Abstract
Chromium (Cr) is a toxic metal in soil–plant system, hence causing possible health risks prominently in the areas with forgoing industrial activities. Copper nanoparticles (Cu NPs) have been reported as an excellent adsorbent for pollutants. Therefore, this study investigates how copper nanoparticles enhance onion growth while decreasing chromium uptake in onion plants. Additionally, it examines the potential health risks of consuming onion plants with elevated chromium levels. The results demonstrated that the addition of CuNPs at 15 mg kg−1 significantly improved the plant height (48%), leaf length (37%), fresh weight of root (61%), root dry weight (70%), fresh weight of bulb (52%), bulb dry weight (59%), leaves fresh weight (52%) and dry weight of leaves (59%), leaf area (72%), number of onion leaves per plant (60%), Chl. a (42%), chl. b (36%), carotenoids (40%), total chlorophyll (40%), chlorophyll contents SPAD value (56%), relative water contents (35%), membrane stability index (16%), total sugars (25%), crude protein (21%), ascorbic acid (19%) and ash contents (64%) at 10 mg kg−1 Cr. Whereas, maximum decline of Cr by 46% in roots, 68% in leaves and 92% in bulb was found with application of 15 mg kg−1 of Cu NPs in onion plants under 10 mg kg−1 Cr toxicity. The health risk assessment parameters of onion plants showed minimum values 0.0028 for average daily intake (ADI), 0.001911 for Non-cancer risk (NCR), and 0.001433 for cancer risk (CR) in plants treated with Cu NPs at 15 mg kg−1 concentration grown in soil spiked with 10 mg kg−1 chromium. It is concluded that Cu NPs at 15 mg kg−1 concentration improved growth of plants in control as well as Cr contaminated soil. Therefore, use of Cu NPs at 15 mg kg−1 concentration is recommended for improving growth of plants under normal and metal contaminated soils.
Keywords: Nanoparticles, Plant physiology, Cr toxicity, Nano-remediation, Crop quality
Introduction
In regions of water scarcity, the irrigation of plants with residential and industrial wastewater is a usual practice [1]. Of the total production of vegetables in Pakistan, it is reported that 26% vegetables are grown with untreated wastewater [2, 3]. Both natural and human-activities like mining, smelting, metallurgical processes, industrial activities, and agricultural processes are the major factors contributing the entry of chromium (Cr) metal in the environment and degeneration of environmental quality [4]. The textile industry also utilizes chromium metal in various dyeing processes. However, the major source of Cr is tanning industry as 60–70% tanning process requires Cr-containing compounds. Therefore, release of untreated waste from these industries contaminate the environment [5]. Vegetables are an important part of dietary needs of humans besides staple foods [6]. However, various industrial processes and poor treatment of wastewater is solely responsible for increased uptake of heavy metals in vegetables in Pakistan [7, 8].
Cr is a heavy metal widely distributed within the earth’s crust [9]. Compounds comprised of Cr metal have also been located in atmosphere as a fine dust particle which later on settled on lithosphere and hydrosphere. It is reported previously that from contaminated sites, leakage and leaching of Cr to under-ground water resources caused serious health risks. Due to recalcitrant and toxic nature [10], heavy metals are considered as a continuing hazard to both the environmental quality and human lives, in commercial and residential surroundings.
Cr is a non-essential element as it doesn’t involve in any metabolic processes but, when certain concentration exceed in plants, it becomes toxic [11]. Chromium metal disturbs the two most important processes (photosynthesis and respiration) in plants, causing severe oxidative damage, hindering activities of certain enzymes, which eventually leads to death [12, 13]. Cr contamination significantly affects the productivity of agricultural crops by reduced rate of seed germination, stunted growth, increase cell damage, loss of chlorophyll and other pigments, inhibition of enzymatic activities and nutrients imbalance [14].
The management of abiotic stress for agricultural produce could be possible by using chemical approaches like hormones, organic substances, ascorbic acid to mitigate stress caused by abiotic environmental factors for improved plant growth [15, 16], whereas, the use of chemical amendments like biochar, zeolite and activated carbon due to high sorption properties serves as an efficient way to control heavy metal toxicity. Many studies have shown improved agricultural production by using chemical approaches like nanoparticles for the amendment of abiotic stress in plants. The smaller size with high surface area of nanoparticles are ideal bases for the sorption of contaminants like heavy metals, making them a perfect solution for long term cleaning of pollutants and safe reusability of wastewater [17]. Presence of various active sites, low production and operational cost with excellent sorption properties, contributed to the popularity of nanoparticles in water remediation approaches [18]. Cu NPs were reported as an efficient sorbent for heavy metals. The large surface to volume ratio, along with functional groups, are characteristic features of Cu NPs as an efficient sorbent of pollutants in soil [19]. As a fertilizer, growth regulator, pesticides, herbicides, and remediation of soil pollutants, Cu NPs have shown their traits for promotion of agricultural produce [20]. Previously, positive impact of Cu NPs on pigeon pea growth [21], Vigna radiata [22] and Cajanus cajan [23] have been reported. Moreover, inhibitory effects of Cu NPs were also found in pea plants by [24] and in Hordeum vulgare by [25]. In comparison to other NPs, further investigation needed to find out the impact of CuO NPs in plants at the physiological and biochemical levels.
Onion (Allium cepa L.) is an important biennial crop belonging to the Alliaceae family, used as both condiment and vegetable [26, 27]. As a highly economic crop, onion is a food commodity serving a high nutritional value with excellent source of essential oils and use as a medicine [28]. According to FAO [29], onion serves as an important commercial crop for the economy of Pakistan [30].
It has been acknowledged that Cu as a micronutrient helps in growth and development of plants. Various researchers used Cu NPs for improved growth and yield of plants under normal conditions. The application of Cu NPs as a sorbent material is also well reported. However, Cu NPs are least studied for their growth regulated potential under heavy metal toxicity. Following all these reports, we hypothesized that Cu NPs could be used as a nano-fertilizer for onion plants while providing an excellent sorption material for safe removal of heavy metals like Cr. The objectives of this experiment were to assess the potential of Cu NPs to improve growth of onion plants, immobilization of Cr metal in soil, reduced Cr associated health risks, and to evaluate the effective application rate of Cu NPs for growth regulation and remediation of pollutants in Cr contaminated soil.
Materials and methods
Production of copper nanoparticles
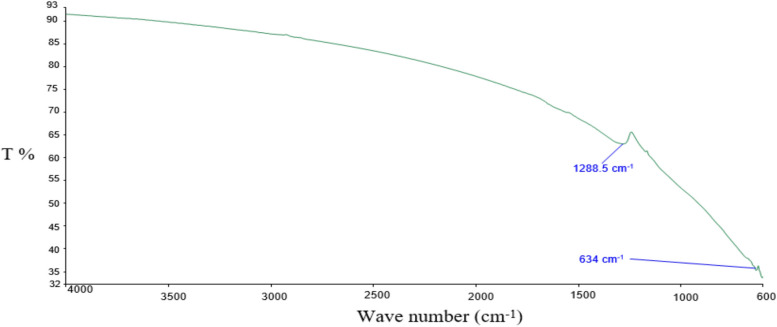
The copper oxide nanoparticles were synthesized by following the previous method [31] with slight modifications. 500 mL of copper acetate and glacial acetic acid (10 mL) were dissolved in a glass beaker. The solution was mixed while constant stirring at 500 rpm at 40 °C. 1 M NaOH (120 mL) was added in the solution. The mixture was heated while stirring for 2 hours. The mixture was allowed to cool at room temperature. Cu NPs precipitates were then subjected to centrifuge for 20 min at 1000 rpm. The supernatant solution was removed, and black-brownish particles were washed 3 times with ethanol and 5 times with distilled water. The nanoparticles were dried at room temperature for 24 h and 5 h in oven at 50 °C. The dried particles were stored for further analysis. Zeta size and zeta potential were analyzed (Nano-ZS; Malvern Instruments) for the copper oxide nanoparticle sample. FTIR analysis (Perkin Elmer System 2000 FTIR spectrometer (USA)) for the formation of copper oxide nanoparticles was also performed (Fig. 1).
Fig. 1.
FTIR analysis of copper nanoparticles (Cu NPs)
Plant growth room experiment
Growth room experiment was conducted at the Institute of Soil and Environmental sciences, University of Agriculture, Faisalabad, Pakistan. The region is classified as semi-arid climate and soil is characterized as sandy loam to clayey soil. The soil collected from the local farm of University of Agriculture, Faisalabad was passed through 8 mm sieve to remove plant based residues, and was air dried for three days. The basic properties of experimental soil are shown in (Table 1).
Table 1.
Physical and chemical properties of the experimental soil
| Soil property | Characteristic value |
|---|---|
| pH | 7.85 |
| EC (dS m−1) | 1.46 |
| Soil texture | Sandy clay loam |
| Cation exchange capacity (cmolc kg−1) | 13.9 |
| Organic matter (%) | 0.64 |
| Chromium | Not detected |
| Nitrogen (%) | 0.046 |
| Phosphorous (g kg−1) | 0.35 |
| Potassium (g kg−1) | 1.16 |
| Copper (mg kg−1) | 0.2 |
Surface sterilized onion (Alium cepa) seeds friendly donated by Department of Horticulture University of Agriculture Faisalabad were placed in plastic glasses (with 200 g soil) in triplicates. In soil chromium metal was applied at 10 mg kg−1. The doses of copper nanoparticles 10 and 15 mg kg−1, were also mixed in soil for each designated treatment. The temperature of the growth room was 25 ℃ during daytime and 18 ℃ by night. The experimental design was completely randomized. Two weeks after the sowing, one healthy seedling per plastic glass was maintained. The onion plants were harvested after 10 weeks and analyzed for various parameters.
The length of root, and leaves were measured by the help of scale. Number of leaves (LN) per plant were recorded. Length and width of onion leaf were recorded to find leaf area (LA) cm2. The fresh weight (g) of roots, bulb, and leaves, per plant was measured with digital balance scale and then oven dried for three days at 55 °C for the estimation of dry weight (g) of roots, bulb, and leaves. Leaf dry matter content, bulb dry matter content, and root dry matter content were observed. The dry biomass of onion plant part was divided with corresponding fresh biomass to get dry matter content of onion plant part (g). Chlorophyll SPAD value was also analyzed. Relative water content (RWC) of onion leaves was analyzed by the protocol given previously [32].
To determine electrolyte leakage (EL), the leaves were cut into identical discs, then leaves were placed in test tubes (10 mL) having distilled water and first electrical conductivity (EC1) was recorded. Tubes were then kept in a mechanical shaker for 2 h and electrical conductivity (EC2) was noted. Later on, the test tubes were autoclaved, and electrical conductivity (EC3) was measured [33].
Total sugar (quantitatively) was analyzed for each onion sample. The volume differences between standard sugar used for the standardization and for back titration was analyzed [34], and calculated as follows:
where, FS = volume of standard sugar solution required to reduce 10 mL mixed Fehling’s solution; SS = volume of standard sugar solution used in back titration of the sample; GS = gram sugar per mL working standard solution; W = weight of sample.
To estimate crude protein contents, a 2 g onion plant sample was taken in 250 ml flask, to which 15 ml 95%concentrated sulphuric acid was added and the sample was heated till sample solution turn colorless. Cooled (filtered) sample solution was taken in 100 ml volumetric flask and maintained with adding distilled water till the mark followed by distillation by (steamed) Markham distillation apparatus for 15–18 min. Afterwards, in a 100 ml conical flask provided with 2% boric acid with mixed indicator (1–2 drops) were placed under the condenser in such a way that condenser tip was covered with the liquid. Later on, a 5 ml colorless sample solution was pipetted in apparatus. The sample solution was washed down with distilled water along with adding 40% NaOH solution and 2–3 drops of phenolphthalein. Sample solution in the condenser was steamed and Boric acid plus indicator solution was added followed by changing in color. Hydrochloric acid was used to titrate solution (to get purple color-indicator of end point). The % nitrogen was calculated [35] using the formula:
Moisture contents in onion plant sample were found by drying (in oven at 105 °C) following method as given in protocol [36]. Moisture contents in onion plant were analyzed with 2 g onion plant sample, taken in a china dish (pre-weighed) followed by oven drying (105 ± 2 °C) till constant weight of dry plant sample was attained. The moisture in onion plant sample was found as:
Onion plant sample was homogenized in a 100 mL beaker with 50 mL HPO3.CH3COOH and then homogenized plant sample was placed in a 100 mL volumetric flask and diluted to volume with HPO3.CH3COOH solution. Blank test (7 mL HPO3.CH3COOH solution) into a 50 mL Erlenmeyer flask was measured and titrated with indophenol solution till rose pink color showed for 10 s. The volumes (mL) used was recorded and mean was calculated.
Titration of the sample: Aliquots of 5 mL of sample was pipette out containing Ca, 2 mg ascorbic acid into each of two 50 ml Erlenmeyer flasks. Enough HPO3.CH3COOH solution was added to make a total volume of 7 mL. Indophenol solution was titrated using a digital burette. The volume was recorded in mL. Calculation of mg ascorbic acid per mL indophenols solution (Factor) [35]:
Calculation of mg ascorbic acid per 100 g sample:
For determination of ash contents [35] around 5 g of dried and finely grounded onion plant sample was placed in a porcelain crucible and burnt at 55 ⁰C for 6 h. After wards, the ash was allowed to cool in desiccators and reweighed and analyzed according to following equation:
Cr analysis
The onion plant was washed, oven dried (65 °C) until constant weight followed by digestion with di-acid method [37]. A mixture of acids (HNO3 and HClO4) was added to grinded plant sample for over-night and digested at hot plate for half an hour till it turned colorless. Later on, filtered the digested solution and with the distilled water made the volume of each sample up to 25 mL. The digested samples were analyzed with the help of Atomic Absorption Spectrophotometer (AAS) for the determination of chromium (Cr) metal.
Remediation of Cr by onion plants
Bio Accumulation factor (BAF)
Bio accumulation factor (BAF) of onion plants was analyzed as [38]:
Bio Accumulation Coefficient (BAC)
Bio accumulation factor (BAC) was calculated as:
Translocation factor (TF)
The translocation factor of Cr metal by onion plants was calculated as:
Cr Health risk assessment parameters
Average Daily Intake (ADI)
The ADI index of Cr metal was calculated by using the following method:
where M is the concentration of Cr in plant (mg kg−1), I is the daily intake of onion, and W is the average body weight (BW). The average adult BW was considered 60 kg, while the average daily onion intake for adults was considered 0.345 kg−1 person−1.
Non cancer risk (NCR)
The NCR for Cr was due to consumption of contaminated onion, calculated as [38]:
Oral reference doses (RFD) for Cr, is 1.5 [39].
Cancer Risk (CR)
The cancer risk (CR) through Cr contamination in onion ingestion was calculated using following formula:
CSF here indicates cancer slope factor, and for chromium CSF is 0.5 [40].
Statistical analysis
The results of this experiment were analyzed with dose rate of copper nanoparticles and heavy metal chromium concentration levels and their interactions as the main factors, through analysis of variance (two-way ANOVA) to estimate the difference among the mean (replications = 3) values by comparing means of each treatment by LSD at 5% probability level using computer-based software (R studio). Normal distribution of data was performed for each parameters by Shapiro–Wilk test in R studio. Normality assumption was not violated. The Heat map was analyzed in R Studio, and for the construction of heat map plot Pheatmap package was used and the version of R studio was 4.0.1.
Results and discussion
Characterization
The data presented in (Table 2) showed zeta size and zeta potential values for copper nanoparticles.
Table 2.
Zeta size and potential of CuNPs
| Sample | Zeta size | Zeta potential |
|---|---|---|
| Cu Nps | 992 nm | -8.38 mV |
By zeta size analysis, the average particle size distribution of 998 nm has been observed for Cu NPs and -8.38 mV zeta potential for Cu NPs was found (Table 2). The FTIR analysis (Fig. 1) showed that band at 1272 cm−1 revealed formation of Cu–O-C linkage and at 624 cm−1 can be attributed to vibrations of Cu–O, leading towards the formation of CuO nanoparticles [41].
Plant growth analysis
Impact of copper nanoparticles on onion growth under chromium stress
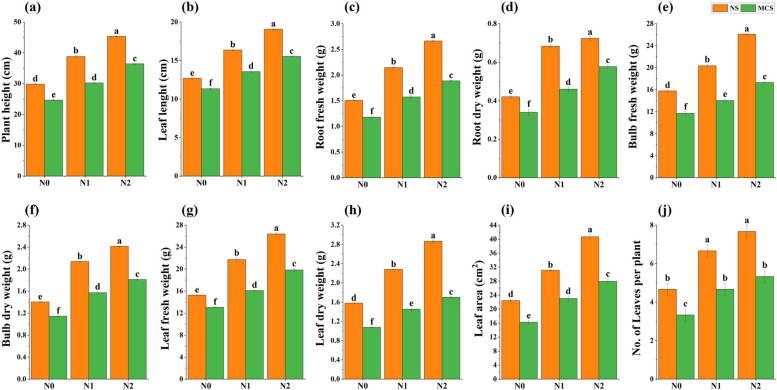
Onion growth parameters were significantly influenced by Cr toxicity (Fig. 2). Chromium concentration at 10 mg kg−1 negatively affected the onion plant growth attributes. However, the application of CuNPs at both concentrations significantly improved the plant growth under both normal soil (NS) and chromium metal contaminated soil (MCS), compared to the control treatment. The results of this study showed that addition of CuNPs at 15 mg kg−1 significantly improved the plant height (48%), leaf length (37%), fresh weight of root (61%), root dry weight (70%), fresh weight of bulb (52%), bulb dry weight (59%), fresh weight of leaves (52%) and dry weight of leaves (59%),leaf area (72%) and number of onion leaves per plant (60%) at 10 mg kg−1 Cr over treatment set as control (Cr 10 mg kg−1) as illustrated in Fig. 2 a, b, c, d, e, f, g, h, i, and j, respectively.
Fig. 2.
Impact of copper nanoparticles on onion (a) plant height, (b) leaf length, (c) root fresh weight, (d) root dry weight, (e) bulb fresh weight, (f) bulb dry weight, (g) leaf fresh weight, (h) leaf dry weight, (i) leaf area, (j) number of leaves per plant, under chromium stress. Treatments are presented as, N0 = without Cu NPs, N1 = 10 mg kg−1 Cu NPs, N2 = 15 mg kg−1 Cu NPs, with normal soil (NS) and with metal Cr (10 mg kg.−1) contaminated soil (MCS). Results are represented as means of 3 replications. Means sharing same letter donot vary significantly at p < 0.05
Impact of copper nanoparticles on chlorophyll pigments and physiology of onion plant under chromium stress
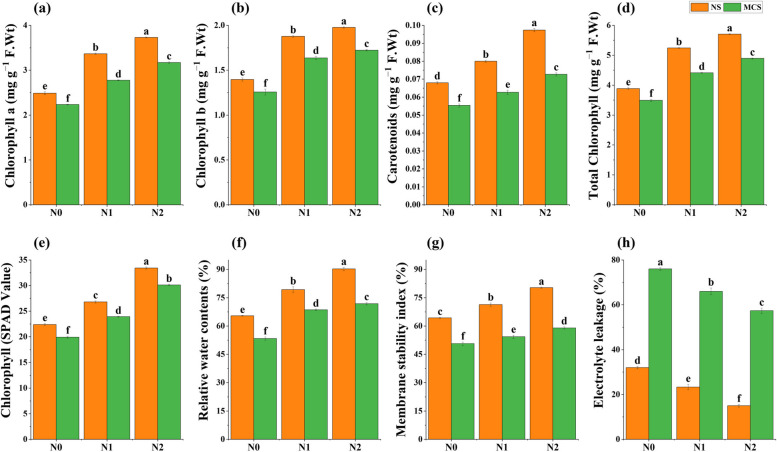
Chlorophyll pigments and physiological activities were remarkably declined due to Cr toxicity. Whereas, when Cr toxicity of 10 mg kg−1 was applied in soil CuNPs at 15 mg kg−1 significantly improved the plant Chl. a (42%), chl. b (36%), carotenoids (40%), total chlorophyll (40%), chlorophyll contents SPAD value (56%), relative water contents (35%), membrane stability index (16%), and reduced the electrolyte leakage by 0.99% as compared to control, demonstrated in Fig. 3 a, b, c, d, e, f, g, and h, respectively.
Fig. 3.
Impact of copper nanoparticles on onion (a) chlorophyll a, (b) chlorophyll b, (c) carotenoids, (d) total chlorophyll, (e) chlorophyll contents (SPAD value), (f) relative water contents, (g) membrane stability index, and (h) electrolyte leakage under chromium stress. Treatments are presented as, N0 = without Cu NPs, N1 = 10 mg kg−1 Cu NPs, N2 = 15 mg kg−1 Cu NPs, with normal soil (NS) and with metal Cr (10 mg kg−1) contaminated soil (MCS). Results are represented as means of 3 replications. Means sharing same letter donot vary significantly at p < 0.05
Impact of copper nanoparticles on proximate analysis of onion plant under chromium stress
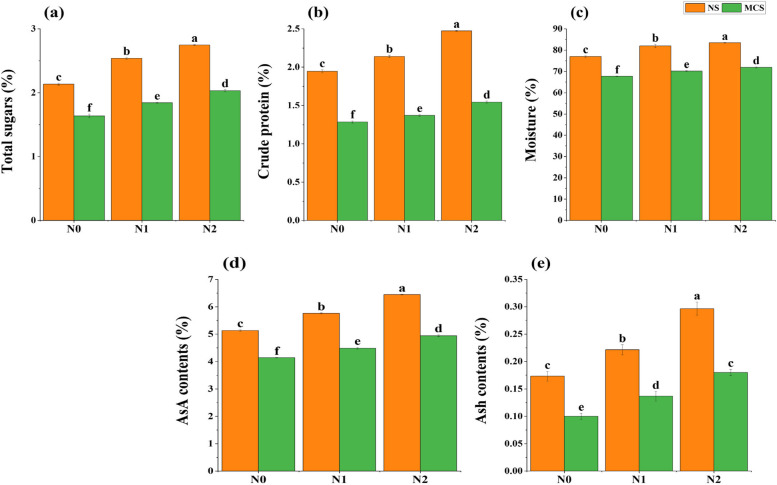
A maximum increase of 29% in total soluble sugars (Fig. 4a), 27% in crude protein (Fig. 4b), 7% in moisture (Fig. 4c), 26% in ascorbic acid (Fig. 4d) and 75% in ash contents (Fig. 4e) of onion plants was noticed with supplementation of CuNPs at 15 mg kg−1 as compared to control (normal soil). Conversely, under Cd stress it was observed that there was a prominent increase of 25% in total sugars, 21% in crude protein, 6% in moisture, 19% in ascorbic acid and 64% in ash contents in plants with supplementation of CuNPs at application rate of 15 mg kg−1 as compared to control (Cr metal contaminated soil).
Fig. 4.
Impact of copper nanoparticles on onion (a) total sugars, (b) crude protein, (c) moisture, (d) ascorbic acid and, (e) ash contents, under chromium stress. Treatments are presented as, N0 = without Cu NPs, N1 = 10 mg kg−1 Cu NPs, N2 = 15 mg kg−1 Cu NPs, with normal soil (NS) and with metal Cr (10 mg kg.−1) contaminated soil (MCS). Results are represented as means of 3 replications. Means sharing same letter donot vary significantly at p < 0.05
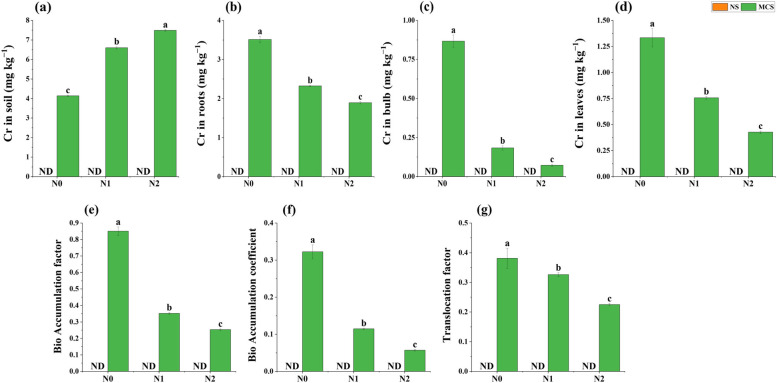
Impact of copper nanoparticles on uptake of chromium metal in onion plant under chromium stress
Data regarding Cr concentration in soil, root, bulb, and leaves was analyzed to evaluate the impact of Cu NPs in immobilization of Cr. In control treatment (no amendment), Cd concentration in soil was observed at 4.13 mg kg−1 while, in contrast to control, application of CuNPs at 10 mg kg-1, 15 mg kg−1 concentration declined the Cr concentration in soil by 60% and 81% , respectively (Fig. 5a). A maximum decline of 46% in Cr uptake was observed in roots of onion (Fig. 5b) was found when 15 Cu NPs were used at 15 mg kg−1 concentration. Likewise, a maximum reduction in Cr uptake by 92% in bulb (Fig. 5c) and 68% in leaves (Fig. 5d) of onion plants were observed compared to control under 10 mg kg−1 Cr toxicity with application of Cu NPs (15 mg kg−1).
Fig. 5.
Impact of copper nanoparticles on onion (a) Cr in soil, (b) Cr in root, (c) Cr in bulb, (d) Cr in leaves (e) Bio accumulation factor, (f) Bio accumulation coefficient, and (g) Translocation factor, under chromium stress. Treatments are presented as, N0 = without Cu NPs, N1 = 10 mg kg−1 Cu NPs, N2 = 15 mg kg−1 Cu NPs, with normal soil (NS) and with metal Cr (10 mg kg.−1) contaminated soil (MCS). Results are represented as means of 3 replications. Means sharing same letter donot vary significantly at p < 0.05
Impact of Cr phytoremediation in onion with use of Cu NPs
The data obtained from the Cr phytoremediation by analyzing parameters of bio accumulation factor (BAF), bio accumulation coefficient (BAC) for Cr and translocation factor (TF), is presented in Fig. 5 e, f, and g. A maximum value (0.85) for BAF, (0.53) for BAC, (0.63) for TF was indicated by data of plants kept as control (10 mg Kg−1). However, with the use of Cu NPs at 15 mg kg−1 concentration level, minimum value (0.25) for BAF, (0.07) for BAC, (0.26) for TF was shown in plants of onion grown in 10 mg kg−1 chromium contaminated soil.
Impact of Cr on health risk assessment parameters
The health risk assessment parameters of onion plants showed minimum values 0.0028 for ADI, 0.001911 for NCR, and 0.001433 for CR in plants treated with Cu NPs at 15 mg kg−1 concentration grown in soil spiked with 10 mg kg−1 chromium (Table 3).
Table 3.
Health risk assessment parameters in adults by consuming Onion plants from soil applied with copper nanoparticles and chromium metal contaminated soil
| Treatments | DIM in adults | NCR | CR | |
|---|---|---|---|---|
| Metal level | Nanoparticle | |||
| NS | N0 | ND | ND | ND |
| NS | N1 | ND | ND | ND |
| NS | N2 | ND | ND | ND |
| MCS | N0 | 0.0126 a | 0.008433 a | 0.006325 a |
| MCS | N1 | 0.0054 b | 0.003603 b | 0.002702 b |
| MCS | N2 | 0.0028 c | 0.001911 c | 0.001433 c |
Here, NS = Normal Soil, MCS = Cr metal (10 mg kg−1) contaminated Soil, N0 = without Cu NPs, N1 = 10 mg kg−1 Cu NPs, N2 = 15 mg kg−1 Cu NPs
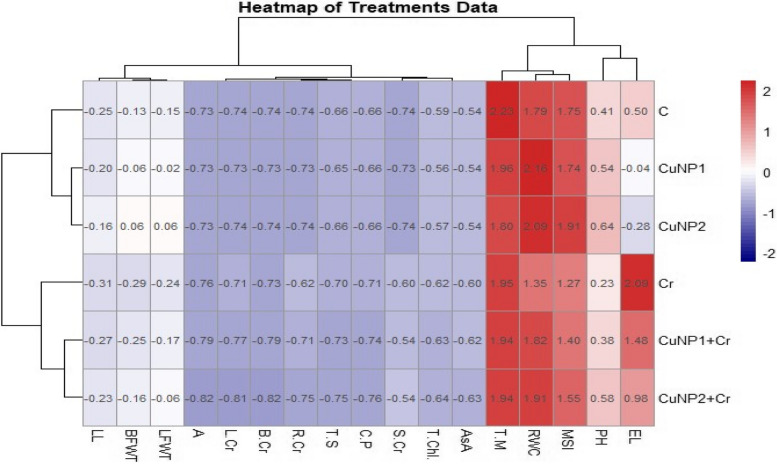
Heat map analysis for onion plants under application of copper nanoparticles and Cr toxicity
The heat map analysis of treatments and parameters has been displayed in (Fig. 6). The negative impact of Cr metal on onion plants can be prominently observed by strongly positive correlation between electrolyte leakage (EL) and Cr contaminated soil. Onion growth, yield and physiological parameters like PH (plant height), MSI (membrane stability index), RWC (relative water contents), and T.M (total moisture) are strongly correlated with each other under normal soils receiving Cu NPs. While AsA (ascorbic acid), T. Chl. (total chlorophyll), S.Cr (Cr in soil), C.P (Crude protein), T.S (total sugars), R.Cr (Cr in root), B.Cr (Cr in bulb), L.Cr (Cr in leaves), and A (ash contents) indicated weakly negative correlation among treatments. LFWT (leaf fresh weight), BFWT (bulb fresh weight), and LL (leaf length), indicated neutral impact of treatments under normal and metal contaminated soils.
Fig. 6.
Heat map analysis of onion plant growth, yield and physiological analysis as well as concentration of heavy metal Cr in soil and plants under application of Cu NPs at different concentrations in both normal and metal contaminated soils. Parameters studied were PH (plant height), electrolyte leakage (EL), MSI (membrane stability index), RWC (relative water contents), and T.M (total moisture), AsA (ascorbic acid), T. Chl. (total chlorophyll), S.Cr (Cr in soil), C.P (Crude protein), T.S (total sugars), R.Cr (Cr in root), B.Cr (Cr in bulb), L.Cr (Cr in leaves), A (ash contents), LFWT (leaf fresh weight), BFWT (bulb fresh weight), and LL (leaf length)
Discussion
Due to the burgeoning environmental concerns allied with heavy metal pollution in agriculture produce the use of nanoparticles for plants is gaining impetus as it promises to provide a sustainable approach to promote plant growth in wastewater driven agriculture while immobilizing heavy metals in soil for better plants growth and yield. In the present study the reduced plant growth in onion plant under Cr toxicity was found in plants without application of Cu NPs. This significant decrease in onion plants could be happened due to the limited or restricted availability of macro nutrients, micronutrients and increased uptake of Cr. Seed germination has been declined under metal stress in different plants as reported by Baruah et al. [42] due to Cr toxicity. Deficiency of macro and micronutrients and reduced growth of pea plants were observed under Cr toxicity in plants [43, 44]. Moreover, reduced uptake of essential macro and micronutrients (N, P, K, Mg and Fe) were widely reported by researchers [13]. Whereas, in both normal soil (NS) and metal contaminated soil (MCS) the results indicated increased growth of root, bulb and leaves of onion plants when supplemented with CuNPs at 15 mg kg−1 as compared to control (Cr metal contaminated soil) (Fig. 2). Metal based nanoparticles like Cu NPs are found to increase plant growth. Ji et al. [45] reported that increased growth of Medicago polymorpha L. showed increased biomass production when Cu NPs were used. Copper oxide nanoparticles are important sources of micro nutrients in plants thereby, acting as nanofertilizer [46]. Reddy and Roth, [47] showed that copper oxide nanoparticles are capable of removing metal from the water. Therefore, the immobilizing property of Cu NPs helped in reduced uptake of Cr metal in onion plants while providing essential nutrients for onion growth. Hence, Cu NPs have prominent contribution in growth of onion plants.
Declined Chlorophyll contents were observed in treatments getting only Cr contaminated water, causing reduced physiological activities and proximate contents as well. This could be because the Cr metal competes with Fe for binding sites in chlorophyll, disturbs the absorption of Fe. This would result in decreased accumulation of iron for the genesis of chlorophyll and heme synthesis [48]. Similarly, Cr stress also inhibit uptake of Kþ and Hþ in maize plants suggesting interference with transport activities of plasma membrane. Naseem et al. [49] also reported that heavy metals contaminated water led to decline tomato plants physiological activities and hence negatively impacting plant growth. The reduced chlorophyll contents [50] and relative water contents [51] have been observed in plants due to heavy metal contamination. The application of Cu NPs showed significant production of photosynthetic pigments and improved physiological analysis of onion plants under Cr toxicity. The improved photosynthetic pigments and photosynthetic activity was found by various researchers previously when Cu NPs were used [45, 52]. The provision of nutrients like Fe, Mg and Ni in onion plants under application of Cu NPs could regulate the N metabolism, production and activity of photosynthetic pigments [53]. This eventually provides the basis for improved physiological activities in onion plants.
The proximate analysis of onion plants showed reduction due to Cr phytotoxicity (Fig. 4). The reduced or imbalance nutrient provision occurred during Cr phytotoxicity resulted in degraded and limited production of biomolecules. Under heavy metals toxicity the reactive oxygen species are produced [54] due to which production of biomolecules like proteins, sugars would be disturbed. The disturbed or reduced production of biomolecules under Cr toxicity was found to be mitigated when Cu NPs were applied to plants. The results of proximate analysis showed improved production of biomolecules under Cu NPs application despite Cr toxicity. The large surface area, small size and presence of functional groups are the key traits of Cu NPs for the sorption of Cr metal [19, 55] in soil, thus ensuring proper and balanced provision of nutrients in onion plants [56].
The plants growing in MCS showed maximum concentration of Cr in soil samples followed by roots, leaves, and bulb of onion plants when no Cu NPs were applied. Chromium transport from the soil to aerial parts of plant was found highest as compared to plants receiving Cu NPs. The least uptake of Cr in root, leaves and bulb of onion was found when Cu NPs at 15 mg kg−1 concentration were used. The increased translocation of Cr could be associated with ionic imbalance [57] where instead of nutrients, Cr metal was taken up by the roots. The uptake of heavy metals may influence the cellular metabolism of aerial parts of plants causing impairments of nutrients and water related mechanisms [12, 58]. This could lead to deficiency or imbalanced nutrient supply to onion plants causes and hence stunted growth [59] with increased metal contents in plant tissues. Whereas application of Cu NPs at both concentrations showed reduced metal concentration in roots, leaves and bulb of onion plant. The nanomaterials are reported to have enough capability for removal of heavy metals due to small size and high surface area [60]. It is reported that various metal-based nanoparticles are recommended for the reusability of wastewater due to their potential to immobilize heavy metals on their surface [17]. When high concentration of heavy metals are present the number of available active sites on nanoparticles will start to decrease. Whereas the higher concentration of Cu NPs (15 mg kg−1) was found to be most effective as compared to other concentration (10 mg kg−1) in this study [61]. This could be explained by the fact that with increasing dose of Cu NPs, the remediation of heavy metals were found to be increased. The increased concentration of Cu NPs showed increased removal of Pb, Ni, and Cd when increased dose were used. This could be due to the increased availability of binding sites on the surface of nanoparticles [62]. Hence, improved remediation of Cr metal by application of Cu NPs (at 15 mg kg−1) was observed. Increased metal contents in edible parts of plants could lead to various health issues [63] as they can accumulate in human tissues [64], therefore, plants grown and irrigated with metal contaminated water should be assessed for the essential parameters like average daily intake of metals (ADI) [38] and cancer risk (CR) assessment [65]. The application of copper nanoparticles under Cr metal contamination showed that all values were less than 1 for analysis of health risk assessment [66]. Hence, under metal toxicity the use of copper nanoparticles makes consumption of onion plants safe without any concomitant health risks for humans.
Conclusions
The results of this study revealed that application of Cu NPs showed improved growth, physiological activities, proximate analysis and yield of onion. Whereas, Cr toxicity negatively impacted the growth, biochemical and physiological processes of onion plants. However, application of Cu NPs at 15 mg kg−1 concentration showed improved remediation of Cr by immobilizing Cr metal in soil while improving growth, yield and physiological processes in onion. The results indicated an increased plant height (48%), root dry weight (70%), fresh weight of bulb (52%), bulb dry weight (59%), and dry weight of leaves (59%), chlorophyll contents SPAD value (56%), relative water contents (35%), total sugars (25%), and crude protein (21%) at 10 mg kg−1 Cr toxicity when supplemented with 15 mg kg−1 of Cu NPs. Moreover, reduced Cr uptake (68%) in leaves and (92%) in bulb was found with application of 15 mg kg −1 of Cu NPs in onion plants under 10 mg kg−1 Cr toxicity. The health risk assessment parameters of onion plants showed safe values for ADI, NCR and CR in onion plants treated with Cu NPs at 15 mg kg−1 concentration grown under chromium contaminated soil. It is concluded from the results that Cu NPs at 15 mg kg−1 concentration can improve growth and yield of plants in control as well as metal contaminated soil. However, the use of Cu NPs at other concentration levels needs to be determined for efficient remediation of metals and other pollutants in soil for improved soil quality, and growth of plants. With the addition of UV–visible spectrum, SEM–EDS images and, XRD analysis more information could be gain in surface characterization of Cu NPs. Molecular level studies explaining the mechanism of Cu NPs in plants for improved growth and reduced metal uptake under metal toxicity should also be studied. Plant roots and shoot samples for cell morphology, stress-induced ROS levels and antioxidant enzyme activity and expression of genes associated with it, under stress and after application of nanoparticles should be assessed.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project number (RSP2024R194), King Saud University, Riyadh, Saudi Arabia. The authors would also like to thank Soil and Environmental Microbiology Lab, Institute of Soil and Environmental Sciences, University of Agriculture, Faisalabad, Pakistan for support in completion of this projection.
Authors’ contributions
Conceptualization, Z.N., M.N., A.M.., and M.A.; methodology, A.M., Z.N., S.N. and M.H.S.; software, Z.N. and M.H.S.; formal analysis, Z.N., S.A., A.M., M.A., S.N. and M.N.; investigation, M.N., A.M. and Z.N.; data curation, A.M., Z.N.; writing—original draft preparation, Z.N. and M.N.; writing—review and editing, A.M., S.A, M.H.S., S.N. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received an external funding for this study under the Researchers Supporting Project number (RSP2024R194), King Saud University, Riyadh, Saudi Arabia.
Availability of data and materials
The data can be made available on a reasonable request from corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Muhammad Naveed, Email: muhammad.naveed@uaf.edu.pk.
Adnan Mustafa, Email: adnanmustafa780@gmail.com.
References
- 1.Faryal R, Tahir F, Hameed A. Effect of wastewater irrigation on soil along with its micro and macro flora. Pak J Bot. 2007;39:193–204. [Google Scholar]
- 2.Ensink JH, Mahmood T, Van der Hoek W, RaschidSally L, Amerasinghe FP. A nationwide assessment of wastewater use in Pakistan: An obscure activity or a vitally important one? Water Policy. 2004;6:197–206. 10.2166/wp.2004.0013 [DOI] [Google Scholar]
- 3.Rafiq A, Bhatti IA, Tahir AA, Ashraf M, Bhatti HN, Zia M. Solar photocatalytic treatment of textile effluent for its potential reuse in irrigation. Pak J Agri Sci. 2019;56(4):993–1001. [Google Scholar]
- 4.López-Bucio JS, Ravelo-Ortega G, López-Bucio J. Chromium in plant growth and development: Toxicity, tolerance and hormesis. Environ Pollut. 2022;312:120084. 10.1016/j.envpol.2022.120084 [DOI] [PubMed] [Google Scholar]
- 5.Xu S, Yu C, Wang Q, Liao J, Liu C, Huang L, Liu Q, Wen Z, Feng Y. Chromium Contamination and Health Risk Assessment of Soil and Agricultural Products in a Rural Area in Southern China. Toxics. 2023;11:27. 10.3390/toxics11010027. 10.3390/toxics11010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Liu J, McGrouther K, Huang H, Lu K, Guo X, He L, Lin X, Che L, Ye Z, Wang H. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ Sci Pollut Res Int. 2016;23:974–84. 10.1007/s11356-015-4233-0 [DOI] [PubMed] [Google Scholar]
- 7.Naseem Z, Naveed M, Asghar HN, Hameed M. Metal Resistant Enterobacter cloacae ZA14 Enhanced Seedling Vigor and Metal Tolerance through Improved Growth, Physiology and Antioxidants in Tomato (Solanum lycopersicum) Irrigated with Textile Effluents. Sustainability. 2022;2022(14):13619. 10.3390/su142013619. 10.3390/su142013619 [DOI] [Google Scholar]
- 8.Naveed M, Mustafa A, Majeed S, Naseem Z, Saeed Q, Khan A, Nawaz A, Baig KS, Jen-Tsung C. Enhancing Cadmium Tolerance and Pea Plant Health through Enterobacter sp. MN17 Inoculation Together with Biochar and Gravel Sand. Plants. 2020;9:530. 10.3390/plants9040530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang B, Gong Y, Gao J, Sun T, Liu Y, Oturan N, Oturan MA. The reduction of Cr(VI) to Cr(III) mediated by environmentally relevant carboxylic acids: state-of-the-art and perspectives. J Hazard Mater. 2019;365:205–26. 10.1016/j.jhazmat.2018.10.070 [DOI] [PubMed] [Google Scholar]
- 10.Kumar M, Kushwaha A, Goswami L, Singh AK, Sikandar M. A review on advances and mechanism for the phycoremediation of cadmium contaminated wastewater. Clean Eng Technol. 2021;5:100288. 10.1016/j.clet.2021.100288 [DOI] [Google Scholar]
- 11.Famielec S, Wieczorek-Ciurowa K. Waste from leather industry. Threats to the environment. Czasopismo Techniczne Chemia. 2011;108:43–8. [Google Scholar]
- 12.Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S. Chromium toxicity in plants. Environ Int. 2005;31:739e753. 10.1016/j.envint.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 13.Nazir A, Wahid A, Nawaz S, Gulshan AB, Leghari SK, Hussain F, Nijabat A, Khan MA, Awan AN, Shafqat U, Naseem Z. Vicissitudes in Morphological and Photosynthetic Attributes in Maize (Zea mays) plant by elevating the Cobalt Concentration in soil. GU J Phytosciences. 2022;2(1):42–7. [Google Scholar]
- 14.Karthik C, Oves M, Thangabalu R, Sharma R, Santhosh SB, Arulselvi PI. Cellulosimicrobium funkei-like enhances the growth of Phaseolus vulgaris by modulating oxidative damage under Chromium (VI) toxicity. J Adv Res. 2016;7:839–50. 10.1016/j.jare.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alves R, Rossatto DR, Silva J, Checchio MV, Oliveira K, Oliveira F, Queiroz S, Cruz M, Gratão PL. Seed priming with ascorbic acid enhances salt tolerance in micro-tom tomato plants by modifying the antioxidant defense system components. Biocatal Agric Biotechnol. 2021;31:101927.10.1016/j.bcab.2021.101927 10.1016/j.bcab.2021.101927 [DOI] [Google Scholar]
- 16.Utsumi Y, Utsumi C, Tanaka M, Ha HV, Takahashi S, Matsui A, Matsunaga TM, Matsunaga S, Kanno Y, Seo M, Okamoto Y, Moriya E, Seki M. Acetic Acid Treatment Enhances Drought Avoidance in Cassava (Manihot esculenta Crantz). Front Plant Sci. 2019;10:521. 10.3389/fpls.2019.00521. 10.3389/fpls.2019.00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun L, Xin X, An X, Qian X. Nano-Cu2O-loaded paper: Green preparation and high visible light photocatalytic performance for formaldehyde removal. Paper Biomater. 2019;4:117. 10.26599/PBM.2019.9260017 [DOI] [Google Scholar]
- 18.Hassan KH, Mahdi ER. Preparation and characterization of copper oxide nanoparticles used to remove nickel ions from aqueous solution. Diyala J Pure Sci. 2017;13:217–34. 10.24237/djps.1302.265B [DOI] [Google Scholar]
- 19.Jain M, Yadav M, Chaudhry S. Copper oxide nanoparticles for the removal of divalent nickel ions from aqueous solution. Toxin Reviews. 2020. 10.1080/15569543.2020.1799407. 10.1080/15569543.2020.1799407 [DOI] [Google Scholar]
- 20.Xiong T, Dumat C, Dappe V. Copper oxide nanoparticle foliar uptake, phytotoxicity, and consequences for sustainable urban agriculture. Environ Sci Tech. 2017;51(9):5242–51. 10.1021/acs.est.6b05546 [DOI] [PubMed] [Google Scholar]
- 21.Shende S, Rathod D, Gade A, Rai M. Biogenic copper nanoparticles promote the growth of pigeon pea (Cajanus cajan L.). IET Nanobiotech. 2017;11(7):773–81. 10.1049/iet-nbt.2016.0179 [DOI] [Google Scholar]
- 22.Dhoke SK, Mahajan P, Kamble R, Khanna A. Effect of nanoparticles suspension on the growth of mung (Vigna radiata) seedlings by foliar spray method. Nanotech Devel. 2013;3(1):e1. 10.4081/nd.2013.e1 [DOI] [Google Scholar]
- 23.Rajak J, Bawaskar M, Rathod D. Interaction of copper nanoparticles and an endophytic growth promoter Piriformospora indica with Cajanus cajan. J Sci Food Agri. 2017;97(13):4562–70. 10.1002/jsfa.8324 [DOI] [PubMed] [Google Scholar]
- 24.Ochoa L, Medina-Velo IA, Barrios AC. Modulation of CuO nanoparticles toxicity to green pea (Pisum sativum Fabaceae) by the phytohormone indole-3-acetic acid. Sci Tot Environ. 2017;598:513–24. 10.1016/j.scitotenv.2017.04.063 [DOI] [PubMed] [Google Scholar]
- 25.Shaw AK, Ghosh S, Kalaji HM. Nano-CuO stress induced modulation of antioxidative defense and photosynthetic performance of Syrian barley (Hordeum vulgare L.). Environ Experi Bot. 2014;102:37–47. 10.1016/j.envexpbot.2014.02.016 [DOI] [Google Scholar]
- 26.Adamicki F, Kepka AK. Storage of onions in controlled atmospheres. Acta Horti Culturae. 1974;38:53–74. 10.17660/ActaHortic.1974.38.5. 10.17660/ActaHortic.1974.38.5 [DOI] [Google Scholar]
- 27.Sravani V, Saravaiya SN, Patel BN, Chhatrola HN, Himani B, Vashi JM. Response of plant bioregulators on growth parameters and plant growth analysis of onion (Allium cepa L.). Int J Chem Studies. 2020;8(3):1312–6. 10.22271/chemi.2020.v8.i3r.9380 [DOI] [Google Scholar]
- 28.Haryanta D, Thohiron M, Gunawan B. Study of onion growth (Allium ascalonicum L.) using sediment soil media and urban waste compos. IOP Conf. Series: Earth and Environmental Science 230 (2019) 012086. 10.1088/1755 –1315/230/1/012086
- 29.FAO. The State of Food and Agriculture. Retrieved in 2012 from http://www.fao.org/publications/sofa/2012/en/. 2012.
- 30.Ghani MA, Mushtaq A, Ziaf K, Ali B, Jahangir MM, Khan RW, Khan I, Azam M, Noor A. Exogenously applied GA3 promotes plant growth in onion by reducing oxidative stress under saline conditions. J Agri Sci (Tarim Bilimleri Dergisi). 2021;27(2):122–8. [Google Scholar]
- 31.Ahamed M, Alhadlaq HA, Khan MAM, Karuppiah P, Al-Dhabi NA. Synthesis, Characterization, and Antimicrobial Activity of Copper Oxide Nanoparticles. J. Nanomaterials. 2014; Article ID 637858:4 pages 10.1155/2014/637858.
- 32.Weatherly PE. Studies in water relations in cotton plants. The field measurement of water deficit in leaves. New Phytol. 1950;49:81–7. 10.1111/j.1469-8137.1950.tb05146.x [DOI] [Google Scholar]
- 33.Yang G, Rhodes D, Joly RJ. Effect of high temperature on membrane stability and chlorophyll fluorescence in glycinebetaine-containing maize lines. Aust J Plant Physiol. 1996;23:431–43. [Google Scholar]
- 34.Greenfield H, Southgate DAT. Food composition data: production, management and use. UK: Elsevier Applied Science; 1992. [Google Scholar]
- 35.AOAC, Official Methods of Analysis of Association of Official Analytical Chemists, 15th ed., Arlington Va, USA: AOAC. 1990:pp.1–50.
- 36.AACC, Approved methods of the American Association of Cereal Chemists, vol. 1 (Method No. 30–25, 44 –15A), USA: American Association of Cereal Chemists, 2000.
- 37.Wolf B. The comprehensive system of leave analysis and its use for diagnosing crop nutrients status. Comm Soil Sci Plant Anal. 1982;13:1035–59. 10.1080/00103628209367332 [DOI] [Google Scholar]
- 38.Jan FA, Ishaq M, Khan S, Ihsanullah I, Ahmad I, Shakirullah M. A comparative study of human health risks via consumption of food crops grown on wastewater irrigated soil (Peshawar) and relatively clean water irrigated soil (lower Dir). J Hazard Mater. 2010;179:612–21. 10.1016/j.jhazmat.2010.03.047 [DOI] [PubMed] [Google Scholar]
- 39.Yahaya TO, Oladele EO, Fatodu IA, Abdulazeez A, Yeldu YI. The concentration and health risk assessment of heavy metals and microorganisms in the groundwater of Lagos, Southwest Nigeria. J Adv Environ Health Res. 2020;8:234–42. [Google Scholar]
- 40.USDOE. U.S. Department of Energy’s Oak Ridge Operations Office (ORO); 2011. The Risk Assessment Information System (RAI).
- 41.Liu J, Huang X, Li Y, Sulieman K, He X, Sun F. Hierarchical nanostructures of cupric oxide on a copper substrate: controllable morphology and wettability. J Mater Chem. 2006;16(45):4427–34. 10.1039/b611691d [DOI] [Google Scholar]
- 42.Baruah N, Mondal SC, Farooq M, Gogoi N. Influence of Heavy Metals on Seed Germination and Seedling Growth of Wheat, Pea, and Tomato. Water Air Soil Pollut. 2019;230:273. 10.1007/s11270-019-4329-0 [DOI] [Google Scholar]
- 43.Islam F, Yasmeen T, Arif MS, Riaz M, Shahzad SM, Imran Q, Ali I. Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and salicylic acid (SA) in attenuation of chromium stress in maize plants. Plant Physiol Biochem. 2016;108:456e467. 10.1016/j.plaphy.2016.08.014 [DOI] [PubMed] [Google Scholar]
- 44.Naveed M, Fatima M, Naseem Z, Ahmad Z, Gaafar AZ, Shabbir M, Farooq QUA, Hodhod MS, Khan MI, Shahid D, Mustafa A. Improving the Growth of Pea Plant by Biochar-Polyacrylamide Association to Cope with Heavy Metal Stress under Sewage Water Application in a Greenhouse. Front. Environ. Sci. 2024;12–2024. 10.3389/fenvs.2024.1380867.
- 45.Ji H, Guo Z, Wang G, Wang X, Liu H. Effect of ZnO and CuO nanoparticles on the growth, nutrient absorption, and potential health risk of the seasonal vegetable Medicago polymorpha L. PeerJ;2022. 10.7717/peerj.14038. [DOI] [PMC free article] [PubMed]
- 46.Singh SB. Green and sustainable copper-based nanomaterials – an environmental perspective. In Green and Sustainable Advanced Materials, John Wiley & Sons, Ltd: 2018; pp159–175.
- 47.Reddy KJ, Roth TR. Arsenic removal from natural groundwater using cupric oxide. Ground Water. 2013;51(1):83–91. 10.1111/j.1745-6584.2012.00926.x [DOI] [PubMed] [Google Scholar]
- 48.Gopal R, Rizvi AH, Nautiyal N. Chromium alters iron nutrition and water relations of spinach. J Plant Nutr. 2009;32:1551e1559. 10.1080/01904160903094313 [DOI] [Google Scholar]
- 49.Naseem Z, Naveed M, Imran M, Saqlain M, Asif M, Bashir M, Alamri S, Siddiqui MH, Brtnicky M, Mustafa A. Elucidating the Potential of Dye-Degrading Enterobacter cloacae ZA14 for Cultivation of Solanum lycopersicum Plants with Textile Effluents. Water. 2023;15:3163. 10.3390/w15173163
- 50.Tripathi P, Singh PC, Mishra A, Srivastava S, Chauhan R, Awasthi S, Mishra S, Vedi SW, Tripathi P, Kalra A. Arsenic tolerant Trichoderma sp. reduces arsenic induced stress in chickpea (Cicer arietinum). Environ Pollut. 2017;223:137–45. 10.1016/j.envpol.2016.12.073 [DOI] [PubMed] [Google Scholar]
- 51.Bhuiyan MR, Rahman MM, Shaid A, Bashar MM, Khan MA. Scope of reusing and recycling the textile wastewater after treatment with gamma radiation. J Clean Prod. 2016;112:3063–71. 10.1016/j.jclepro.2015.10.029 [DOI] [Google Scholar]
- 52.Dimkpa CO, White JC, Elmer WH, Gardea-Torresdey JL. Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J Agri Food Chem. 2017;65:8552–9. 10.1021/acs.jafc.7b02961. 10.1021/acs.jafc.7b02961 [DOI] [PubMed] [Google Scholar]
- 53.Rui MM, Ma CX, Hao Y, Guo J, Rui YK, Tang XL, Zhao Q, Fan X, Zhang ZT, Hou TQ, Zhu SY. Iron oxide nanoparticles as a potential iron fertilizer for peanut (Arachis hypogaea). Front Plant Sci. 2016;7:815. 10.3389/fpls.2016.00815. 10.3389/fpls.2016.00815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabir M, Naseem Z, Ahmad W, Usman M, Nadeem F, Ahmad HR. Alleviation of adverse effects of nickel on growth and concentration of copper and manganese in wheat through foliar application of ascorbic acid. Int J Phytoremediation. 2022;24:695–703. 10.1080/15226514.2021.1962801 [DOI] [PubMed] [Google Scholar]
- 55.Hassan KH, Jarullah AA, Saadi SK. Synthesis of copper oxide nanoparticle as an adsorbent for removal of Cd(II) and Ni(II) ions from binary system. Int J Appl Env Sci. 2017;12:1841–51. [Google Scholar]
- 56.Liu LL, Ji HT, An JP, Shi KJ, Ma JF, Liu B, Tang L, Cao WX, Zhu Y. Response of biomass accumulation in wheat to low-temperature stress at jointing and booting stages. Environ Experim Bot. 2019;157:46–57. 10.1016/j.envexpbot.2018.09.026. 10.1016/j.envexpbot.2018.09.026 [DOI] [Google Scholar]
- 57.Singh R, Rathore D. Effects of Fertilization with Textile Effluent on Germination, Growth and Metabolites of Chilli (Capsicum annum L.) Cultivars. Environ Process. 2021;8:1249–66. 10.1007/s40710-021-00531-1 [DOI] [Google Scholar]
- 58.Chatterjee J, Kumar P, Sharma PN, Tewari RK. Chromium toxicity induces oxidative stress in turnip. Indian J Plant Physiol. 2015;20:220e226. 10.1007/s40502-015-0163-6 [DOI] [Google Scholar]
- 59.Gill RA, Zang L, Ali B, Farooq MA, Cui P, Yang S, Zhou W. Chromium induced physio-chemical and ultrastructural changes in four cultivars of Brassica napus L. Chemosphere. 2015;120:154e164. 10.1016/j.chemosphere.2014.06.029 [DOI] [PubMed] [Google Scholar]
- 60.Mahmoud AED, Stolle A, Stelter M. Sustainable synthesis of high-surface-area graphite oxide via dry ball milling. ACS Sustain Chem Eng. 2018;6:6358–69. 10.1021/acssuschemeng.8b00147 [DOI] [Google Scholar]
- 61.Mahmoud AED. Graphene-based nanomaterials for the removal of organic pollutants: Insights into linear versus nonlinear mathematical models. J Environ Manage. 2020;270:110911. 10.1016/j.jenvman.2020.110911 [DOI] [PubMed] [Google Scholar]
- 62.Mahmoud AED, Al-Qahtani KM, Alfaij SO, Al-Qahtani SF, Alsamhan FA. Green copper oxide nanoparticles for lead, nickel, and cadmium removal from contaminated water. Sci Rep. 2021;11:12547. 10.1038/s41598-021-91093-7. 10.1038/s41598-021-91093-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zulfiqar U, Jiang W, Xiukang W, Hussain S, Ahmad M, Maqsood MF, Ali N, Ishfaq M, Kaleem M, Haider FU. Cadmium Phytotoxicity, Tolerance, and Advanced Remediation Approaches in Agricultural Soils. A Comprehensive Review Front Plant Sci. 2022;13:773815. 10.3389/fpls.2022.773815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Haq QMR. Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidant. Int J Mol Sci. 2015;16:29592–630. 10.3390/ijms161226183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hannah AA, Longinus NK, Olatunde AM, Alex AA, Omosileola JA. Potential Human Health Risk Assessment of Heavy Metals Intake via Consumption of some Leafy Vegetables obtained from Four Market in Lagos Metropolis. Nigeria Appl Sci Environ Manage. 2016;20(3):530–9. [Google Scholar]
- 66.Naveed M, Tanvir B, Xiukang W, Brtnicky M, Ditta A, Kucerik J, Subhani Z, Nazir MZ, Radziemska M, Saeed Q, Mustafa A. Co-composted Biochar Enhances Growth, Physiological, and Phytostabilization Efficiency of Brassica napus and Reduces Associated Health Risks Under Chromium Stress. Front Plant Sci. 2021;12:775785. 10.3389/fpls.2021.775785 10.3389/fpls.2021.775785 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data can be made available on a reasonable request from corresponding author.