ΔΔG‡ = 3.6 kcal/mol). The NO2 radical addition at the [Si] site is computationally predicted to possess only a marginally higher barrier than for [Ge] to form adduct I (ΔΔG‡ = 0.8 kcal/mol) at M06-2X (and also at PBE0-D3, wB97XD; 1.0 kcal/mol at MN15L but only 0.5 kcal/mol at the DLPNO-CCSD(T) level of theory). However, the subsequent silyl radical loss as compared to Ge radical loss is significantly less favored. In fact, it is associated with a free energy that lies energetically at similar level (or above for other tested methods: 1.8 kcal/mol at PBE0-D3, 3.0 kcal/mol at MN15L, 4.3 kcal/mol at wB97XD, and even 7.6 kcal/mol at the DLPNO-CCSD(T) level of theory)32 as the initial nitro radical addition transition state. This suggests that the process likely reverses back to starting material, making the dissociation of the silyl radical potentially rate-determining,32 depending on the substrate’s substitution pattern. See the SI for an assessment of the reaction profile with other levels of theory. The GeEt3 radical is subsequently readily oxidized to the corresponding cation (calculated potential is −0.68 V vs SCE), which regenerates the catalyst (Scheme 2b).33,34
Scheme 2. DFT Study on Photocatalyzed ipso-Nitration.
TS2 could not be located and was approximated as the energy maximum obtained from a relaxed scan of the C-LG bond length.
Values (in kcal/mol) refer to Gibbs free energies at the at CPCM (MeCN) M06-2X/def2-TZVP//B3LYP-D3(BJ)/6-31++G(d,p) (LANL2DZ for Ru) level of theory.31,32
Given the exceptional robustness of organogermanes toward various reaction conditions, we also explored the possibility to nitrate in the presence of the Ge functionality (Scheme 1c).
To this end, we engaged Pd-catalysis developed by Buchwald and co-workers20a and attempted the nitration of (4-chlorophenyl)triethylgermane. Despite the employed harsh reaction conditions (130 °C), there was no consumption of the Ge functionality, and instead C–Cl was exclusively functionalized to give 16 in a 90% yield. By contrast, under photocatalysis conditions, the Ge functionality is exclusively nitrated while leaving C–Cl untouched (15). These results further manifest the orthogonal reactivity features of trialkyl aryl germanes.
We next explored the scope for the ipso-nitration more generally in the absence of competing molecular handles (Scheme 3). Pleasingly, the protocol proved to be effective for a wide range of aryl germanes bearing electron-donating and electron-withdrawing substituents. Alkyl- and methoxy-substituted aryl germanes reacted smoothly to give the corresponding nitroarenes in high yields (17–24), also when positioned ortho to the [Ge]-site (25). Cyanide and sulfonyl (26–28) functional groups as well as the heterocycles isoxazole and pyrrolidinone were similarly tolerated (29 and 30).
Scheme 3. Substrate Scope of ipso-Nitration.
NaBF4 (0.45 mmol, 1.5 equiv) was used.
Reaction conditions: aryl germane (0.3 mmol, 1.0 equiv), N-nitrosuccinimide (0.6 mmol, 2.0 equiv), [Ru(bpy)3](PF6)2 (0.0075 mmol, 2.5 mol %), and MeCN (1.2 mL). Yields of isolated products are given.
In conclusion, we showcased the chemoselective ipso-nitration of aryl germanes under photocatalytic conditions using bench-stable and readily available N-nitrosuccinimide as a nitrating reagent. This transformation represents the first C–N bond formation of aryl germanes to access a wide range of nitroarenes while tolerating a diverse array of functional groups on the aryl moiety. Intra- and intermolecular competitions of aryl germanes versus silanes and boronic esters and halogens gave selective functionalization at the Ge site, while established nitration protocols (e.g., on aryl halides) were shown to tolerate the Ge functionality owing to its exquisite robustness. These results further underscore the privileged reactivity and potential of aryl germanes and overall broaden the repertoire of modular syntheses campaigns to nitration.
Acknowledgments
We thank the European Research Council (ERC-864849) for funding. Calculations were performed with computing resources granted by JARA-HPC from RWTH Aachen University under project “jara0091”.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.3c02822.
Experimental procedures, NMR spectra, characterization data of compounds, and computational details (PDF)
Author Contributions
‡ A.D. and A.G.G. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Fricke C.; Schoenebeck F. Organogermanes as Orthogonal Coupling Partners in Synthesis and Catalysis. Acc. Chem. Res. 2020, 53, 2715–2725. 10.1021/acs.accounts.0c00527. [DOI] [PubMed] [Google Scholar]
- a Faller J. W.; Kultyshev R. G. Palladium-Catalyzed Cross-Coupling Reactions of Allyl, Phenyl, Alkenyl, and Alkynyl Germatranes with Aryl Iodides. Organometallics 2002, 21, 5911–5918. 10.1021/om020578c. [DOI] [Google Scholar]; b Nakamura T.; Kinoshita H.; Shinokubo H.; Oshima K. Biaryl Synthesis from Two Different Aryl Halides with Tri(2-furyl)germane. Org. Lett. 2002, 4, 3165–3167. 10.1021/ol026613t. [DOI] [PubMed] [Google Scholar]; c Spivey A. C.; Gripton C. J. G.; Hannah J. P.; Tseng C.-C.; de Fraine P.; Parr N. J.; Scicinski J. J. The development of a ‘safety-catch’ arylgermane for biaryl synthesis by palladium-catalysed germyl-stille cross-coupling. Appl. Organomet. Chem. 2007, 21, 572–589. 10.1002/aoc.1270. [DOI] [Google Scholar]
- a Fricke C.; Sherborne G. J.; Funes-Ardoiz I.; Senol E.; Guven S.; Schoenebeck F. Orthogonal Nanoparticle Catalysis with Organogermanes. Angew. Chem., Int. Ed. 2019, 58, 17788–17795. 10.1002/anie.201910060. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Fricke C.; Dahiya A.; Reid W. B.; Schoenebeck F. Gold-Catalyzed C–H Functionalization with Aryl Germanes. ACS Catal. 2019, 9, 9231–9236. 10.1021/acscatal.9b02841. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Sherborne G. J.; Gevondian A. G.; Funes-Ardoiz I.; Dahiya A.; Fricke C.; Schoenebeck F. Modular and Selective Arylation of Aryl Germanes (C–GeEt3) over C–Bpin, C–SiR3 and Halogens Enabled by Light-Activated Gold Catalysis. Angew. Chem., Int. Ed. 2020, 59, 15543–15548. 10.1002/anie.202005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahiya A.; Schoenebeck F. Orthogonal and Modular Arylation of Alkynylgermanes. ACS Catal. 2022, 12, 8048–8054. 10.1021/acscatal.2c02179. [DOI] [Google Scholar]
- Fricke C.; Deckers K.; Schoenebeck F. Orthogonal Stability and Reactivity of Aryl Germanes Enables Rapid and Selective (Multi)Halogenations. Angew. Chem., Int. Ed. 2020, 59, 18717–18722. 10.1002/anie.202008372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahiya A.; Gevondian A. G.; Schoenebeck F. Orthogonal C–O Bond Construction with Organogermanes. J. Am. Chem. Soc. 2023, 145, 7729–7735. 10.1021/jacs.3c01081. [DOI] [PubMed] [Google Scholar]
- a Selmani A.; Schoenebeck F. Anti-Markovnikov Hydrogermylation of Alkenes via Lewis Acid Catalysis. Synthesis 2023, 55, 1792–1798. 10.1055/a-2036-3868. [DOI] [Google Scholar]; b Selmani A.; Schoenebeck F. Transition-Metal-Free, Formal C–H Germylation of Arenes and Styrenes via Dibenzothiophenium Salts. Org. Lett. 2021, 23, 4779–4784. 10.1021/acs.orglett.1c01505. [DOI] [PubMed] [Google Scholar]; c Dahiya A.; Schoenebeck F. Base-Mediated Direct C–H Germylation of Heteroarenes and Arenes. Org. Lett. 2021, 23, 6010–6013. 10.1021/acs.orglett.1c02079. [DOI] [PubMed] [Google Scholar]
- Effective coupling protocols with germatranes have also recently been developed. However, the Ge functionality is less reactive than, for example, BPin or alternatives in these reactivity regimes, see:Xu M.-Y.; Xiao B. Germatranes and carbagermatranes: (hetero)aryl and alkyl coupling partners in Pd-catalyzed cross-coupling reactions. Chem. Commun. 2021, 57, 11764–11775. 10.1039/D1CC04373K. [DOI] [PubMed] [Google Scholar]
- Few isolated examples of ipso-nitration of aryl-GeEt3 have been reported, albeit none of them were site-selective and all required prolonged heating at 100 °C in fuming nitric acid:; a Eaborn C.; Leyshon K.; Pande K. C. Organogermanium Compounds. Part II. The Preparation of Triethyl-m- and -p-nitrophenylgermane. J. Chem. Soc. 1960, 3423–3424. [Google Scholar]; b Moerlein S. M. Synthesis and spectroscopic characteristics of aryltrimethyl-silicon, -germanium, and -tin compounds. J. Organomet. Chem. 1987, 319, 29–39. 10.1016/0022-328X(87)80344-3. [DOI] [Google Scholar]
- Ono N.The Nitro Group in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- a Ju K.-S.; Parales R. E. Nitroaromatic Compounds, from Synthesis to Biodegradation. Microbiol. Mol. Biol. Rev. 2010, 74, 250–272. 10.1128/MMBR.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Nepali K.; Lee H.-Y.; Liou J.-P. Nitro-Group-Containing Drugs. J. Med. Chem. 2019, 62, 2851–2893. 10.1021/acs.jmedchem.8b00147. [DOI] [PubMed] [Google Scholar]
- Fiedler H.; Mücke W.. Nitro Derivatives of Polycyclic Aromatic Hydrocarbons (NO2-PAH). In Anthropogenic Compounds; Hutzinger O., Ed.; The Handbook of Environmental Chemistry, Vol. 3; Springer: Berlin, Germany, 1991; pp 97–137. [Google Scholar]
- a Muto K.; Okita T.; Yamaguchi J. Transition-Metal-Catalyzed Denitrative Coupling of Nitroarenes. ACS Catal. 2020, 10, 9856–9871. 10.1021/acscatal.0c02990. [DOI] [Google Scholar]; b Li G.; Nykaza T. V.; Cooper J. C.; Ramirez A.; Luzung M. R.; Radosevich A. T. An Improved PIII/PV=O-Catalyzed Reductive C–N Coupling of Nitroaromatics and Boronic Acids by Mechanistic Differentiation of Rate- and Product-Determining Steps. J. Am. Chem. Soc. 2020, 142, 6786–6799. 10.1021/jacs.0c01666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formenti D.; Ferretti F.; Scharnagl F. K.; Beller M. Reduction of Nitro Compounds Using 3d-Non-Noble Metal Catalysts. Chem. Rev. 2019, 119, 2611–2680. 10.1021/acs.chemrev.8b00547. [DOI] [PubMed] [Google Scholar]
- Zou D.; Wang W.; Hu Y.; Jia T. Nitroarenes and nitroalkenes as potential amino sources for the synthesis of N-heterocycles. Org. Biomol. Chem. 2023, 21, 2254–2271. 10.1039/D3OB00064H. [DOI] [PubMed] [Google Scholar]
- a Olah G. A.; Narang S. C.; Fung A. P. Aromatic substitution. 47. Acid-catalyzed transfer nitration of aromatics with N-nitropyrazole, a convenient new nitrating agent. J. Org. Chem. 1981, 46, 2706–2709. 10.1021/jo00326a020. [DOI] [Google Scholar]; b Calvo R.; Zhang K.; Passera A.; Katayev D. Facile access to nitroarenes and nitroheteroarenes using N-nitrosaccharin. Nat. Commun. 2019, 10, 3410. 10.1038/s41467-019-11419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Mondal S.; Samanta S.; Hajra A. Regioselective C-7 Nitration of 8-Aminoquinoline Amides Using tert-Butyl Nitrite. Adv. Synth. Catal. 2018, 360, 1026–1031. 10.1002/adsc.201701555. [DOI] [Google Scholar]; b Blum S. P.; Nickel C.; Schäffer L.; Karakaya T.; Waldvogel S. R. Electrochemical Nitration with Nitrite. ChemSusChem 2021, 14, 4936–4940. 10.1002/cssc.202102053. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Patra S.; Mosiagin I.; Giri R.; Nauser T.; Katayev D. Electron-Driven Nitration of Unsaturated Hydrocarbons. Angew. Chem., Int. Ed. 2023, 62, e202300533. 10.1002/anie.202300533. [DOI] [PubMed] [Google Scholar]
- a Zhang W.; Lou S.; Liu Y.; Xu Z. Palladium-Catalyzed Chelation-Assisted Aromatic C–H Nitration: Regiospecific Synthesis of Nitroarenes Free from the Effect of the Orientation Rules. J. Org. Chem. 2013, 78, 5932–5948. 10.1021/jo400594j. [DOI] [PubMed] [Google Scholar]; b Xie F.; Qi Z.; Li X. Rhodium(III)-Catalyzed Azidation and Nitration of Arenes by C–H Activation. Angew. Chem., Int. Ed. 2013, 52, 11862–11866. 10.1002/anie.201305902. [DOI] [PubMed] [Google Scholar]; c Katayev D.; Pfister K. F.; Wendling T.; Gooßen L. J. Copper-Mediated ortho-Nitration of (Hetero)Arenecarboxylates. Chem. Eur. J. 2014, 20, 9902–9905. 10.1002/chem.201403363. [DOI] [PubMed] [Google Scholar]
- Aitken K. M.; Aitken R. A.. Product Class 21: Nitroarenes. In Science of Synthesis; Thieme, 2007; Vol. 31b, pp 1183–1320. [Google Scholar]
- a Fors B. P.; Buchwald S. L. Pd-Catalyzed Conversion of Aryl Chlorides, Triflates, and Nonaflates to Nitroaromatics. J. Am. Chem. Soc. 2009, 131, 12898–12899. 10.1021/ja905768k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Prakash G. K. S.; Mathew T. ipso-Nitration of Arenes. Angew. Chem., Int. Ed. 2010, 49, 1726–1728. 10.1002/anie.200906940. [DOI] [PubMed] [Google Scholar]
- a Zarei M.; Noroozizadeh E.; Moosavi-Zare A. R.; Zolfigol M. A. Synthesis of Nitroolefins and Nitroarenes under Mild Conditions. J. Org. Chem. 2018, 83, 3645–3650. 10.1021/acs.joc.7b03289. [DOI] [PubMed] [Google Scholar]; b Natarajan P.; Chaudhary R.; Venugopalan P. Silver(I)-Promoted ipso-Nitration of Carboxylic Acids by Nitronium Tetrafluoroborate. J. Org. Chem. 2015, 80, 10498–10504. 10.1021/acs.joc.5b02133. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Budinská A.; Passera A.; Katayev D. N-Nitroheterocycles: Bench-Stable Organic Reagents for Catalytic Ipso-Nitration of Aryl- and Heteroarylboronic Acids. Org. Lett. 2020, 22, 2714–2719. 10.1021/acs.orglett.0c00671. [DOI] [PubMed] [Google Scholar]
- a Reddy K. R.; Maheswari C. U.; Venkateshwar M.; Kantam M. L. Selective Oxidation of Aromatic Amines to Nitro Derivatives using Potassium Iodide-tert-Butyl Hydroperoxide Catalytic System. Adv. Synth. Catal. 2009, 351, 93–96. 10.1002/adsc.200800641. [DOI] [Google Scholar]; b Patil V. V.; Shankarling G. S. Steric-Hindrance-Induced Regio- and Chemoselective Oxidation of Aromatic Amines. J. Org. Chem. 2015, 80, 7876–7883. 10.1021/acs.joc.5b00582. [DOI] [PubMed] [Google Scholar]
- Tani K.; Lukin K.; Eaton P. E. Nitration of Organolithiums and Grignards with Dinitrogen Tetroxide: Success at Melting Interfaces. J. Am. Chem. Soc. 1997, 119, 1476–1477. 10.1021/ja963658e. [DOI] [Google Scholar]
- For isolated examples of Ar–COOH functionalization in the presence of halogens (Cl and Br), see ref (21). For examples of Ar–B(OH)2 functionalization in the presence of halogens (Cl, Br, I), see ref (22).
- When our work was being submitted, the nitration of silanes had been reported, please see:; Mosiagin I.; Fernandes A. J.; Budinská A.; Hayriyan L.; Ylijoki K. E. O.; Katayev D. Catalytic ipso-Nitration of Organosilanes Enabled by Electrophilic N-Nitrosaccharin Reagent. Angew. Chem., Int. Ed. 2023, e202310851. 10.1002/anie.202310851. [DOI] [PubMed] [Google Scholar]
- See the SI for further information.
- For an example of electrophilic C–N bond formation of silanes, see:; Zhang Q.; Hitoshio K.; Saito H.; Shimokawa J.; Yorimitsu H. Copper-Catalyzed Electrophilic Amination of Alkoxyarylsilanes. Eur. J. Org. Chem. 2020, 2020, 4018–4021. 10.1002/ejoc.202000562. [DOI] [Google Scholar]
- Zhang K.; Jelier B.; Passera A.; Jeschke G.; Katayev D. Synthetic Diversity from a Versatile and Radical Nitrating Reagent. Chem. Eur. J. 2019, 25, 12929–12939. 10.1002/chem.201902966. [DOI] [PubMed] [Google Scholar]
- Light is required during the entire reaction time and not just as an initiator. When the light was turned off after 1 h, only traces of 1-fluoro-4-nitrobenzene (2) were obtained under otherwise unaltered reaction conditions.
- The following program was used for calculations:; Frisch M. J., et al. Gaussian 16, Revision A.03, rev. A.03; Gaussian, Inc.: Wallingford, CT, 2016.; See the Supporting Information for a complete reference and further details on computational methods and results.
- Energies were also computed using other DFT functionals as well as DLPNO-CCSD(T), which provided the same conclusions on a qualitative level, albeit predicted slightly higher selectivities as a result of TS2Si being higher in energy compared to TS1Si and TS1Ge. For details, please refer to the SI.
- Calculations of reduction potentials were done at SMD (MeCN) MN15/def2-TZVPP//SMD (MeCN) wB97XD/6-31+G(d,p). For the choice of method, see:; Bouayad-Gervais S.; Nielsen C. D. T.; Turksoy A.; Sperger T.; Deckers K.; Schoenebeck F. Access to Cyclic N-Trifluoromethyl Ureas through Photocatalytic Activation of Carbamoyl Azides. J. Am. Chem. Soc. 2022, 144, 6100–6106. 10.1021/jacs.2c02004. [DOI] [PubMed] [Google Scholar]
- The corresponding succinimide anion with a calculated potential of 1.47 V vs SCE is less readily oxidized than the Et3Ge radical.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.