Abstract
The African Research Group for Oncology (ARGO) was formed in 2013 to undertake methodologically rigorous cancer research in Nigeria, and to strengthen cancer research capacity in the country through training and mentorship of physicians, scientists, and other healthcare workers. Here, we describe how ARGO’s work in colorectal cancer (CRC) has evolved over the past decade. This includes the consortium’s scientific contributions to the understanding of CRC in Nigeria and globally and its research capacity-building program.
Keywords: cancer research, colorectal cancer, global oncology, Nigeria, research collaborative
1 |. INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer worldwide, and a growing challenge for countries and health systems. The total number of global CRC cases is predicted to increase from 1.9 million new cases in 2020 to 3.2 million new cases by 2040.1 While CRC incidence and mortality rates have stabilized and declined in many high-income countries (HICs), the incidence and mortality are rising in many low- and middle-income countries (LMICs), including in western Africa.2 Currently, there is marked variation in overall survival (OS) after a cancer diagnosis between countries and World Bank income groups2: 5-year OS after a CRC diagnosis in the United States is 65%,3 compared to ~22% in Nigeria.4 Advanced stage at presentation due to low access to timely and affordable screening, early detection, diagnosis, and treatment are thought to explain much of this difference.5–7 However population-, country-, and context-specific knowledge of cancer biology, epidemiology, and outcomes are lacking in many LMICs.5,7 In the absence of context-specific research, evidence, and treatment paradigms from HICs—often from predominantly European-ancestry populations—have been used to inform guidelines for oncology patients globally, including LMICs such as Nigeria.7,8 HIC data are often not applicable outside the populations and environments in which they were generated. For CRC specifically, patients in Nigeria typically present at a younger median age,9 with advanced stage disease,9 and a unique pattern of clinical and molecular features10,11 compared to North American and European populations, all of which impact treatment needs and responses.10 Moreover, patients seek and receive care in a health system with a highly variable range of resources and capabilities and face differing geographic, financial, and sociocultural barriers to accessing care compared to the majority of patients in HICs.7,12
Recognizing the importance of context-specific knowledge about cancer risk factors, epidemiology, early detection, treatment, and outcomes,7,13 and the need to build local and national cancer research capacity8 the African Research Group for Oncology (ARGO) was formed in 2013. ARGO’s founding mission was to improve cancer outcomes in the country and the wider sub-Saharan African region through research and training.13 The relationship underpinning ARGO was forged when the Nigerian founding partner (O. I. A.), a surgical oncologist, spent 3 months as a Soudavar Traveling Fellow at Memorial Sloan Kettering Cancer Center (MSKCC), New York, USA. The professional relationships developed during the Fellowship led to a reciprocal trip to Obafemi Awolowo University Teaching Hospital (OAUTH) in Ife, Nigeria by MSKCC colleagues, including the founding US partner (T. P. K.), also a surgical oncologist. The observed differences between the US and Nigerian patient populations in CRC clinical presentation, response to treatment, and outcomes were documented in a collaborative research paper between the two institutions,9 and acted as the starting point of many discussions on further research questions, needs, and priorities. This prompted the establishment of a formal research and training collaboration between OAUTH in Nigeria and MSKCC in the United States.8,14
Today, ARGO is a collaborative, multi-institutional, international cancer research consortium (Figure 1), which is recognized by the National Cancer Institute (NCI, USA). ARGO aims to generate methodologically rigorous, regionally representative evidence on cancer epidemiology and risk factors, prevention and early detection, diagnosis, treatment, and survivorship (Figures 2 and 3) and to strengthen research capacity in Nigeria through education, training, and mentorship of physicians, scientists, and other healthcare workers.15 What began as a partnership between two institutions (OAUTH in Ife, Nigeria and MSKCC, New York, USA) has over the past decade grown to include 28 member institutions across Nigeria, alongside three member institutions and four collaborating partners in North America (Supporting Information: Appendix). ARGO members are supported by an established research infrastructure, biobanks, and clinical sites for research and training and over $8 million in competitive research and training funding.
FIGURE 1.
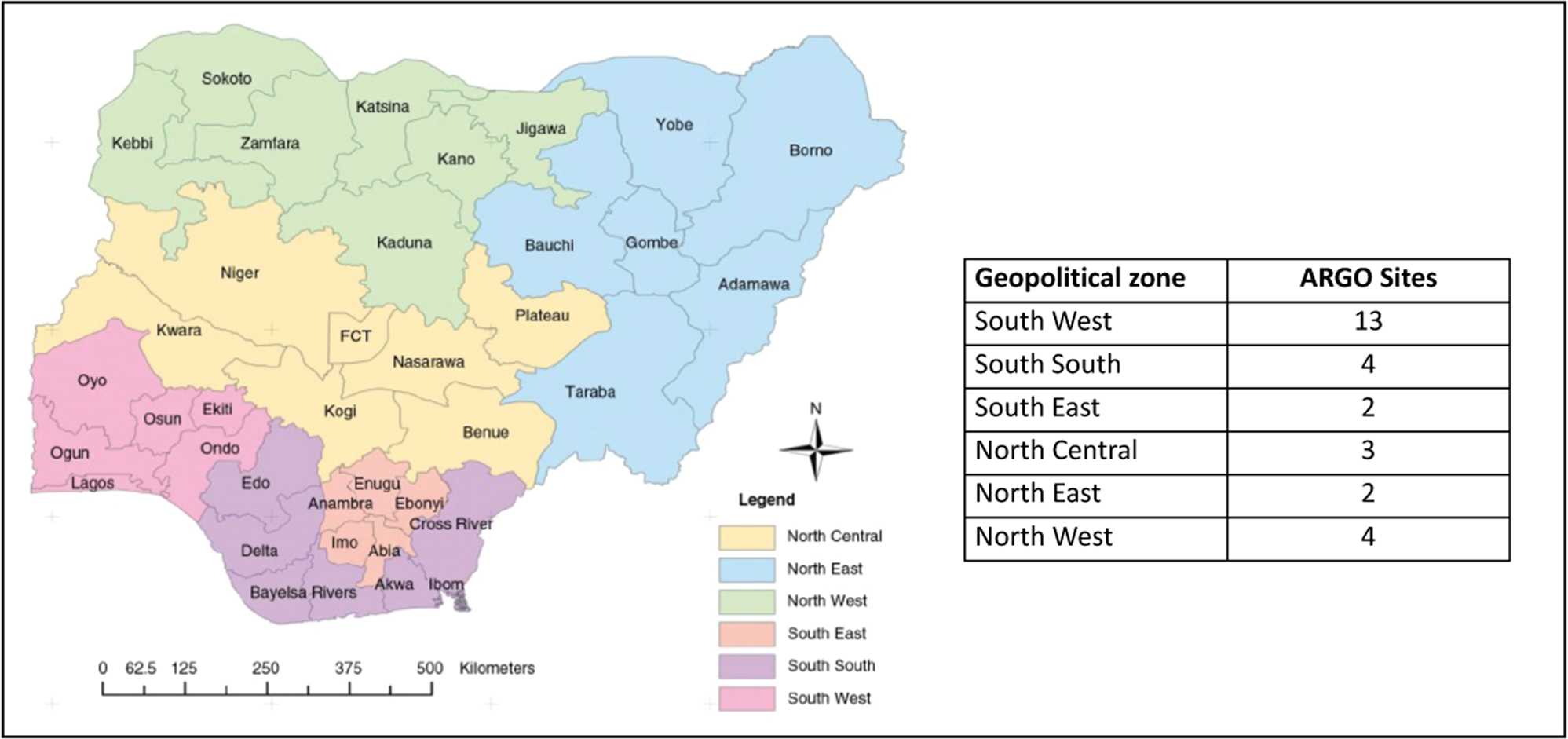
African Research Group for Oncology (ARGO) members, by state and geopolitical zone in Nigeria, 2023. The full list of Nigerian and North American ARGO institutions, by state and geopolitical zone is listed in Supporting Information: Appendix 1.
FIGURE 2.
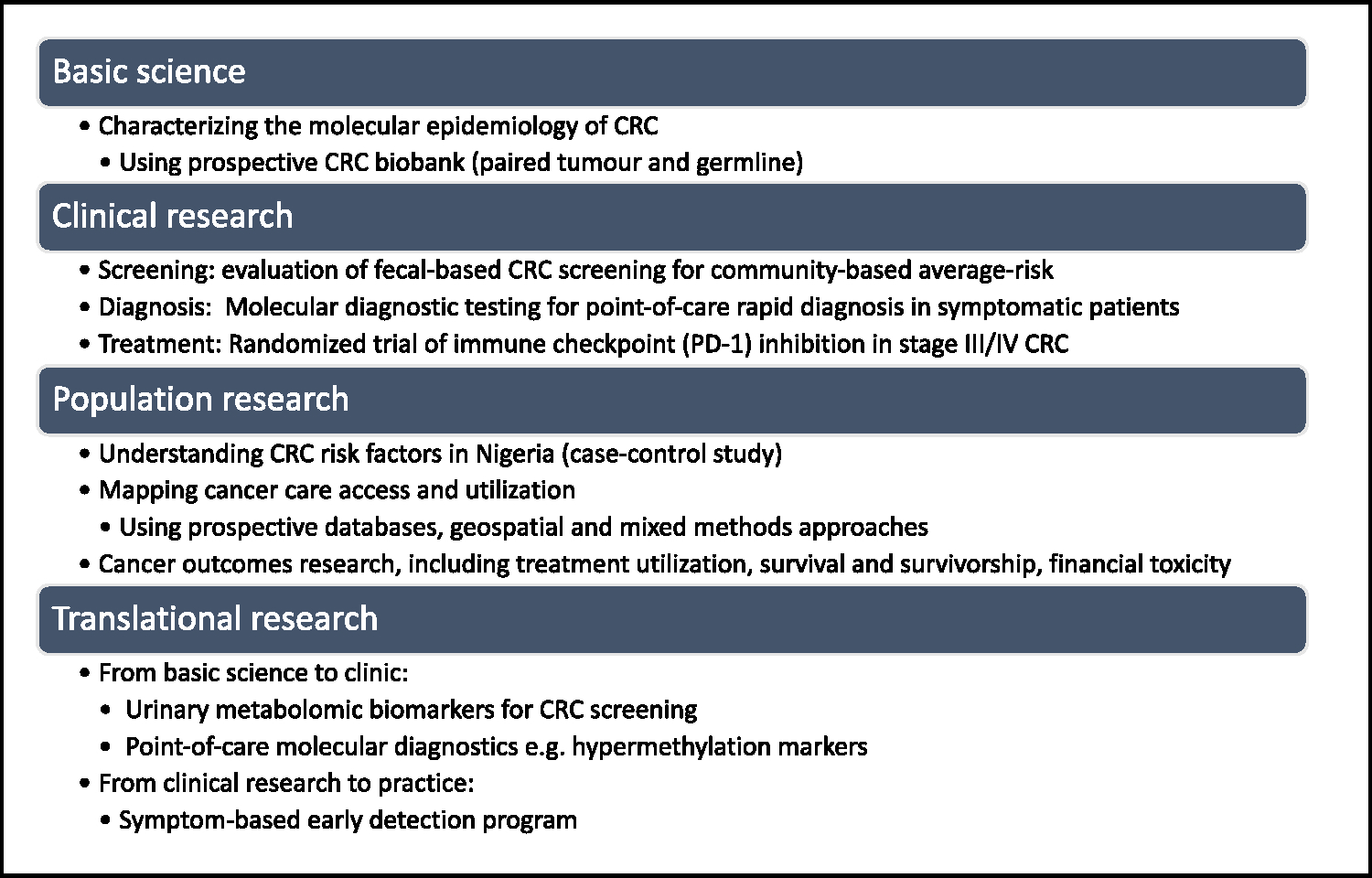
Mapping African Research Group for Oncology colorectal cancer (CRC) research activity to the four broad categories of cancer research.
FIGURE 3.
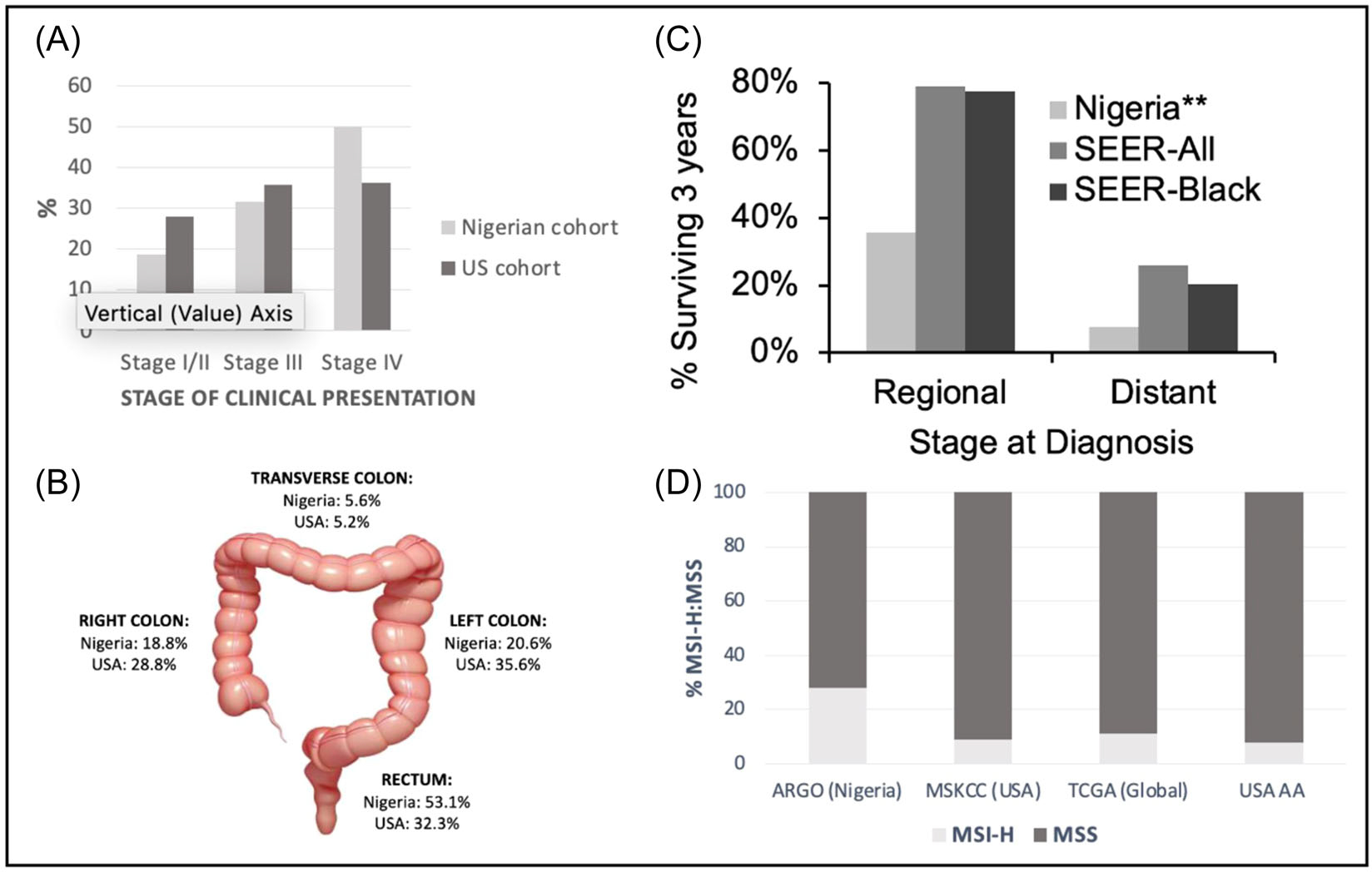
Select key findings on the clinical and molecular epidemiology of colorectal cancer in Nigeria. ARGO’s first paper demonstrated that the stage of colorectal cancer presentation (A), and anatomic site of disease (B) were significantly different between a southwest Nigerian (n = 160) and a single institution North American cohort (n = 1947). Survival at 3 years after colorectal cancer diagnosis was also markedly different compared in Nigeria compared to the Surveillance Epidemiology and End Results (SEER) US database, for both regional and distant disease and on subgroup analysis (SEER-Black) (C). **Significantly different than both the seer-all and seer-black groups. (D) More recently, ARGO has demonstrated that the proportion of microsatellite instability-high (MSI-H) in a Nigerian cohort is significantly higher than in other North American (MSKCC) and global (TCGA) genomic datasets, including when compared to African American (AA) populations in the United States (drawn from MSKCC and TCGA combined genomic datasets). ARGO, African Research Group for Oncology; MSKCC, Memorial Sloan Kettering Cancer Center; MSS, microsatellite stable; TCGA, The Cancer Genome Atlas.
In this article, we describe how ARGO’s work in CRC has evolved over the past decade. This includes the consortium’s scientific contributions to understanding CRC in Nigeria and globally and its training and research capacity-building program which has been developed in parallel.
2 |. DEVELOPING A CRC RESEARCH AGENDA
CRC has been a primary focus of ARGO’s research agenda since its inception.16 This focus reflects the rising incidence of CRC within Nigeria16; it’s inclusion as a priority within Nigeria’s National Cancer Control Plan17; the paucity of clinical, molecular, and population-based CRC research within the country and the wider West African subregion12; and the institutional expertise of ARGO collaborators.9 CRC is in the top five causes of cancer death in Nigeria, with a lower median age of diagnosis than in HICs, a rising yearly incidence, and high mortality: incidence ratio.10,18 Geographic access to CRC diagnostics and treatment at a population level is low and most patients pay out-of-pocket for cancer services including treatment.14,19
At the time the ARGO Consortium was established, relatively little was known about the epidemiology of CRC in Southwest Nigeria, clinical presentation, healthcare utilization patterns, barriers to care, and oncologic outcomes. Single-center retrospective experiences in Nigeria, typically with small patient numbers over relatively long time periods, had compromised the majority of a sparse literature in the late 20th and early 21st century. A need to move beyond this approach to better understand CRC patterns in Nigeria was a key motivation for establishing ARGO and fostering a multicenter partnership in Nigeria. Putting in place the research infrastructure to prospectively collect sociodemographic and clinical information represented a natural starting point for the consortium, required only modest seed funding, and leveraged expertise in developing and maintaining clinical databases at MSKCC, the North American partner. At the same time, increasing recognition of different molecular subtypes of CRC, and their clinical importance including targetable mutations, was occurring at a global level.20 Most of this work was based on high-income, predominantly European ancestry populations.12,20 Recognizing the utility of linking clinical information to molecular data to both better understand fundamental cancer biology in West African populations, inform prognostic and clinical decision-making making, and identify treatment opportunities, the consortium also worked to establish a CRC biobank at OAUTH, the primary Nigerian site, linked to the clinical databases. This provided a foundational agenda around which to build relevant infrastructure, education, and training opportunities and build research capacity.
Funding for ARGO’s research has occurred in three phases. The initial phase was obtaining pilot grants from the NCI Center for Global Health and internal MSKCC (US founding partner) funds. The next phase in funding was obtaining philanthropic funds to expand to larger multi-institutional studies. This allowed ARGO to generate enough preliminary data to apply for and obtain larger National Institutes of Health (NIH) grants (R01, UG3/UH3, D43), while continuing to receive philanthropic and MSK support. This stepwise approach allowed for the steady expansion of the ARGO research team. The team initially had one research assistant (RA) at its hub site OAUTH in 2013 and has grown to over 30 RAs based across eight Nigerian institutions.
3 |. UNDERSTANDING CRC RISK FACTORS, PRESENTATION, MANAGEMENT, AND OUTCOMES IN NIGERIA
Over the past decade, the ARGO Consortium has sought to characterize patterns of CRC presentation and to better define sociodemographic characteristics of patients, their clinical trajectory, and outcomes. To date, ARGO investigators have established >13 prospective cancer databases and led 14 prospective cohort studies to characterize CRC presentation, management, and outcomes across Nigeria. This work was initially concentrated in Osun State, southwest Nigeria, in an ethnically homogenous population (Yoruba), based out of OAUTH (the founding Nigerian academic partner). The databases now include geographic, demographic, ethnic, and socioculturally diverse cohorts of CRC patients across all six geopolitical zones in the country, as more hospitals and investigators have joined ARGO and established data collection and reporting processes.
Key findings from this work have been the younger age of CRC onset among Nigerian patients compared to in the United States and other HICs (even after accounting for differences in demography),10 more advanced disease stages at presentation,10 a higher rate of rectal primary tumors,11,12 higher rates of peritoneal only metastases but lower rates of liver-only metastases,10 and an increased prevalence of mucinous differentiation.12,21 OS, stage-for-stage, is worse among Nigerian patients, especially those presenting emergently, and those who did not receive recommended multimodality treatment.4,11 In one prospective series of 535 CRC patients presenting to ARGO centers between 2013 and 2020, the median OS was 13.3 months (95% confidence interval [CI]: 10.5–15.5 months), and 46% of patients presented with stage IV disease.4 Even among patients presenting with stage I/II disease, the median OS was 34.3 months, considerably lower than that reported in HIC cohorts.
Survival analyses have shown that lower access to, and utilization of multimodal treatment in Nigeria, contributes to some of the survival discrepancies seen. For example, among 300 CRC patients whose treating physician recommended multimodal management, 71% received a surgical operation, 50% received chemotherapy as recommended, and 4% received radiation as recommended.11 Financial barriers were the main reason cited for not initiating or completing surgical and systemic treatment as recommended.11 In addition to cost, geographic access to radiation facilities (out of state) was a major factor in low radiation utilization. Analyses of out-of-pocket expenditure by breast cancer patients in the ARGO network have demonstrated that 95% and 86% of households experience catastrophic health expenditure (at a threshold of 10% and 25% of annual income, respectively) paying for cancer care22; although not specific to CRC, these results reflect the financial toxicity associated with multimodal cancer treatment in Nigeria.
Nationally-representative, population-based cancer registry data is lacking in Nigeria; however, hospital-based and local cancer registry data demonstrate that the incidence of CRC is rising in Nigeria, similar to other African middle-income countries.18 Rising CRC incidence in transitioning economies is often attributed to changes in lifestyle and diet that more closely resemble those found in many HICs.23 However, population-specific epidemiologic risk factors for CRC remain poorly characterized in Nigeria. Variation in the clinical patterns of disease, and differences in underlying cancer biology prompted ARGO researchers to consider whether different epidemiologic risk factors exist for CRC in Nigeria, and whether these may be modifiable. To answer this, investigators are undertaking a case-control study of 600 Nigerian CRC patients and 1200 cancer-free hospital-based controls recruited from six ARGO centers in three of the six geopolitical zones in Nigeria, namely the south west, north central, and north east. A subset of cases will undergo somatic and germline molecular profiling using next-generation sequencing (NGS), to better understand the links between epidemiologic and molecular cancer risk factors in southwest Nigeria, supplementing other molecular epidemiology studies the collaborative has undertaken (see below). This work is funded by an NIH NCI R01 (research project) grant. The study is still accruing.
4 |. FINDING CANCERS EARLIER: EXAMINING THE ROLE OF CRC EARLY DETECTION AND SCREENING
Delays in seeking care and receiving a timely diagnosis have been identified as contributors to advanced CRC presentation in the ARGO prospective cancer database analyses, and in mixed-methods patient and provider surveys.4,11,14,24 Cancer knowledge and general health literacy are variable and often low in parts of Nigeria, correlating with general education levels.24 An ARGO survey published in 2017 of patients with rectal bleeding, and primary and secondary health care providers in Nigeria highlighted that the majority (60%) of patients referred for colonoscopy for rectal bleeding did not know this could be caused by cancer.24 Failure to recognize the symptom as problematic was a leading reason for the delay in seeking care (~40% of those surveyed), followed by financial barriers (~22%). On the healthcare provider side, although physicians regularly saw patients with rectal bleeding most provided a diagnosis of hemorrhoids, and few referred patients for colonoscopy even when they had more than one risk factor for CRC.24
ARGO’s research findings demonstrating delays in cancer diagnosis and their contributions to advanced presentation, along with research prioritization discussions led the group to focus on the role and feasibility of CRC screening and early detection programs. Several strategies have been investigated, building iteratively on knowledge generated through the group’s research efforts. These include clinical risk stratification tools for primary care providers,25 stool-based screening with qualitative fecal immunohistochemical testing (FIT),26,27 a novel urinary metabolomics approach to identify a CRC biomarker panel,28 development of a point-of-care molecular diagnostics test using methylated DNA markers,29 and a state-wide early detection program at the primary and secondary care levels.
Between 2014 and 2016, a symptom-based clinical risk prediction score for CRC was developed for Nigerian patients aged >45 years with rectal bleeding.25 Rectal bleeding is a relatively common symptom in Nigeria, with hemorrhoids as a leading benign cause.26 Given the very limited endoscopic capacity particularly outside of urban centers (~150 endoscopy providers serve a population of 215 million people, Olusegun I. Alatise [personal communication August 1, 2023]), differentiating between rectal bleeding likely to be from benign causes and that worrisome for malignancy requiring further investigation is important. Using a training and validation cohort of patients from three ARGO centers, and logistic regression methods, rectal bleeding in combination with weight loss and change in bowel habit significantly predicted CRC (odds ratio [OR]: 12.8; 95% CI: 4.6–35.4, p < 0.001), with 56% of patients with these three symptoms in the validation cohort having a diagnosis of CRC, compared to 2% of patients with bleeding as the sole symptom.25 Most recently, this work has informed the development of an early diagnosis pathway which will be piloted at the state level in primary and secondary care facilities, in collaboration with the Osun State Ministry of Health. The program will use patient navigators to link high-risk patients to an early diagnosis clinic at the tertiary hub site.
Population-based screening has been shown to reduce CRC incidence and mortality in several HICs.30,31 In these settings screening is feasible and cost-effective using fecal-based and/or endoscopic screening modalities. There has been growing interest in the role of CRC screening in middle-income countries, where CRC incidence is rising and late presentation is common.5 The World Health Organization does not currently recommend organized or opportunistic screening for CRC outside of HICs,32 however there has been public and political interest in CRC screening in many LMICs including Nigeria.33 The Nigerian Cancer Control Plan (2018–2022) identified CRC screening as a priority and endorsed the establishment of a national screening program, identifying stool-based screening as the most appropriate method because of scarce endoscopic resources.17 The Society for Gastroenterology and Hepatology in Nigeria supports the use of FIT-based screening while recognizing the lack of context-specific evidence.34 Most CRC screening in Nigeria is opportunistic.33 The target age range, most appropriate screening test, time interval for screening, health system readiness, and cost-effectiveness of CRC screening for Nigeria—and across sub-Saharan Africa—are ill-defined. To evaluate the performance of fecal-based CRC testing, and understand acceptability, feasibility, and cost-effectiveness in the Nigerian context, ARGO undertook a single-center pilot study,27 followed by a larger community-based, cross-sectional study of qualitative FIT-based screening for CRC.26 The latter study recruited 2330 participants from the community in 2021, who were average-risk (asymptomatic, no personal or family history of CRC), aged 45–75 years, and residing in the southwest (Osun, Lagos) and north central (Kwara) regions. A high proportion of returned FIT screens were positive (21%, n = 432/2109, 95% CI: 20–21), but on endoscopic evaluation, most of these were false positive for cancer often in the setting of benign causes of lower gastrointestinal (GI) bleeding (e.g., hemorrhoids). This generated a high endoscopic follow-up burden, for a low positive predictive value (PPV) of the screening test. PPV was 1.1% (95% CI: 0.3–3.3) for invasive CRC and 7.0% (95% CI: 4.5–10.8) for advanced adenoma. In contrast, the reported PPV for CRC after average-risk FIT testing in HICs is at least 5%, and for advanced adenoma detection can be up to 54%. CRC detection rates after average-risk FIT screening were also three to five times higher in HICs than shown in Nigeria. Participantr-eported acceptability of FIT screening was high, and colonoscopy follow-up participation rates were comparable to those in HICs (two-thirds of participants with a FIT-positive screen attended colonos-copy follow-up). Willingness to pay thresholds was much lower than the cost of the screening test. The cost of a colonoscopy to investigate a positive result (which was covered by the study) was equivalent to half of the median monthly household income reported. These are all important considerations in a country where cancer screening and follow-up are typically paid for out-of-pocket. The study highlighted again to ARGO members the importance of using country-level and context-specific data on CRC screening modalities, because key performance characteristics may not translate across diverse populations.
Recognizing the challenges of using fecal-based hemoglobin testing for CRC in a setting with high rates of benign causes for GI bleeding, and in light of the poor performance of average risk screening with FIT, ARGO members sought to develop novel screening technologies and point-of-care testing for CRC using metabolomic and epigenetic based approaches. With scientists in Canada and the United States, a urine metabolomic-based biomarker panel for the detection of CRC has been developed and is under investigation in Nigeria. Two urine-based metabolites, diacetylspermine and kynurenine have demonstrated high specificity (80.0%) and sensitivity (80%) for detecting CRC in North American populations,28 and are now being further investigated using urine samples from CRC patients in the ARGO biobanks.
Another approach using hypermethylated biomarkers for early CRC detection in patients presenting with rectal bleeding is an area of active investigation through an academic-industry collaboration between US- and Nigerian-based ARGO partners (led by OAUTH, Johns Hopkins, and MSKCC) and Cepheid, a molecular diagnostics company. Epigenetic alterations are drivers of tumorigenesis in all cancer types, and specific genes that become aberrantly methylated uniquely, early, and frequently in cancer provide excellent biomarkers for cancer detection, prognosis, and prediction of therapeutic response.29 The collaborative and partners aim to develop an inexpensive point-of-care CRC molecular diagnostic assay based on quantitative measurement of tumor-specific methylated DNA in plasma, and then technically validate this in primary and secondary care settings in Nigeria in patients presenting with rectal bleeding and at least one additional risk factor for CRC. Improved differentiation of high- and low-risk rectal bleeding is especially important in Nigeria given the very limited colonoscopy capacity for full endoluminal evaluation and the frequency of benign lower GI bleeding in the same age groups as those in which CRC is also diagnosed. The study findings will provide data needed to move towards future product development by Cepheid, which has already successfully deployed point of care molecular diagnostics at scale for other global health challenges including human immunodeficiency virus, tuberculosis, COVID, and estrogen receptor/progesterone receptor/human epidermal growth factor receptor 2 testing for breast cancer.35
5 |. UNRAVELING THE MOLECULAR BASIS OF CRC IN NIGERIA: FROM CANCER BIOLOGY TO CLINICAL TRIAL
Implementing cancer-control strategies in a limited-resource setting such as Nigeria demands knowledge of disease biology to optimize diagnostic and treatment pathways, minimize ineffective clinical interventions, reduce patient and system-level costs, and improve cancer outcomes. However, the natural history and molecular basis of CRC are poorly characterized in West African populations. Over the past decade, the importance of addressing this in ARGO’s basic science and clinical research has become apparent, as globally molecular subtypes of CRC are increasingly relevant to prognosis and treatment selection. Several ARGO members are active in delineating CRC molecular epidemiology in Nigeria. Early work demonstrated higher rates (34%–43%) of microsatellite instability (MSI) on CRC specimens from Southwest Nigeria than that reported in other high-income, European-ancestry populations.36 Higher incidence of wild-type BRAF and KRAS has also been found in Nigerian CRC patients compared to European-ancestry patients in HICs.37 Most of these studies relied on formalin-fixed, paraffin-embedded specimens that can be difficult to examine accurately with IHC in low resource environments,12 owing to variable warm ischemia and fixation times, and challenging laboratory conditions (heat, humidity, reagent access). To overcome these challenges, fresh-frozen tumors and blood specimens from CRC patients have been collected in the prospectively maintained ARGO CRC biobank since 2013. These samples are linked to clinical outcome data which is collected during routine 3-monthly follow up.
The ARGO biobank has provided a unique opportunity to perform paired tumor and germline genomic profiling with multigene, NGS. To date, this analysis has been performed in the United States using the MSK-IMPACT assay with samples shipped from Nigeria, owing to an absence of local NGS capacity. In 2020, the Consortium reported in Nature Communications that the rate of MSI (determined using both NGS and IHC approaches on fresh frozen specimens) is significantly higher among Nigerian CRC patients (28%) than those in The Cancer Genome Atlas (14%) and a matched patient cohort at MSKCC (9%).12 Nigerian CRC patients with microsatellite-stable tumors were significantly less likely to have APC mutations compared to the North American cohort (39% vs. 76%), less likely to have WNT pathway alterations (48% vs. 82%), and significantly more likely to have RAS pathway alteration (76% vs. 60%).12 These findings suggest that the dominant biological pathways underpinning CRC in Nigeria are different from those seen in HIC, predominantly European-ancestry populations on which the majority of our current treatment paradigms are based. This may explain the clinical variation seen between Nigerian and US patient cohorts in the age of CRC onset, anatomic site, and metastatic pattern, as well as differences in polyp burden.10 The finding of a higher proportion of microsatellite instability-high (MSI-H) tumors also has immediate clinical applications. MSI-H is a prognostic marker, that identifies patients more likely to possess germline mutations and is associated with poor response to conventional cytotoxic chemotherapy.38 These findings highlight the importance of subnational, national, and regional clinical and molecular evidence to appropriately inform the development of cancer guidelines and treatment pathways in Nigeria, and other LMICs.
Immune checkpoint blockade, including PD-1 blockade (referred to as “immunotherapy”) has been shown to be highly effective as a systemic treatment (including as first line) for patients with MSI-H metastatic CRC in high-income settings, with clinically significant response durability, fewer adverse events and prolonged OS compared to conventional (5-fluorouracil based) chemotherapy.38,39 It has not been evaluated in a low-resource setting such as Nigeria, although global access programs exist that could be utilized to reduce drug costs if they were shown to be safe and effective. Around half of all CRC patients who present to ARGO centers in Nigeria do so with metastatic disease,11 and a higher proportion may benefit from immune checkpoint blockade than in HICs given the higher rates of MSI-H disease.12 These findings prompted discussion several years ago among ARGO members about the possibility of a PD-1 blockade clinical trial in the metastatic setting in Nigeria. In 2022, researchers at MSKCC published a landmark study in the New England Journal of Medicine assessing the role of single-agent PD-1 blockade immunotherapy in patients with locally advanced MSI-H rectal cancer in North America.40 This showed that first-line treatment with PD-1 blockade (in this case, the single agent monoclonal antibody dorstarlimab, administered every 3 weeks for 6 months) resulted in a complete clinical response, with no evidence of tumor on clinical exam, endoscopic evaluation, biopsy, or multimodality imaging, allowing patients to omit neoadjuvant chemoradiation and surgical resection. While the long-term durability of this response is still being followed, it represents a major scientific and therapeutic advance for the (relatively small) proportion of patients in HICs with locally advanced MSI-H tumors. In Nigeria, the finding is potentially even more impactful given the proportion of MSI-H CRC tumors is closer to 30%. Access to multimodality therapy for CRC is also much lower in Nigeria, and so a single-agent treatment for locally advanced rectal cancer that could obviate the need for chemotherapy, radiation, and surgery would remove many existing access barriers—if it were available, affordable, safe to administer, and monitor and clinically efficacious in this setting.
The diffusion of scientific innovations and new therapies for cancer from HICs to LMICs such as in Nigeria has typically taken decades. Recognizing the unique opportunity to ensure cutting-edge science and a potential major therapeutic breakthrough could be rapidly translated to, and evaluated in an appropriate lower-resource country, ARGO partners have committed to delivering a pragmatic randomized clinical trial of PD-1 blockade in MSI-H locally advanced rectal cancer, and metastatic CRC in Nigeria. This has been designed as an investigator-initiated trial. The PD-1 inhibitor will be provided by a pharmaceutical company partner, with the broader trial costs supported through institutional and philanthropic funds. ARGO has partnered with Bioventures for Global Health to support in-country trial delivery, including regulatory requirements, as well as longer-term market access options should the trial show a positive outcome. Recruitment of patients is planned to commence in late 2023. If PD-1 blockade has a similar clinical impact in the management of locally advanced and metastatic CRC in Nigeria’s MSI-H patients as it has in North America and Europe, and is safe and feasible in terms of drug delivery, monitoring, and longer-term surveillance, this will represent important proof of principle of the power of long-term, locally embedded, funded research collaborations to advance our understanding of cancer biology, epidemiology and treatment in Nigeria, and other LMICs.
6 |. THE NEXT GENERATION: BUILDING CRC RESEARCH CAPACITY IN NIGERIA
ARGO was founded following an international educational exchange for academic surgical oncologists, and education and research training have been the foundations upon which the collaboration has grown and through which it hopes to have a lasting impact on cancer research in Nigeria and globally. Needs assessments undertaken by ARGO members have consistently identified a need and strong desire to improve local capacity for cancer research, a high level of interest in research training, and the importance of multidisciplinary research to tackle the challenges in cancer prevention, diagnosis, and management in Nigeria.41 Establishing reciprocal opportunities for educational and research training exchange (e.g., 3–12 month clinical and research fellowships in both Nigeria and North America) has been an important part of the HIC/LMIC partnership, facilitating the regular exchange of people, ideas, and resources both within and between countries. Since ARGO was established, 18 Nigerian clinicians including surgeons, pathologists, radiologists, and public health specialists have spent time at MSKCC on Soudavar Fellowships (established to fund qualified physicians from around the world to undertake 3 months of educational and clinical observership activities at MSKCC and in which author OIA participated in 2010, catalyzing the establishment of ARGO). Since 2018 MSKCC has offered a year-long Global Cancer Disparities Fellowship for North American surgeons which combines clinical exposure and research training at MSKCC (6 months) and in Nigeria (6 months). A number of other research exchange opportunities exist for Nigerian-based and North-American-based academics and clinicians, including, formal degree and certificate programs (e.g., several ARGO members have participated in the Principles and Practice of Clinical Research postgraduate course offered by the Harvard T.H. Chan School of Public Health, and several NIH-funded postgraduate students have undertaken a period of their research training at ARGO sites in Nigeria).
Research capacity-building efforts initially focused on equipping clinician-investigators with the skills, formal training, and opportunities to succeed in local and international cancer research. However, the collaboration quickly realized the need to also develop strong research teams and infrastructure and has broadened both its training scope and target audiences. This has included providing community, clinical, and research teams affiliated with ARGO with training in data management, ethics and regulatory requirements, quality assurance, finances and budgeting, grant writing and publication, and health systems leadership.9,41
Formal short-term research training and educational programs designed and delivered by ARGO have included a Fundamentals of Clinical Research course, in which select senior trainees and junior faculty within 3 years of completing training from ARGO-affiliated sites undertake an in-person, multiday research training workshop at OAUTHC, supported by longitudinal research mentorship; a 2-day open training event “Improving Clinical Oncology Research Capacity in Sub-Saharan Africa”; the annual ARGO Symposium and ad hoc topic-specific virtual and in-person workshops. In addition, ARGO research managers and RAs have developed collaborative approaches to on-the-job training including upskilling in data management software, budget management, and developing ARGO-wide protocols and training manuals for new research team members.
In 2021, ARGO launched a comprehensive multidisciplinary research training program, the Nigeria Cancer Research Training (NCAT) program supported by an NIH D43 (International Research Training) grant. Academic careers in cancer are challenging in all settings, but in Nigeria—and other LMICs—access to resources such as research funding, online journals, research courses, data software, and other academic infrastructure as well as protected time for research, and longitudinal research mentorship are often lacking. As a result, exposure to cancer research is variable and often low. During previous ARGO-led research courses, 92% of participants reported having no experience with clinical trials, 63% had no experience with epidemiological studies related to cancer, 90% had no previous experience writing grants and 95% had no grant management experience.41 The NCAT program was designed to address some of these challenges and utilizes a US-based funding opportunity designed specifically for international research training. The NCAT program provides focused training and mentorship to Nigerian clinical faculty and to full-time PhD-level researchers using a team-science approach. Each year, seven PhD-level trainees from Nigeria (NCAT fellows) pursue postdoctoral training in cancer epidemiology, behavioral science, or statistics at MSKCC before returning to their cancer research or health-system position in Nigeria. A further 36 Nigerian Clinical Research Scholars (physicians, nurses, allied health, behavioral sciences) annually undertake a year-long cancer research program within Nigeria, using a combination of coursework, mentorship, and the Harvard University online certificate course in clinical research. An option to pursue a funded master ‘s-level degree at a Nigerian institution is also available. Each cohort of NCAT scholars also participates in a longitudinal pilot research project and team-science training.
An early and sustained focus on research capacity building has enabled ARGO to move relatively quickly from single-center retrospective studies to nationally representative, prospective cross-sectional and cohort studies and more recently clinical trials. It has also allowed for translational science research to occur, including through biobanking. Approaches to data analysis, ownership, and stewardship have also evolved across the decade, reflecting increased capacity at a local level to collect, store, analyze, and curate data, and a commitment at the leadership level to data sovereignty. Finding the right balance between performing data analysis and specimen processing where the personnel, equipment, infrastructure, and funding already exist (e.g., in the HIC partner sites) versus developing this in Nigerian partner centers where little or no research infrastructure exists has been an important and ongoing learning for the collaboration. Some work, such as molecular and genomic profiling is still carried out in HICs because of an absence of infrastructure to perform this within Nigeria, and no financially feasible way to build this in the immediate term. Most pathological and radiological assessment is performed in Nigeria, with the option of telemedicine support from partner sites if local expertise is lacking.
7 |. CONCLUSIONS
It has been 10 years since the ARGO was first established. What began as an institutional partnership between one academic hospital in Nigeria and one academic cancer center in the United States has developed into a thriving collaboration involving clinicians and research teams across all geopolitical zones in Nigeria, and several leading North American academic institutions. The collaboration has demonstrated that it is possible to undertake prospective, methodologically rigorous, cancer research at scale in Nigeria, across the cancer control continuum, and from bench to bedside. Partnership with HIC institutions has facilitated access to research funding, infrastructure, and expertise, but the collaborative’s reach and research agenda have been determined and delivered by Nigerian clinicians and investigators. Importantly, key research findings are now being used to inform national and regional clinical guidelines and cancer policy, as well as everyday practice. Insights into cancer biology and molecular epidemiology hold relevance not only for Nigerian cancer patients but also for developing a more representative global understanding of CRC. The CRC early diagnosis studies and the planned immunotherapy trial highlight the importance of population and context-specific cancer research and provide proof of principle that these studies can be undertaken in countries of all income and resource levels. Research capacity building has been at the heart of ARGO’s mission since its inception, and will ensure the early impact the collaborative has had on the global cancer research landscape—in Nigeria and internationally—continues to multiply in the decades ahead.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Memorial Sloan Kettering Cancer Center P30 Cancer Center Support Grant (P30 CA008748) and the Thompson Family Foundation.
Footnotes
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Morgan E, Arnold M, Gini A, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72(2):338–344. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 3.Cancer.Net. Colorectal cancer—statistics [Internet]. 2012. Accessed August 1, 2023. https://www.cancer.net/cancer-types/colorectal-cancer/statistics
- 4.Aderibigbe A, Dare A, Knapp G, Alatise O, Kingham TP. Abstract 111: colorectal cancer presentation and survival outcomes in Nigeria: a prospective multi-centre cohort study of 543 patients. Cancer Epidemiol Biomarkers Prevent. 2021;30(7_suppl):111. [Google Scholar]
- 5.Dare AJ, Knapp GC, Romanoff A, et al. High-burden cancers in middle-income countries: a review of prevention and early detection strategies targeting at-risk populations. Cancer Prev Res. 2021;14(12):1061–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelband H, Sankaranarayanan R, Gauvreau CL, et al. Costs, affordability, and feasibility of an essential package of cancer control interventions in low-income and middle-income countries: key messages from disease control priorities, 3rd edition. Lancet. 2016;387(10033):2133–2144. [DOI] [PubMed] [Google Scholar]
- 7.Ngwa W, Addai BW, Adewole I, et al. Cancer in sub-Saharan Africa: a lancet oncology commission. Lancet Oncol. 2022;23(6):e251–e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pramesh CS, Badwe RA, Bhoo-Pathy N, et al. Priorities for cancer research in low- and middle-income countries: a global perspective. Nature Med. 2022;28(4):649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer SE, Alatise OI, Komolafe AO, et al. Establishing a cancer research consortium in low- and middle-income countries: challenges faced and lessons learned. Ann Surg Oncol. 2017;24(3):627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saluja S, Alatise OI, Adewale A, et al. A comparison of colorectal cancer in Nigerian and North American patients: is the cancer biology different? Surgery. 2014;156(2):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Alatise OI, Adisa AO, et al. Treatment of colorectal cancer in sub-Saharan Africa: results from a prospective Nigerian Hospital Registry. J Surg Oncol. 2020;121(2):342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alatise OI, Knapp GC, Sharma A, et al. Molecular and phenotypic profiling of colorectal cancer patients in West Africa reveals biological insights. Nat Commun. 2021;12(1):6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingham TP, Alatise OI. Establishing translational and clinical cancer research collaborations between high- and low-income countries. Ann Surg Oncol. 2015;22(3):741–746. [DOI] [PubMed] [Google Scholar]
- 14.Sharma A, Alatise OI, O’Connell K, et al. Healthcare utilisation, cancer screening and potential barriers to accessing cancer care in rural South West Nigeria: a cross-sectional study. BMJ Open. 2021;11(7):e040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ARGO. African Research Group for Oncology (ARGO) [Internet]. 2023. Accessed August 1, 2023. https://www.argo-research.org/
- 16.Irabor D, Adedeji OA. Colorectal cancer in Nigeria: 40 years on. A review. Eur J Cancer Care. 2009;18(2):110–115. [DOI] [PubMed] [Google Scholar]
- 17.ICCP. National Cancer Control Plan 2018–2022—Nigeria [Internet]. Federal Ministry of Health, Government of Nigeria. 2023. Accessed August 1, 2023. https://www.iccp-portal.org/system/files/plans/NCCP_Final%20%5B1%5D.pdf [Google Scholar]
- 18.Awedew AF, Asefa Z, Belay WB. Burden and trend of colorectal cancer in 54 countries of Africa 2010–2019: a systematic examination for Global Burden of Disease. BMC Gastroenterol. 2022;22(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knapp GC, Tansley G, Olasehinde O, et al. Mapping geospatial access to comprehensive cancer care in Nigeria. J Glob Oncol. 2019;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdulkareem FB, Abudu EK, Awolola NA, et al. Colorectal carcinoma in lagos and sagamu, southwest Nigeria: a histopathological review. World J Gastroenterol. 2008;14(42):6531–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knapp GC, Wuraola FO, Olasehinde O, Romanoff A, Kingham PT, Alatise OI. The out-of-pocket cost of breast cancer care at a public tertiary care hospital in Nigeria: an exploratory analysis. Pan Afr Med J. 2022;41:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bishehsari F Epidemiological transition of colorectal cancer in developing countries: environmental factors, molecular pathways, and opportunities for prevention. World J Gastroenterol. 2014;20(20):6055–6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alatise OI, Fischer SE, Ayandipo OO, Omisore AG, Olatoke SA, Kingham TP. Health-seeking behavior and barriers to care in patients with rectal bleeding in Nigeria. J Glob Oncol. 2017;3(6):749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alatise OI, Ayandipo OO, Adeyeye A, et al. A symptom-based model to predict colorectal cancer in low-resource countries: results from a prospective study of patients at high risk for colorectal cancer. Cancer. 2018;124(13):2766–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alatise OI, Dare AJ, Akinyemi PA, et al. Colorectal cancer screening with fecal immunochemical testing: a community-based, cross-sectional study in average-risk individuals in Nigeria. Lancet Global Health. 2022;10(7):e1012–e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knapp GC, Alatise O, Olopade B, et al. Feasibility and performance of the fecal immunochemical test (FIT) for average-risk colorectal cancer screening in Nigeria. PLoS One. 2021;16(1):e0243587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng L, Ismond K, Liu Z, et al. Urinary metabolomics to identify a unique biomarker panel for detecting colorectal cancer: a multicenter study. Cancer Epidemiol Biomarkers Prevent. 2019;28(8):1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klein Kranenbarg RAM, Vali AH, IJzermans JNM, et al. High performance methylated DNA markers for detection of colon adenocarcinoma. Clin Epigenetics. 2021;13(1):218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106–1114. [DOI] [PubMed] [Google Scholar]
- 31.Cardoso R, Guo F, Heisser T, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 2021;22(7):1002–1013. [DOI] [PubMed] [Google Scholar]
- 32.WHO. WHO report on cancer: setting priorities, investing wisely and providing care for all [Internet]. 2023. Accessed August 1, 2023. https://www.who.int/publications-detail-redirect/9789240001299
- 33.Knapp GC, Alatise OI, Olasehinde OO, et al. Is colorectal cancer screening appropriate in Nigeria? J Glob Oncol. 2019;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alatise O, Olasehinde O, Olokoba A, et al. Colorectal cancer screening guidelines for Nigeria in 2019. Nigerian J Gastroenterol Hepatol. 2019;11(2):42. [Google Scholar]
- 35.Drain PK, Garrett NJ. The arrival of a true point-of-care molecular assay—ready for global implementation? Lancet Global Health. 2015;3(11):e663–e664. [DOI] [PubMed] [Google Scholar]
- 36.Irabor DO, Oluwasola OA, Ogunbiyi OJ, et al. Microsatellite instability is common in colorectal cancer in native Nigerians. Anticancer Res. 2017;37(5):2649–2654. [DOI] [PubMed] [Google Scholar]
- 37.Abdulkareem FB, Sanni LA, Richman SD, et al. KRAS and BRAF mutations in Nigerian colorectal cancers. West Afr J Med. 2012;31(3):198–203. [PubMed] [Google Scholar]
- 38.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol. 2020;38(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cercek A, Lumish M, Sinopoli J, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med. 2022;386(25):2363–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owoade I, Wuraola F, Olasehinde O, et al. Unveiling research training gaps in oncology: evaluating a research capacity building effort among Nigerian physicians. Niger J Clin Pract. 2022;25(7):1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


