Abstract
Background
Physical activity is associated with improved brain health and cognition in humans. However, the validity, range, and quality of evidence for the beneficial outcomes linked to exercise in experimental models of vascular dementia (VaD) have not been evaluated. We performed a systematic review and meta-analysis of studies that assessed the effect of exercise intervention on models of VaD to provide an unbiased and comprehensive determination of the cognitive function and brain morphology benefits of exercise.
Summary
A systematic search in three databases as well as study design characteristics and experimental data extraction were completed in December 2021. We investigated the effects of exercise on cognitive function and brain-morphology outcomes in VaD models. Twenty-five studies were included for systematic review, while 21 studies were included in the meta-analysis. These studies included seven models of VaD in rats (60%, 15 studies), mice (36%, 9 studies), and pigs (4%, 1 study). None of the included studies used aged animals, and the majority of studies (80%) used only male animals.
Key Message: Exercise improves cognition but increased neuro-inflammation in VaD models.
Exercise improved cognitive function as well as some markers of brain morphology in models of VaD. However, exercise increased anxiety and neuro-inflammatory signals in VaD models. Further, we observed increased reporting anomalies such as a lack of blinding to group treatment or data analysis and randomization of animals to groups. Our report could help in the appropriate design of experimental studies seeking to investigate the effects of exercise as a non-pharmacological intervention on VaD models with a high translational impact.
Keywords: Physical activity, vascular dementia, systematic review, meta-analysis, preclinical, cognitive function
Introduction
Vascular dementia (VaD) is the second most diagnosed form of dementia after Alzheimer’s disease and currently lacks effective treatment.1–3 VaD describes a spectrum of memory and cognitive impairments associated with cerebrovascular and cardiovascular diseases.4–6 The debilitating inability of people with VaD to undertake normal daily tasks impacts millions of unpaid caregivers, most of whom are family members and friends. 7 Also, the global economic cost of dementia was US$ 818 billion in 2015 and is estimated to double by 2030. 8 Although intense effort has been directed towards the development of pharmaceutical therapy to improve memory function prior to and following the onset of VaD,2, 3 emerging evidence indicates that non-pharmaceutical lifestyle interventions such as physical activity better preserve brain health and cognition as well as slow the onset of dementia even when initiated after mid-life.9–19
Hence, physical activity has the potential to become a desired therapy as it can be self-administered and is not associated with the side effects that pharmacotherapies may present. Physical activity is strongly associated with a decreased risk of cardiovascular diseases such as hypertension, dyslipidemia, stroke, and myocardial infarction, which are common comorbidities in VaD patients.20–23 These vascular risk factors and physical inactivity have been linked directly to poor brain health and cognitive decline in humans.20–23 Importantly, accumulating body of work shows that exercise, whether forced or voluntary, improves memory and cognitive performances in preclinical models.24–27 Nonetheless, few studies have reported the modest clinical benefit of exercise in older people with unknown or mild cognitive impairments.28–32 While the exercise interventions in those studies have been characterized as suboptimal, other studies have shown positive outcomes of physical activities in older patients with mild cognitive impairments33–35 and dementia. 36 Refining the effect of exercise on brain outcome measures will advance knowledge in this realm by providing consensus findings on the mechanism(s) of a non-pharmacological approach to improving VaD. Preclinical models of VaD are vital tools to investigate the potential of exercise intervention and may provide relevant answers to translational questions.
In addition, it is now known that exercise attenuates oxidative stress, upregulates brain expression, and increases serum circulation of molecules such as brain-derived neurotrophic factor (BDNF) and secreting insulin-like growth factor 1 (IGF1), which are known to promote neurogenesis and synaptic plasticity in the cortex and hippocampus.24–27 However, despite growing clinical research interests in exercise as it relates to improved cognitive function, little is known about the relationship between exercise and preclinical models of VaD. A major pathway to attaining understanding in this area is to review the reported outcomes linked to exercise in experimental models of VaD while considering the validity and quality of the evidence. Here we provide an impartial and comprehensive determination of the cognitive and cerebrovascular benefits of exercise in experimental models of VaD. Our objectives were to conduct a systematic review and meta-analysis of identified reports of physical activity in animal models of VaD to investigate the effects of physical activity on cognitive and histological outcome measures.
Methods
Search Strategy
This review and meta-analysis followed the preferred reporting items for systematic review and meta-analysis (PRISMA) guidelines and was accepted for registration on the International Prospective Register of Systematic Reviews (PROSPERO, CRD42020212001). 37 A literature search was conducted on PubMed, EMBASE, and EBSCO host databases from 1980 to 2020 to screen and identify eligible studies reported in English (by BIJ and CWH). The search strategy was built in PubMed and adapted to the other databases (Supplementary Material 1). The full text of eligible studies was uploaded and processed for risk of bias assessment and data extraction using the systematic review software Covidence (https://app.covidence.org/reviews/117430; extraction 1.0).
Search Term
We conducted an electronic literature search on PubMed, EMBASE, and EBSCO from 1980 to 2020 for:
[(“physical activity” OR “voluntary physical activity” OR “voluntary physical exercise” OR “voluntary exercise” OR “exercise” OR “fitness” OR “environmental enrichment” OR “locomotion” OR “ambulation” OR “walk” OR “run” OR “treadmill” OR “wheel” OR “swim” OR “physical therapy” OR “physical exercise” OR “physical conditioning” OR “physical activity” OR “physical exertion” OR “forced exercise”)]
AND
[(“vascular dementia” OR “VaD” OR “vascular cognitive impairment” OR “VCI” OR “Vascular contributions to vascular dementia” OR “VCID” OR “chronic cerebral hypoperfusion” OR “cch” OR “cerebral hypoperfusion” OR “severe hypoperfusion” OR “bilateral common carotid artery stenosis” OR “bilateral carotid artery stenosis” OR “BCAS” OR “white matter lesion” OR “white matter injury” OR “two vessel stenosis” OR “two vessel occlusion” OR “cerebral small vessel disease” OR “multi-infarct dementia” OR “strategic infarct” OR “cerebral amyloid angiopathy” OR “cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy” OR “cadasil” OR “asymmetric CCA” OR “Asymmetric carotid artery stenosis” OR “ACAS” OR “gradual carotid artery stenosis” OR “GCAS” OR “atherosclerosis” OR “bilateral carotid artery stiffness” OR “unilateral-carotid artery occlusion” OR “unilateral carotid artery stenosis” OR “carotid artery calcification” OR “internal artery calcification” OR “bilateral carotid artery calcification” OR “cerebrovascular” OR ((“cerebral” OR “cerebellar” OR “brain” OR “vertebrobasilar” OR “chronic hypertension” OR “renovascular occlusion” OR “diabetes melitus” OR “hyperhomocysteinemia”)]
AND [(“dementia” OR “cognition” OR “cognitive function” OR “mild cognitive impairment” OR “cognitive impairment” OR “memory” OR “executive function”)]
AND
[(“animal” OR “mice” OR “mouse” OR “rat” OR “gerbil” OR “rodent” OR “murine” OR “feline” OR “cat” OR “canine” OR “dog” OR “porcine” OR “pig” OR “ferret” OR “rabbit” OR “monkeys” OR “non-human primates”)].
Selection Criteria
To be eligible for inclusion, studies must include (1) a model of vascular dementia (as described in search term 2 of Supplementary Material), (2) exercise intervention, (3) an appropriate control paradigm, and (4) specified outcome(s). Examined outcomes are cognitive, motor, and social function assessments; neuroinflammatory changes; cerebral blood flow changes; myogenic tone; blood–brain barrier changes; or alterations in dendritic branches, dendritic spines, or synaptic pruning. Studies with VaD modeled in neonates or new-born animals were not included. Housing conditions that encourage or model voluntary physical activity or forced physical activity by use of treadmills prior to, during, or following VaD, including continuous, intermittent, or no restriction, were applied for study inclusion. Physical activity was characterized as either voluntary or forced. Voluntary physical activity was defined as any exercise treatment that encourages the animals to engage in physical activity without stimulus prompts (such as unrestricted access to exercise apparatus), while forced physical activity was defined as any exercise paradigm that depends on the administration of stimulus prompts (such as an electric shock or a puff of air) to the animals to achieve exercise performance. Only studies written in English or translated into English were eligible for consideration.
Two authors (IJB and WHC) independently screened titles and abstracts for potential eligibility. The full-text review was completed independently by these two authors to determine if the studies were eligible for inclusion. A third author (SB) adjudicated any discrepancies.
Data Extraction
A standardized data extraction format was developed using Covidence and agreed upon by all authors. Predefined data were independently extracted by four authors (WHC, SB, RJS, or VSG). Discrepancies were resolved by a third reviewer (IJB). Authors of eligible studies requiring supplemental or missing data were contacted by e-mail. Where numerical data were unavailable after e-mailing authors, measured values were obtained from graphs using Web Plot Digitizer (https://automeris.io/WebPlotDigitizer/). The number of experimental groups, study duration, longitudinal timepoints, sample size, sex, species, strain, average age, weight, comorbidities, distribution of groups, model of vascular dementia, and duration of vascular dementia from induction were extracted from all studies. Also extracted from all included studies were physical activity interventions (i.e., time of day for exercise, type of exercise, duration of exercise per timepoint, total duration and/or distance performed over the study period, duration of exercise) administered prior to and following vascular dementia induction to the point of study termination, individual outcome measures between animals treated to physical activity and control group animals that were not administered physical activity treatment, and type of functional assessment.
Data Analysis
A random-effect meta-analysis was conducted with inverse variance for continuous variables using Review Manager (RevMan, Version 5.4, The Cochrane Collaboration, 2020). Overall, heterogeneity (I2), z-values, and p-values were computed for each comparison. At least two studies for cognitive function or morphological outcomes were deemed sufficient to run the meta-analysis. When appropriate, meta-analyses were subgrouped by measurement type. For morphological outcomes, analytical methods (i.e., ELISA, qPCR, western blot) were pooled. For all analyses, forest plots were produced to visually assess the pooled outcomes. An effect size for each study was calculated using Hedge’s g with a 95% confidence interval as well as Hedges and Olk’s standard error based on a standardized mean comparison between treated and control animals. Subgroup analysis by exercise type (forced, involuntary, and voluntary) was performed within the program (STATA 16 [version 16.1]) to generate an overall effect size for each exercise type. In addition, to detect publication bias, we performed a regression-based Egger’s test with a random-effects model and nonparametric trim-and-fill analysis using STATA 16 (version 16.1). Risk of bias graphs were plotted with Microsoft Excel.
Study Quality
The SYRCLE’s risk of bias tool, CAMARADES, and ARRIVE guideline checklists for study quality were adapted from Covidence to assess the risk of bias and quality of the eligible studies. Four independent reviewers (WHC/SB or RJS/VSG) screened and scored studies based on the following checklists: a clearly stated hypothesis, randomization of animals into study groups, blinding consideration (i.e., a statement that experimenter[s] where blinded to group identification during experimentation or data analysis), animal model use of appropriate age/comorbid animals, sex consideration, animal regulation/ethics approval, species-specific details of animals, source/vendor of animals used, physiological control for surgery, timeline/frequency of treatment interventions, inclusion/exclusion criteria, summary statistic report, appropriateness of statistical test used, sample size/power calculation, strategy to reduce confounds, conflict of interest, and stated reason(s) for incomplete outcome data. A third reviewer (IJB) was consulted to resolve any discrepancies. Studies that met the inclusion criteria but failed to adequately report data (sample size, mean, and a measure of variance) for meta-analysis were included in the assessment of study quality.
Results
Study Selection and Characteristics
A total of 320,554 articles were identified from databases, and 246,663 articles were screened per the stated eligibility criteria after accounting for duplicates. The PRISMA flow diagram (Figure 1) shows that 246,641 articles were excluded due to a priori exclusion criteria which was due to the use of neonate animals, unavailable full-text articles, a lack of a VaD model, or no exercise intervention. Of the 45 selected reports evaluated for eligibility, 20 articles were specifically excluded for lack of a VaD model (n = 10), use of neonates (n = 3), missing data (n = 6), and review paper (n = 1). Twenty-five studies were included in the systematic review, comprising 21 studies used for meta-analysis. These studies entailed various models of VaD: bilateral carotid artery occlusion or two vessel occlusion (52%, 13 studies),38–50 diet-induced vasculopathy/cognitive dysfunction (16%, 4 studies),51–54 bilateral carotid artery stenosis (8%, 2 studies),55, 56 diabetes (8%, 2 studies),57, 58 transgenic/hypertension-induced vasculopathy (8%, 2 studies),59, 60 unilateral carotid artery occlusion (4%, 1 study), 61 and aortic band (4%, 1 study). 62 Species of animals used in the selected study include rats38–50, 58–61 (64%, 16 studies), mice51–57, 60 (32%, 8 studies), and pigs 62 (4%, 1 study), which included studies of only male animals (80%, 20 studies), male and female animals53, 54, 57, 60 (16%, 4 studies), and female animals only 43 (4%, 1 study). All studies used animals below 12 months of age, accounting for 100% of the use of young animals. Of all included studies, forced exercise (only) was the most administered intervention38, 39, 44, 47, 48, 50, 56–59, 61, 62 (48%, 12 studies), followed by voluntary exercise41–43, 49, 51–55 (36%, 9 studies). Only four studies (16%) reported the use of more than one exercise form (i.e., environmental enrichment, voluntary, involuntary, or forced) administered to distinct experimental groups.40, 45, 46, 60 All animals were made to perform at least 1 week of longitudinal (forced, involuntary, or voluntary) exercise, which amounted to a minimum distance of 150 m per day (Table 1).
Figure 1. PRISMA Flow diagram for Study Selection, Showing the Number of Studies Identified, Screened, and Included for Descriptive Assessment, Meta-Analysis, and Study Quality Evaluation.
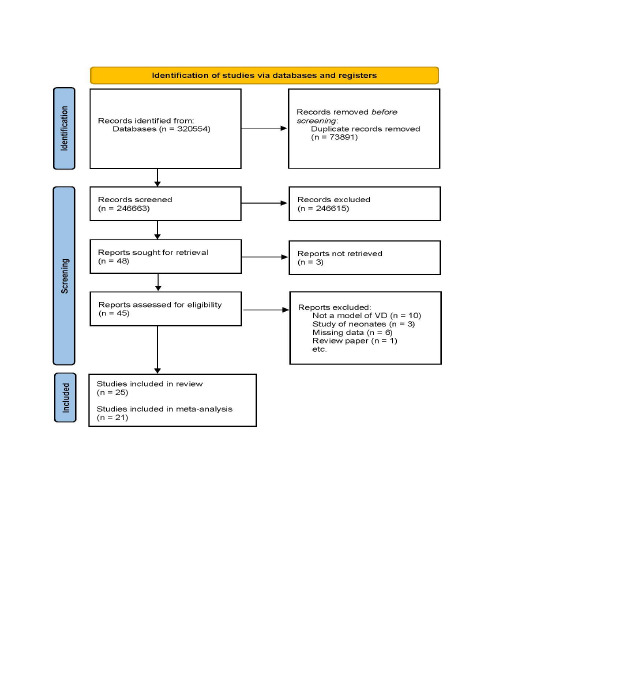
Table 1. Summary Table.
| Author | Model of Dementia | Species or Strain | Age | Sex | Exercise Type | Time of Exercise Intervention | Time of Day | Period of Exercise | Exercise Duration/Day | Exercise Frequency/Wk | Total Distance (m) |
| Banoujaafar et al. 60 | Unilateral common carotid artery occlusion | Wistar, Wistar-Kyoto, SHR rats | 13 wks | Male | F, treadmill | Pre | 09–12 | 7 d | 30 min | 7 d | 540 m/d |
| Cechetti 2012 25 | 2 VO | Wistar rat | 12 wks | Male | F, treadmill | Pre and/ or post | 17- 19 | 12 wks | 20 min | 3 d | 924 m/d |
| Choi et al. 38 | bilateral common carotid artery occlusion | Wistar rat | 8 wks | Male | F, treadmill | 3 wks post | Not given | 4 wks | 30 min | 7 d | 450 m/d |
| de Souza et al. 50 | HFECD | Swiss albino mice | 12 wks | Male | V, running wheel | 4 wks post | Not given | 4 wks | Not given | Not given | Not given |
| Dong et al. 39 | bilateral common carotid artery occlusion | Wistar rat | Not given | Male | V, F, and I; running wheel for V and F | 1 wk post | Not given | 2 wks | 30 min | 14 dsys | V target: 350 circles |
| F: 12 circles/ min | |||||||||||
| I: Not given | |||||||||||
| Graham et al. 51 | Western diet | C57BL/6J mice | 10 mo | Male | V, running wheel | Coincides with diet | “nightly” | 15 d | 16 h access | 7 d | ~ 45000 m |
| Hase et al. 54 | BCAS | C57BL/6 J mice | 9 wks | Male | V, running wheel | Immediately post | 09–12 | 12 wks | 3 h | 7 daya for first 4 wks, 3 d for remaining 8 wks | Not given |
| 24 h | 7 d | Not given | |||||||||
| Jin et al. 40 | 2VO | Sprague Dawley rat | 8 wks | Male | V, running wheel | 3 d post | 09–21 | 4 wks | 12 h access | 7 d | Not given |
| Langdon et al 41 | 2VO | Sprague Dawley rat | 6 mo | Male | V, running wheel | Concurrent | 24 h access | 28 wks | 24 h access | 7 daya | Not given |
| Author | Model of Dementia | Species or Strain | Age | Sex | Exercise Type | Time of Exercise Intervention | Time of Day | Period of Exercise | Exercise Duration/Day | Exercise Frequency/Wk | Total Distance (m) |
| Langdon et al. 42 | 2VO | Sprague Dawley rat | 6–8 mo | Female | V, running wheel | Concurrent | 24 h access | 28 wks | 24 h access | 7 d | 4 wk post: 4092 ± 1015/ wk |
| 8 wk post: 6246 ± 3415/ wk | |||||||||||
| 12 wk post: 3662 ± 1169/ wk | |||||||||||
| 16 wk post: 2461 ± 892/ wk | |||||||||||
| 20 wk post: 1785 ± 615/ wk | |||||||||||
| 24 wk post: 1323 ± 308/ wk | |||||||||||
| Leardini-Tristão et al. 43 | 2VO | Wistar rat | 12 wks | Male | F, treadmill | 3 d post | 08–10 | 12 wks | 30 min | 3 d | Not given |
| Wistar rat | 12 wks | Male | F, treadmill | 3 d post | 08–10 | 12 wks | 30 min | 3 d | Not given | ||
| Lin et al. 44 | 2VO | Wistar rat | Not given | Male | V, F, and I; running wheel for V and F | 1 wk post | Not given | 2 wks | 30 min | 7 d | 360/ d |
| Lin et al. 45 | 2VO | Wistar rat | Not given | Male | V, F, and I; running wheel for V and F | 1 wk post | Not given | 2 wks | 30 min | 7 d | 360/ d |
| Author | Model of Dementia | Species or Strain | Age | Sex | Exercise Type | Time of Exercise Intervention | Time of Day | Period of Exercise | Exercise Duration/Day | Exercise Frequency/Wk | Total Distance (m) |
| Monnier et al 58 | Hypertension | SHR rats | 12 wks | Male | F, treadmill | Concurrent | Not given | 1 wk | 30 min | 7 d | 540/d |
| Moreira et al. 59 | LDL receptor KO | Mice | 26 wks | Male | V, running wheel | Concurrent | 24 h access | 4 wks | 24 h access | 7 d | Not given |
| LDL receptor KO | Mice | 90 d | Female | EE | Concurrent | 24 h access | 90 d | 24 h access | 7 d | Not given | |
| LDL receptor KO | Mice | 90 d | Male | EE | Concurrent | 24 h access | 90 d | 24 h access | 7 d | Not given | |
| Niu et al. 46 | 2VO | Sprague Dawley | 6 mo | Male | F, treadmill | 3 wk post | Not given | 4 wks | 30 min | 7 d | 450/d |
| Ohtomo et al. 55 | BCAS | C57BL/6J mice | 9 wks | Male | F, treadmill | 7 d post | “Early afternoon” | 6 wks | 60 min | 5 d | Up to 600/d |
| Olver et al. 61 | Aortic band | Yucatan miniature swine | 8 Mo | Male | F, treadmill | 2 Mo post | Not given | 18 wks | 120 min | 3 d | 5203.67 |
| Sarkaki et al. 47 | Permanent bilateral common carotid arteries occlusion | Wistar rat | 12 wks | Male | F, treadmill | Post | NR | 4 wks | 60 min | 7 d | 1080 m |
| Trigiani 2019 53 | TGF-β1; High cholesterol diet | TGF-β1 mice | 12 wks | Male and Female | V, running wheel | Concurrent | Cohort 1: night access | 3–4 mo | 12 h access | 7 d | NR |
| Trigiani et al. 52 | TGF-β1; High cholesterol diet | TGF-β1 mice | 3–4 mo | Male and Female | V, running wheel | Concurrent | 19:00–22:00 | 4–6 mo | 3 h/d | 5 d | 3–4 km/ night |
| Xu et al. 48 | 2VO | Sprague Dawley rats | “Adult” | Male | V, running wheels | Concurrent | 10:00–16:00 | 6 wks | 6 h | 7 d | Not given |
| Yazdanian et al 49 | Bilateral carotid artery occlusion, transient | Wistar rats | Not given | Male | F, treadmill | pre | Not given | 8 wks | 50 min | 5 d | 60,000 |
| Yermakov et al. 56 | Type 2 diabetes | db/db mice | 5–11 wks | Male and female | F, running wheels | Concurrent | 02:00–04:00 | 5 wks | 1 h | 7 d | 16,800 |
| Zarrinkalam et al. 57 | Type 1 diabetes | Wistar rats | 6–8 wks | Male | F, ladder climbing and resistance training | 3 d post injection | Not given | 10 wks | Not given | 5 d | NA |
Abbreviations: All included studies are characterized in alphabetical order. 2VO, 2-vessel occlusion; BCAS, bilateral common carotid artery stenosis; CD, control diet; D, day; E, exercise; EE, enriched environment with running wheel; F, forced; h, hours; HFECD, high-fat, cholesterol-enriched diet; I, involuntary; km, kilometer; M, meter; Min, minute; mo, months; S, sedentary; SHR, spontaneously hypertensive; TGF, transforming growth factor; V, voluntary; wk, week; WT, wild-type; Y, yes; NA, not applicable.
Meta-Analysis of Exercise on Learning, Memory, and Anxiety Outcomes
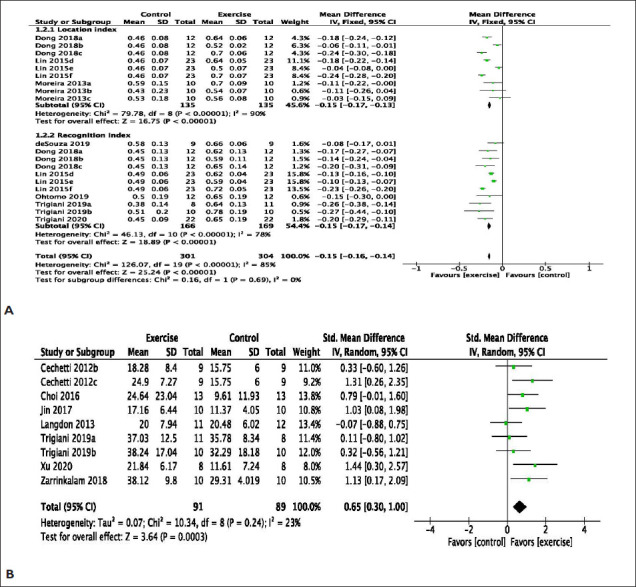
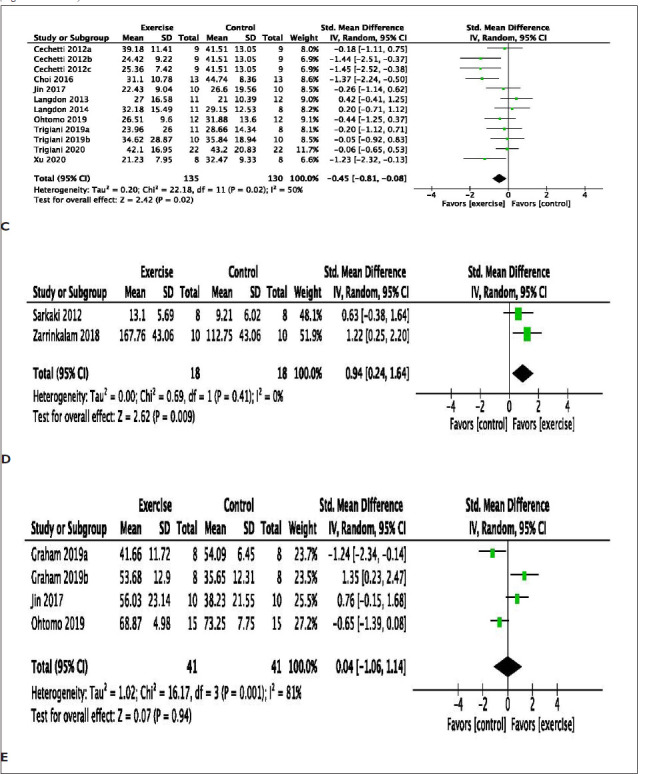
The effect of exercise on learning and memory function assessed in VaD models was reported in 16 studies. In various models of VaD, exercise improved object location index (mean difference, MD: 1.66 [95% CI 0.84 to 2.47]) with considerable heterogeneity (χ2 = 61.14, df = 8; p < .00001; I2 = 87%). Similarly, exercise significantly improved the novel object recognition index (MD: 1.65 [95% CI: 1.14 to 2.16]) with substantial heterogeneity (χ2 = 37.45, df = 10; p < .00001; I2 =73%). The overall effect of exercise on novel object indexes is significant (MD: 1.65 [95% CI: −1.20 to 2.16]) with substantial heterogeneity (χ 2 = 99.55, df = 19; p < .00001; I2 =81%) (Figure 2A). In addition, exercise significantly improves learning in other measures of learning, such as Morris water maze probes (MD: 0.65 [95% CI: 0.30 to −1.00]) with minimal heterogeneity (χ2 = 10.34, df = 8; p < .24; I2 = 23%) (Figure 2B) and platform latency trials (MD: −0.45 [95% CI: −0.81 to −0.08]) with a moderate heterogeneity (χ2 = 22.18, df = 11; p < .02; I2 = 50%)] (Figure 2C). Exercise improves learning memory function, as evaluated by the passive avoidance test (MD: 0.94 [95% CI: 0.24 to 1.64]) with minimal heterogeneity (χ2 = 0.69, df = 1; p = .41; I2 = 0%) (Figure 2D). Novel object indexes, passive avoidance tests, and Morris water maze tests measure learning and memory retention. However, working memory function, as indicated by the Y-maze test, was not improved by exercise (MD: 0.04 [95% CI −1.06 to 1.14]) with substantial heterogeneity (χ2 = 16.17, df = 3; p = .0001; I2 = 81%) (Figure 2E).
Figure 2. (A) Meta-Analysis of Novel Object Location and Recognition Test. Dong a, Involuntary Exercise Group; Dong b, Forced Exercise Group; Dong c, Voluntary Exercise Group; Lin d, Involuntary Exercise Group; Lin e, Forced Exercise Group; Lin f, Voluntary Exercise Group; Moreira a, Running Wheel; Moreira b, Female Enriched Environment; Moreira c, Male Enriched Environment. Trigiani a, Cohort 1; Trigiani b, Cohort 2. (B) Meta-Analysis of Morris Water Maze Probe. Cechetti b, Post-Surgery Exercise; Cechetti, c Pre- and Post-Surgery Exercise; Trigiani a, Cohort 1; Trigiani b, Cohort 2. (C) Meta-analysis of Morris Water Maze latency (seconds). Cechetti a, Pre-Surgery Exercise; Cechetti b, Post-Surgery Exercise; Cechetti, c Pre- and Post-Surgery Exercise; Trigiani a, Cohort 1; Trigiani b, Cohort 2. (D) Meta-Analysis of Passive avoidance. (E) Meta-Analysis of Y-maze (percent spontaneous alterations). Graham a, Chow; Graham b, Western Diet. (F) Meta-Analysis of Open Field.
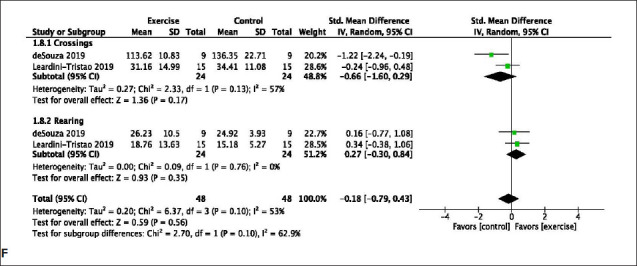
An open field test is commonly used to measure anxiety in rodents. Exercise significantly decreased crossings (MD: −0.66 [95% CI −1.60 to 0.29) with a minimal heterogeneity (χ2 = 2.33, df = 1; p = .13; I2 = 57%), while exercise increased rearing behavior (MD: 0.27 [95% CI: −0.30 to 0.84]) with a minimal heterogeneity (χ2 = 0.09, df = 1; P = .76; I2 = 0%) (Figure 2F).
Meta-Analysis of Exercise on Brain Morphology
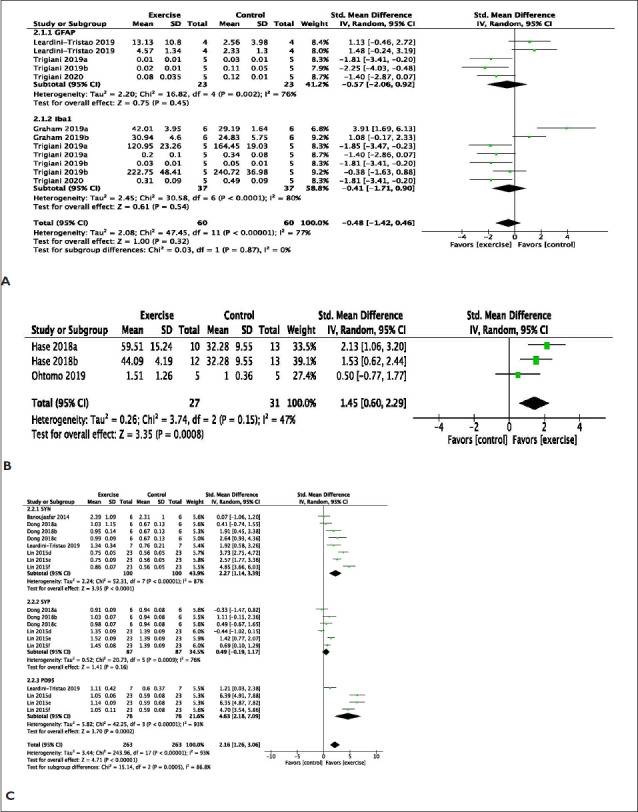
The effect of exercise on brain morphology was evaluated in 12 studies reporting changes in brain markers for synaptic transmission, cellular integrity, neurotrophic factors, cell proliferation, white matter integrity, and neuroinflammation in VaD models. Exercise significantly increased neuro-inflammatory markers for astrocyte reactivity/activation using glial fibrillary acidic protein (GFAP) (MD: −0.57 [95% CI: −2.06 to 0.92]) with substantial heterogeneity (χ2 = 16.82, df = 4; P = .002; I2 =76%) and ionized calcium binding adaptor molecule 1 (Iba-1) (MD: −0.41 [95% CI: −1.71 to 0.90]) with substantial heterogeneity (χ2 = 30.58, df = 6; p < .00001; I2 =80%) (Figure 3A). Also, exercise improves white matter integrity markers (MD: 1.45 [95% CI: 0.60 to 2.29]) with minimal heterogeneity (χ2 = 3.74, df = 2; p < .15; I2 =47%) (Figure 3B). Similarly, exercise improves markers of neuronal synaptic transmission such as synapsin (SYN) (MD: 2.27 [95% CI: 1.14 to 3.39]) with substantial heterogeneity (χ2 = 52.31, df = 7; p < .0001; I2 =87%), synaptophysin (SYP) (MD: 0.49 [95% CI: −0.19 to 1.17]) with substantial heterogeneity (χ2 = 20.73, df = 5; p < .0009; I2 =76%), and postsynaptic density 95 (PSD95) (MD: 4.63 [95% CI: 2.18 to 7.09]) with substantial heterogeneity (χ2 = 42.25, df = 3; p = .00001; I2 =93%) (Figure 3C).
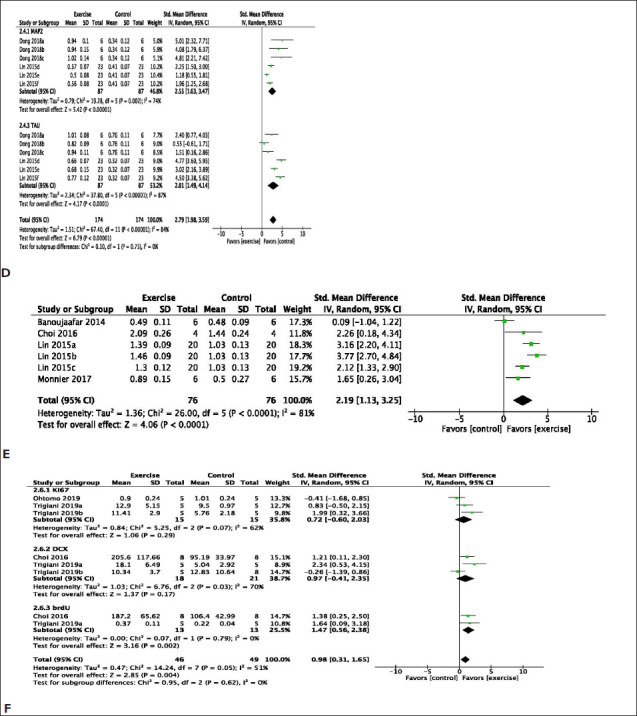
Figure 3. (A) Meta-Analysis of Neuroinflammation Markers. Graham a, Chow; Graham b, Western Diet; Trigiani a, Cohort 1; Trigiani b, Cohort 2. (B) Meta-Analysis of Markers of White Matter Integrity. Hase a, 3 h Environmental Enrichment; Hase b, Full-Time Environmental Enrichment. Data Used in the Analysis Were Total Number of Oligodendrocytes in the Corpus Callosum and Western Blot of Myelin Basic Protein. (C) Meta-Analysis of Markers of Neuronal Synaptic Transmission. Dong a, Involuntary Exercise Group; Dong b, Forced Exercise Group; Dong c, Voluntary Exercise Group; Lin d, Involuntary Exercise Group; Lin e, Forced Exercise Group; Lin f, Voluntary Exercise Group. (D) Meta-Analysis of Markers of Cellular Integrity. Dong a, Involuntary Exercise Group; Dong b, Forced Exercise Group; Dong c, Voluntary Exercise Group; Lin d, Involuntary Exercise Group; Lin e, Forced Exercise Group; Lin f, Voluntary Exercise Group. (E) Meta-analysis of Western Blots of Brain-Derived Neurotrophic Factor. Lin a, Involuntary Exercise Group; Lin b, Forced Exercise Group; Lin c, Voluntary Exercise Group. (F) Meta-Analysis of Cell Proliferation. Trigiani a, Cohort 1; Trigiani b, Cohort 2. brdU.
Abbreviations: Bromodeoxyuridine; DCX, doublecortin.
Furthermore, exercise significantly increased markers of cellular integrity such as microtubule-associated protein 2 (MAP2) (MD: 2.55 [95% CI: 1.63 to 3.47]) with considerable heterogeneity (χ2 = 19.28, df = 5; p < .002; I2 = 74%) and Tau protein (MD: 2.81 [95% CI: 1.49 to 4.14]) with substantial heterogeneity (χ2 = 37.8, df = 5; p < .00001; I2 = 87%) (Figure 3D). Exercise significantly improves brain-derived neurotrophic factor (BDNF) (MD: 2.19 [95% CI: 1.13 to 3.25]) with considerable heterogeneity (χ2 = 26.00, df = 5; p < .0001; I2 = 81%) (Figure 3E).
Exercise significantly improves makers of cell proliferation such as Ki-67 protein (MD: 0.72 [95% CI: −0.60 to 2.03]) with moderate heterogeneity (χ2 = 5.25, df = 2; p < .07; I2 =62%), doublecortin (DCX) (MD: 0.97 [95% CI: −0.41 to 2.35]) with considerable heterogeneity (χ2 = 6.76, df = 2; p < .03; I2 = 70%), and bromodeoxyuridine (BrdU) (MD: 1.47 [95% CI: 0.56 to 2.38]) with minimal heterogeneity (χ2 = 0.07, df = 1; p = .79; I2 =0%) (Figure 3F).
Meta-Analysis of Exercise Type on Cognition and Brain Morphology
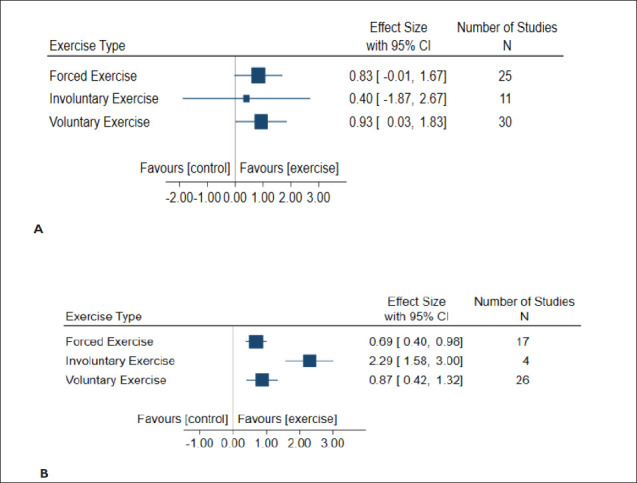
The effect of exercise types on overall cognition function tests and brain morphology was evaluated in all included study cohorts. All exercise types, including forced, involuntary, and voluntary exercise, improved cognitive function (Figure 4A). However, involuntary exercise, albeit with a smaller number of study cohorts (n = 4), had a larger effect size of 2.29 (95% CI: 1.58 to 3.00) when compared to the other types of exercise. Similarly, all exercise types had a favorable effect on brain morphology in the models of VaD (Figure 4B). Involuntary exercise had the least effect size of 0.40 (95% CI: −1.87 to 2.67) when compared to other types of exercise, which had similar weights and comparable effect sizes.
Figure 4. Forest Plots of Exercise Type Effects on Cognitive Function (A) and Brain Morphology (B) Following Vascular Dementia Modeling in Animals. The Size of the Square Represents the Weight.
Abbreviations: CI, confidence interval; N: total number of corresponding exercise cohorts from included studies.
Reporting Quality and Risk of Bias Assessment
All included studies in this systematic review were published at least 2 years after the first edition of ARRIVE guidelines was released in 2010. The evaluation of 25 studies based on our reporting checklist (Figure 5A) indicates that 100% of the included studies did not use aged animals, while 80% of the studies used only male animals. Only 44% of studies reported blinding consideration, and only 32% of studies did not report the random assignment of animals to groups. Twenty-five percent of studies failed to use appropriate statistical tests to analyze their data, and 72% of studies reported their summary statistics as mean ± standard error of mean (SEM). In addition, some studies reported the following information: sex consideration sample size calculation (41.7% of studies), clearly stated hypothesis (64% of studies), and inclusion/exclusion criteria (24% of studies). Hence, reporting quality for appropriate summary statistics, sex consideration, use of aged animals, and inclusion/exclusion criteria was very low.
Figure 5. Study Quality. Summary of the Quality of the 25 Included Studies Based on (A) Reporting Quality Criteria and (B) the Risk of Bias Assessment Specified Reporting and/ or Methodological Quality Criteria. The Number of Studies Assessed for Quality are Indicated Within Colored Zone of the Bars, With the Exception of the “Unclear” Category in Panel (B) for Clarity.
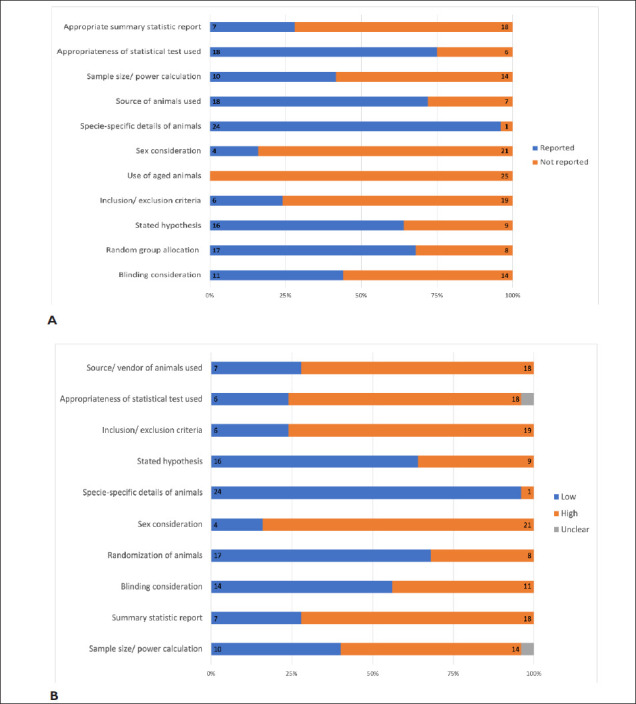
The majority of included articles scored poorly on the risk of bias assessment criteria (Figure 5B). The source and/or vendor of the animals used were specifically mentioned in only 7 (28%) of the 25 articles. Similarly, summary statistics were appropriately reported in only 28% of the articles. Only 6 (24%) of the 25 articles used appropriate statistical tests as well as defined exclusion and inclusion criteria. Also, only 16% of the articles used male and female animals, while sample size and/or power calculation were used in 40% of all included articles. Nevertheless, more than half of all studies had a clear study hypothesis (64%), species-specific details of animals used (96%), randomized animals into study groups (68%), and considered blinding of treatment groups either during experimentation or data analysis (56%).
Publication Bias Assessment
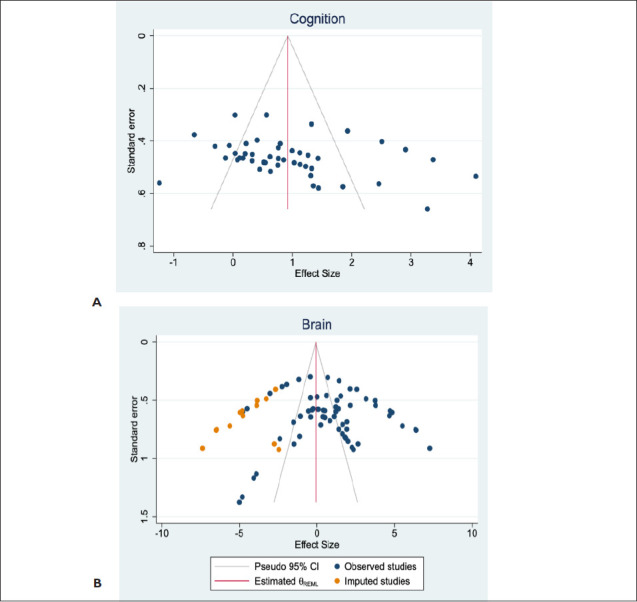
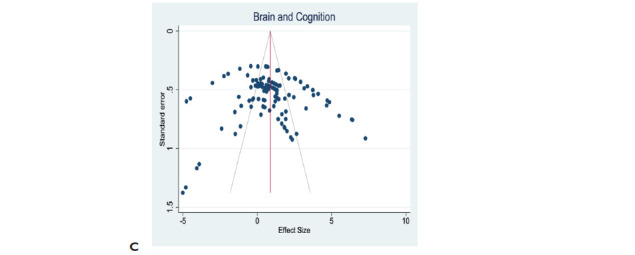
The effect size of cognitive function following exercise intervention is 1.184 [95% CI: 0.826 to 1.542]. Egger’s regression test was insignificant (p = .1206), suggesting no funnel plot asymmetry (Figure 6A). However, the effect size of brain morphology following exercise intervention is 0.810 [95% CI: 0.183 to 1.437]. Egger’s regression test was significant (p = .0058), indicating funnel plot asymmetry and therefore publication bias (Figure 6B). However, the combined effect sizes of cognitive function and brain morphology are 0.87 [95% CI: 0.497 to 1.244]. Egger’s regression test was insignificant (p = .4234), indicating funnel plot symmetry (Figure 6C). Trim-and-fill analysis imputed 13 data points for theoretically missing experiments for only the brain morphology plot with an adjusted SMD of −0.082 [95% CI: −0.784 to 0.62] (Figure 6B). This suggests the presence of publication bias for only brain morphology and not cognitive function data.
Figure 6. Publication Bias. Evaluation of Publication Bias in Outcome Effects of Exercise Intervention Following the Induction of Vascular Dementia Models. (A) The Funnel Plot for Cognitive Function Data Suggests Plot Symmetry Indicating the Absence of Publication Bias. (B) The Funnel Plot of Effect of Exercise on Brain Morphology Shows Asymmetrical Plots Which Suggests the Presence of Publication Bias. (C) The Funnel Plot of Combined Data for Cognitive Function and Brain Morphology Effects Suggests Symmetrical Plots and, Hence Absence of the Publication Bias. The Vertical Red Line Represents the Overall Effect Size. The Gray Lines Indicate the Statistical Significance of Effect Sizes of Cohort Comparisons. Reporting of a Statement Regarding Potential Conflict of Interests and Compliance With Animal Welfare Regulations Were Extracted, But They Were Not Part of the Overall Risk of Bias.
Discussion
The objective of this study is to determine whether physical activity improves cognitive function and brain morphology in models of VaD. Also, we investigated whether a specific type of exercise is more beneficial than others. To our knowledge, this is the first meta-analysis conducted on the effect of exercise in VaD models. Although we sought to include VaD studies from a variety of species, only one study used pigs, while 24 other studies used rodents (15 rat studies and 9 mouse studies). Surgically induced models of VaD such as bilateral carotid artery occlusion, unilateral carotid artery occlusion, bilateral carotid artery stenosis, and aortic band account for a total of 17 studies. Only rodent studies included cognitive outcome measures.
Our analyses suggest that exercise improves cognitive function in models of VaD. Learning and memory function tests such as the Morris water maze, novel object location, and novel object recognition tests were associated with significant improvements in cognitive function across the 16 studies that evaluated cognition in rodent models of VaD. These tests are routinely used in assessing memory functions in laboratory animals and explore the rodents’ ability to investigate new objects and/or to engage brain-associated memory-dependent pathways in the recall of previously explored objects, locations, or spatial cues. 63 The brain regions principally associated with memory function are the hippocampus and prefrontal cortex.64, 65 In addition, the dorsoventral striatum has been associated with learning and memory functions. 66 The surgical models of VaD included in this meta-analysis compromise blood flow to the brain, with subsequent white matter damage that may involve a hippocampal lesion following a cascade of disruptive molecular signaling events.67, 68 The striatum is rich in afferent and efferent white matter bundles, which connect the prefrontal cortex and hippocampal structures. 69 Hence, white matter damage in the striatum will impact memory function, which our analyses suggest exercise ameliorates. However, working and/or spatial memory assessed by Y-maze spontaneous alternation was not improved by exercise interventions in VaD models. It is puzzling to reconcile the differences in overall exercise outcomes between Morris water maze and Y-maze spontaneous alternation tests, given that both tests entail the use of spatial memory as well as the overlap of study cohorts. The Morris water maze test is a more sensitive test for the determination of changes in learning and memory when compared to the Y-maze spontaneous alternation test. 70 This is primarily because the Morris water maze demonstrates the animals’ failure or success to acquire (location) memory over 4 to 5 days when compared to the Y-maze spontaneous alternation test, which is a single trial of maze exploration. The Y-maze spontaneous alternation test is believed to be non-hippocampus-dependent and relies on the animals’ emotional state, which may vary between strains. 71 Further, the passive avoidance test, a fear-conditioned learning memory paradigm, was improved by exercise. The foregoing indicates the need for further investigations of the viability of Y-maze spontaneous alternation for the evaluation of cognition in rodent models of VaD subjected to exercise interventions. While anxiety is not a function of cognition per se, it does influence the outcome of memory function tests, as mouse models of VaD are reported to be hyperactive and have increased levels of anxiety. 68 Our analysis reveals that exercise increased anxiety as measured by the number of crossings and rearing behaviors in rodents subjected to an open field test. This may be attributed to repeated stress from exercise interventions. The relationship between exercise and anxiety is multifaceted. Also, forced, but not voluntary, exercise has been found to increase anxious behavior in rats in the open field 72 Further research is needed to elucidate the effect of forced versus voluntary exercise in VaD animal models.
Exercise is well known to induce inflammation in skeletal muscles,73, 74 nevertheless, our analysis shows that neuro-inflammation is worsened in VaD models subjected to exercise interventions, as evidenced by the increased effect size of GFAP and Iba-1 histological markers. Overall, in tandem with our finding of improved cognitive function, exercise improves white matter integrity as well as markers of neuronal synaptic transmission such as SYN, SYP, and PSD95. This may stem from increased BDNF in addition to improved MAP2 and Tau proteins, which are markers of cellular integrity. Indeed, human and experimental studies have indicated that exercise results in increased BDNF, which helps improve overall brain function as well as slow the progression of diagnosed cognitive impairments by enlarging hippocampal and gray matter volumes,75–78 suggesting that exercise can ameliorate the impacts of VaD on brain structure and function. Although voluntary exercise is a more appealing rodent exercise intervention due to its similarity to human exercise with willful and self-administering features, 79 we note that all exercise types from our included studies improved both cognitive function as well as brain morphology. We did not find any evidence of voluntary exercise being more beneficial for either of the two outcomes.
Although all included studies for the present meta-analysis were published at least two years after the first edition of the ARRIVE guidelines in 2010, a majority of the studies had some reporting anomalies such as blinding consideration (either at the time of experimentation or data analysis), randomization, and use of appropriate statistical tests. This begs for increased reporting quality advocacy among preclinical scientists. Further, 72% of studies expressed their summary statistics as mean ± SEM, whereas the correct statistical summary should be confidence interval or mean ± SD. The difference between SEM and standard deviation, as well as the importance of SD as a measure of variability in data reporting are well established and documented elsewhere.80–82 All the included studies lacked the use of advanced age in the modeling of VaD. VaD is a disease of the elderly that may be diagnosed in middle age; hence, the appropriate rodent age for the translational modeling of VaD should be 13–15 months (for middle age) and 19–25 months (for advanced age).83, 84 One reason for the continuous use of young animals is the high cost of older animals. Nevertheless, sex as a biological consideration is conspicuously lacking in the included studies. It is known that VaD impacts both elderly men and women (although with some differences in early disease progression), 85 yet the use of male-only animals for preclinical investigations has continued, partly due to the neuroprotective effect of estrogen. The neuroprotection conferred by estrogen is lost from middle age in female animals, and the National Institutes of Health provides funded studies requiring aged animals at no cost. It is in the best interest of investigators, for increased potential clinical translation of their experimental findings, to follow the ARRIVE guidelines and consider the appropriateness of age and sex during the experimental design of VaD studies.
Our analysis of the data from the included studies indicates the existence of publication bias for brain morphology outcomes, whereas there was no evidence of publication bias for cognitive function measures. Publication bias, in theory, describes the likelihood of including studies that support a given hypothesis rather than studies showing neutral results for systematic review or meta-analysis. While it is not uncommon to find reports of publication bias in systematic reviews of preclinical studies,86, 87 we took steps to ensure that the process of study selection is in tandem with extant guidelines for systematic reviews (as outlined in the “Methods” section of the present study). Our results could be due to the common practice of preclinical science journals accepting more manuscripts with “positive results” than those with equivocal observations. Also, our data characteristics and statistical analysis may be contributing factors to our observation; hence, reporting bias is not the single most important factor for publication bias.89 Trim and fill analyses have been reported to inaccurately estimate asymmetry and impute missing data plots.
The present study would have benefited from an analysis of the duration of exercise intervention as well as the comparison of VaD models on cognitive function and brain morphology outcomes. However, due to the small number of included studies and the variability in the types of VaD models across the included studies, such analysis would yield little or no knowledge advancement.
Conclusion
Our results from three databases on the effects of physical activity in models of VaD yielded 25 studies for systematic review and 21 for meta-analysis and demonstrate that exercise, a non-pharmacological intervention, improved cognition and brain morphology outcomes in VaD models. Also, we note that future investigations of exercise effects in VaD models should strongly consider advanced age and female sex to boost the translational impact of experimental findings.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the American Heart Association (AHA) Postdoctoral Fellowship (Grant number: 831577).
ORCID iDs: Ifechukwude J. Biose  https://orcid.org/0000-0001-9642- 2803
https://orcid.org/0000-0001-9642- 2803
Abbreviations
ARRIVE, Animal Research: Reporting of In Vivo Experiments; BDNF, brain-derived neurotrophic factor; BrdU, bromodeoxyuridine; CAMARADES, The Collaborative Approach to Meta-Analysis and Review of Animal Experimental Studies; DCX, doublecortin ; ELISA, Enzyme-linked immunosorbent assay; GFAP, glial fibrillary acidic protein; Iba-1, ionized calcium binding adaptor molecule 1; IGF1, insulin-like growth factor 1; MAP2, microtubule-associated protein 2; MD, mean deviation; PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses; PROSPERO, International Prospective Register of Systematic Reviews; PSD95, postsynaptic density 95 protein; qPCR, quantitative polymerase chain reaction; SYN, synapsin; SYP, synaptophysin; SYRCLE, Systematic Review Centre for Laboratory animal Experimentation; VaD, vascular dementia.
Authors’ Contributions
IJB conceptualized, planned, and supervised the study; IJB and WHC conducted literature search; IJB and WHC and SB screening studies; WHC, RJS, VSG and BS conducted data extraction; RJS and HW conducted data analysis; IJB and RJS prepared sections of manuscript; GJB supervised manuscript drafts; all authors edited and approved manuscript.
Statements of Ethics
No experiments were conducted by the author for this Review Article. Hence, ethical approval was not required.
Declaration on ICMJE Recommendation
The authors abide by the recommendations of the International Committee of Medical Journal Editors (ICMJE).
References
- 1.van Norden AG, van Dijk EJ, de Laat KF, et al. Dementia: Alzheimer pathology and vascular factors: from mutually exclusive to interaction. Biochem Biophys Acta 2012; 1822: 340–349. [DOI] [PubMed] [Google Scholar]
- 2.Ligthart SA, Moll van Charante EP, Van Gool WA, et al. Treatment of cardiovascular risk factors to prevent cognitive decline and dementia: a systematic review. Vasc Health Risk Manag 2010; 7(6): 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richard E, Moll van Charante EP, and Van Gool W.. A Vascular risk factors as treatment target to prevent cognitive decline. J Alzheimers Dis 2012; 32(3): 733–740. [DOI] [PubMed] [Google Scholar]
- 4.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American heart association/American Stroke Association. Stroke 2011; 42: 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal NT and Decarli C.. Vascular dementia: emerging trends. Semin Neurol 2007; 27: 66–77. [DOI] [PubMed] [Google Scholar]
- 6.Jellinger KA. Morphologic diagnosis of ’vascular dementia’-a critical update. J. Neurol Sci 2008; 270: 1–12. [DOI] [PubMed] [Google Scholar]
- 7.Riffin C, Van Ness PH, Wolff JL, et al. Family and other unpaid caregivers and older adults with and without dementia and disability. J Am Geriatr Soc 2017; 65(8): 1821–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prince MJ, Wimo A, Guerchet MM, et al. World Alzheimer Report. The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends . London: Alzheimer’s Disease International. (2015). 84 p. [Google Scholar]
- 8.Hörder H, Johansson L, Guo X, et al. Midlife cardiovascular fitness and dementia: A 44-year longitudinal population study in women. Neurology 2018; 90(15): e1298–e1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andel R, Crowe M, Pedersen NL, et al. Physical exercise at midlife and risk of dementia three decades later: a population-based study of Swedish twins. J Gerontol A Biol Sci Med Sci 2008; 63: 62–66. [DOI] [PubMed] [Google Scholar]
- 11.Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol 2005; 4: 705–711. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto H, Ohara T, Hata J, et al. The long-term association between physical activity and risk of dementia in the community: the Hisayama Study. Eur J Epidemiol 2016; 31: 267–274. [DOI] [PubMed] [Google Scholar]
- 13.Tolppanen AM, Solomon A, Kulmala J, et al. Leisure-time physical activity from mid- to late life, body mass index, and risk of dementia. Alzheimers Dement 2015; 11: 434–e6. [DOI] [PubMed] [Google Scholar]
- 14.Najar J, Östling S, Gudmundsson P, et al. Cognitive and physical activity and dementia: a 44-year longitudinal population study of women. Neurology 2019; 92(12): e1322–e1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. The Lancet 2015; 385(9984): 2255–2263. [DOI] [PubMed] [Google Scholar]
- 16.Ahlskog JE, Geda YE, Graff-Radford NR, et al. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clinic Proc 2011; 86(9): 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kling MA, Trojanowski JQ, Wolk DA, et al. Vascular disease and dementias: paradigm shifts to drive research in new directions. Alzheimers Dement 2013; 9(1): 76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Llamas-Velasco S, Contador I, Villarejo-Galende A, et al. Physical activity as protective factor against dementia: a prospective population-based study (NEDICES). J Int Neuropsychol Soc 2015; 21: 861–867. [DOI] [PubMed] [Google Scholar]
- 19.Chang M, Jonsson PV, Snaedal J, et al. The effect of midlife physical activity on cognitive function among older adults: AGES—reykjavik study. J Gerontol A Biol Sci Med Sci 2010; 65(12): 1369–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol 2005; 161(7): 639–651. [DOI] [PubMed] [Google Scholar]
- 21.Dregan A, Stewart R, and Gulliford MC.. Cardiovascular risk factors and cognitive decline in adults aged 50 and over: a population-based cohort study. Age Ageing 2013; 42(3): 338–345. [DOI] [PubMed] [Google Scholar]
- 22.Abete P, Della-Morte D, Gargiulo G, et al. Cognitive impairment and cardiovascular diseases in the elderly. A heart-brain continuum hypothesis. Ageing Res Rev 2014; 18: 41–52. [DOI] [PubMed] [Google Scholar]
- 23.Wysocki M, Luo X, Schmeidler J, et al. Hypertension is associated with cognitive decline in elderly people at high risk for dementia. Am J Geriatr Psychiatry 2012; 20(2): 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radak Z, Toldy A, Szabo Z, et al. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem Int 2006; 49: 387–392. [DOI] [PubMed] [Google Scholar]
- 25.Cechetti F, Worm PV, Elsner VR, et al. Forced treadmill exercise prevents oxidative stress and memory deficits following chronic cerebral hypoperfusion in the rat. Neurobiol Learn Mem 2012; 97: 90–96. [DOI] [PubMed] [Google Scholar]
- 26.van Praag H, Christie BR, Sejnowski TJ, et al. Running enhances neurogenesis, learning and long-term potentiation in mice. Proc Natl Acad Sci U S A 1999; 96: 13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogonovszky H, Berkes I, Kumagai S, et al. The effects of moderate-, strenuous- and over-training on oxidative stress markers, DNA repair and memory, in rat brain. Neurochem Int 2005; 46: 635–640. [DOI] [PubMed] [Google Scholar]
- 28.Young J, Angevaren M, Rusted J, et al. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev 2015; 22(4): CD005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sink KM, Espeland MA, Castro CM, et al. Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the LIFE randomized trial. JAMA 2015; 314: 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gates N, Fiatarone Singh MA, Sachdev P, et al. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Am J Geriatr Psychiatry 2013; 21: 1086–1097. [DOI] [PubMed] [Google Scholar]
- 31.Forbes D, Forbes SC, Blake CM, et al. Exercise programs for people with dementia. Cochrane Database Syst Rev 2015; 15(4): CD006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karssemeijer EG, Aaronson JA, Bossers WJ, et al. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: a meta-analysis. Ageing Res Rev 2017; 40: 75–83. [DOI] [PubMed] [Google Scholar]
- 33.Poulin MJ, Eskes GA, and Hill MD.. Physical activity vs health education for cognition in sedentary older adults. JAMA 2016; 315: 415. [DOI] [PubMed] [Google Scholar]
- 34.Ohman H, Savikko N, Strandberg TE, et al. Effect of physical exercise on cognitive performance in older adults with mild cognitive impairment or dementia: a systematic review. Dement Geriatr Cogn Disord ; 201438: 347–365. [DOI] [PubMed] [Google Scholar]
- 35.Northey JM, Cherbuin N, Pumpa KL, et al. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med 2018; 52: 154–160. [DOI] [PubMed] [Google Scholar]
- 36.Blumenthal JA, Smith PJ, Mabe S, et al. Longer Term Effects of Diet and Exercise on Neurocognition: 1-Year Follow-up of the ENLIGHTEN Trial. J Am Geriatr Soc 2020; 68(3):559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biose IJ, Banerjee S, Chastain W, et al. The effects of physical activity on experimental models of vascular dementia –A systematic review and meta-analysis. PROSPERO 2020. 2020:CRD42020212001. [Google Scholar]
- 38.Choi DH, Lee KH, and Lee J.. Effect of exercise-induced neurogenesis on cognitive function deficit in a rat model of vascular dementia. Mol Med Rep 2016; 13(4): 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong J, Zhao J, Lin Y, et al. Exercise improves recognition memory and synaptic plasticity in the prefrontal cortex for rats modelling vascular dementia. Neurol Res 2018; 40(1): 68–77. [DOI] [PubMed] [Google Scholar]
- 40.Jin X, Li T, Zhang L, et al. Environmental Enrichment Improves Spatial Learning and Memory in Vascular Dementia Rats with Activation of Wnt/β-Catenin Signal Pathway. Med Sci Monit 2017; 23: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Langdon KD, Granter-Button S, Harley CW, et al. Cognitive rehabilitation reduces cognitive impairment and normalizes hippocampal CA1 architecture in a rat model of vascular dementia. J Cereb Blood Flow Metab 2013; 33(6): 872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langdon KD, Granter-Button S, Harley CW, et al. A cognitive rehabilitation paradigm effective in male rats lacks efficacy in female rats. J Cereb Blood Flow Metab 2014; 34(10): 1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leardini-Tristão M, Andrade G, Garcia C, et al. Physical exercise promotes astrocyte coverage of microvessels in a model of chronic cerebral hypoperfusion. J Neuroinflammation 2020; 17(1): 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin Y, Dong J, Yan T, et al. Involuntary, forced and voluntary exercises are equally capable of inducing hippocampal plasticity and the recovery of cognitive function after stroke. Neurol Res 2015; 37(10): 893–901. [DOI] [PubMed] [Google Scholar]
- 45.Lin Y, Lu X, Dong J, et al. Involuntary, Forced and Voluntary Exercises Equally Attenuate Neurocognitive Deficits in Vascular Dementia by the BDNF-pCREB Mediated Pathway. Neurochem Res 2015; 40(9): 1839–1848. [DOI] [PubMed] [Google Scholar]
- 46.Niu Y, Wan C, Zhou B, et al. Aerobic exercise relieved vascular cognitive impairment via NF-βB/miR-503/BDNF pathway. Am J Transl Res 2018; 10(3): 753–761. [PMC free article] [PubMed] [Google Scholar]
- 47.Sarkaki A, Rafieirad M, Hossini S, et al. Cognitive deficiency induced by cerebral hypoperfusion/ischemia improves by exercise and grape seed extract. HealthMED 2012; 6(4): 1097–1104. [Google Scholar]
- 48.Xu L, Qu C, Qu C, et al. Improvement of autophagy dysfunction as a potential mechanism for environmental enrichment to protect blood-brain barrier in rats with vascular cognitive impairment. Neurosci Lett 2020; 739: 135437. [DOI] [PubMed] [Google Scholar]
- 49.Yazdanian M, Moazami M, Shabani M, et al. Effects of exercise preconditioning on neurotrophin-4 and Tropomyosin receptor kinase b expression in the hippocampal CA1 Region following transient global cerebral ischemia/reperfusion in Wistar rats. Mljgoums 2019; 13(6): 23–28. [Google Scholar]
- 50.de Souza RM, de Souza L, Machado AE, et al. Behavioural, metabolic and neurochemical effects of environmental enrichment in high-fat cholesterol-enriched diet-fed mice. Behav Brain Res 2019; 359: 648–656. [DOI] [PubMed] [Google Scholar]
- 51.Graham LC, Grabowska WA, Chun Y, et al. Exercise prevents obesity-induced cognitive decline and white matter damage in mice. Neurobiol Aging 2019; 80: 154–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trigiani LJ, Royea J, Tong XK, et al. Comparative benefits of simvastatin and exercise in a mouse model of vascular cognitive impairment and dementia. FASEB J 2019; 33(12): 13280–13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trigiani LJ, Lacalle-Aurioles M, Bourourou M, et al. Benefits of physical exercise on cognition and glial white matter pathology in a mouse model of vascular cognitive impairment and dementia. Glia 2020; 68(9): 1925–1940. [DOI] [PubMed] [Google Scholar]
- 54.Hase Y, Craggs L, Hase M, et al. The effects of environmental enrichment on white matter pathology in a mouse model of chronic cerebral hypoperfusion. J Cereb Blood Flow Metab 2018; 38(1): 151–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohtomo R, Kinoshita K, Ohtomo G, et al. Treadmill Exercise Suppresses Cognitive Decline and Increases White Matter Oligodendrocyte Precursor Cells in a Mouse Model of Prolonged Cerebral Hypoperfusion. Transl Stroke Res 2020; 11(3): 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yermakov LM, Drouet DE, Griggs RB, et al. Type 2 Diabetes Leads to Axon Initial Segment Shortening in db/db Mice. Front Cell Neurosci 2018; 12: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zarrinkalam E, Ranjbar K, Salehi I, et al. Resistance training and hawthorn extract ameliorate cognitive deficits in streptozotocin-induced diabetic rats. Biomed Pharmacother 2018; 97: 503–510. [DOI] [PubMed] [Google Scholar]
- 58.Monnier A, Garnier P, Quirie A, et al. Effect of short-term exercise training on brain-derived neurotrophic factor signaling in spontaneously hypertensive rats. J Hypertens 2017; 35(2): 279–290. [DOI] [PubMed] [Google Scholar]
- 59.Moreira EL, Aguiar AS Jr., de Carvalho CR, Santos D, de Oliveira J, de Bem A, et al. Effects of lifestyle modifications on cognitive impairments in a mouse model of hypercholesterolemia. Neurosci Lett 2013; 541: 193–198. [DOI] [PubMed] [Google Scholar]
- 60.Banoujaafar H, Van Hoecke J, Mossiat C, et al. Brain BDNF levels elevation induced by physical training is reduced after unilateral common carotid artery occlusion in rats. J Cereb Blood Flow Metab 2014; 34(10): 1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olver TD, Klakotskaia D, Ferguson BS, et al. Carotid Artery Vascular Mechanics Serve as Biomarkers of Cognitive Dysfunction in Aortic-Banded Miniature Swine That Can Be Treated With an Exercise Intervention. J Am Heart Assoc 2016; 5(5): e003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanila H. Testing cognitive functions in rodent disease models: Present pitfalls and future perspectives. Behav Brain Res 2018;352: 23–27. [DOI] [PubMed] [Google Scholar]
- 63.Sigurdsson T and Duvarci S.. Hippocampal-prefrontal interactions in cognition. behavior and psychiatric disease. Front Syst Neurosci 2016; 9: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reinert S, Hübener M, Bonhoeffer T, et al. Mouse prefrontal cortex represents learned rules for categorization. Nature 2021; 593(7859): 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woolley DG, Laeremans A, Gantois I, et al. Homologous involvement of striatum and prefrontal cortex in rodent and human water maze learning. Proc Natl Acad Sci U S A 2013; 110(8): 3131–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du SQ, Wang XR, Xiao LY, et al. Molecular mechanisms of vascular dementia: what can be learned from animal models of chronic cerebral hypoperfusion? Mol Neurobiol 2017; 54(5): 3670–3682. [DOI] [PubMed] [Google Scholar]
- 67.Nishio K, Ihara M, Yamasaki N, et al. A mouse model characterizing features of vascular dementia with hippocampal atrophy. Stroke 2010; 41(6): 1278–1284. [DOI] [PubMed] [Google Scholar]
- 68.Goodroe SC, Starnes J, and Brown TI.. The complex nature of hippocampal-striatal interactions in spatial navigation. Front Hum Neurosci 2018; 12: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolf A, Bauer B, Abner EL, et al. Comprehensive Behavioral Test Battery to Assess Learning and Memory in 129S6/Tg2576 Mice. PLoS One 2016; 11(1): e0147733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Celestine J, Tanti A, Aubert A.. Dissociation between performances in water maze and spontaneous alternation in BALB/c versus A/J Mice. J Behav Brain Sci 20122: 156–161. [Google Scholar]
- 71.Leasure JL, Jones M.. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience 2008; 156(3): 456–465. [DOI] [PubMed] [Google Scholar]
- 72.Kelly ÁM. Exercise-Induced Modulation of Neuroinflammation in Models of Alzheimer’s Disease. Brain Plast 2018; 4(1): 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jensen CS, Bahl JM, Østergaard LB, et al. Exercise as a potential modulator of inflammation in patients with Alzheimer’s disease measured in cerebrospinal fluid and plasma. Exp Gerontol 2019; 121: 91–98. [DOI] [PubMed] [Google Scholar]
- 74.Verdelho A, Madureira S, Ferro JM, et al. Physical activity prevents progression for cognitive impairment and vascular dementia: results from the LADIS (Leukoaraiosis and Disability) study. Stroke 2012; 43(12): 3331–3335. [DOI] [PubMed] [Google Scholar]
- 75.Aarsland D, Sardahaee F, Anderssen S, et al. Is physical activity a potential preventive factor for vascular dementia? A systematic review. Aging Ment Health 2010; 14(4): 386–395. [DOI] [PubMed] [Google Scholar]
- 76.Feter N, Spanevello RM, Soares MS, et al. How does physical activity and different models of exercise training affect oxidative parameters and memory? Physiol Behav 2019; 201: 42–52. [DOI] [PubMed] [Google Scholar]
- 77.Małkiewicz MA, Szarmach A, Sabisz A, et al. Blood-brain barrier permeability and physical exercise. J Neuroinflammation 2019; 16(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manzanares G, Brito-da-Silva G, Gandra PG.. Voluntary wheel running: patterns and physiological effects in mice. Braz J Med Biol Res 2018; 52(1): e7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dirnagl U. Statistics in experimental stroke research: From sample size calculation to data in rodent models of stroke. Neuromethods 2010; 47: 249–262. [Google Scholar]
- 80.Baron JC, Macrae IM, Adams HP Jr., et al. ESC-BRAIN: experimental and clinical stroke research–do they connect? Meeting report of the ESC-BRAIN joint symposium held in London and Shanghai in May 2013. Cerebrovasc Dis 2013; 36(4): 306–321. [DOI] [PubMed] [Google Scholar]
- 81.Barde MP and Barde PJ.. What to use to express the variability of data: Standard deviation or standard error of mean? Perspect Clin Res 2012; 3(3): 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flurkey K, Currer JM, and Harrison DE.. Mouse models in aging research. In: Fox J, Davisson M, Quimby F, Barthold S, Newcomer CE, and Smith A editors. The mouse in biomedical research . Burlington: Academic Press;2007. [Google Scholar]
- 83.Román GC. Vascular dementia may be the most common form of dementia in the elderly. J Neurol Sci 2022; 203: 7–10. [DOI] [PubMed] [Google Scholar]
- 84.Akhter F, Persaud A, Zaokari Y, et al. Vascular dementia and underlying sex differences. Front Aging Neurosci 2021; 13: 720715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Korevaar D, Hooft L, and ter Riet G.. Systematic reviews and meta-analyses of preclinical studies: publication bias in laboratory animal experiments. Lab Anim 2011; 45: 225–230. [DOI] [PubMed] [Google Scholar]
- 86.ter Riet G, Korevaar D, Leenaars M, et al. Publication bias in laboratory animal research: a survey on magnitude, drivers, consequences and potential solutions. PLoS One 2012; 7: e43404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peters JL, Sutton AJ, Jones DR, et al. Contourenhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 2008; 61: 991–996. [DOI] [PubMed] [Google Scholar]


