Abstract
Malignant gastric outlet obstruction (mGOO) is a major condition affecting patients with periampullary tumors, including pancreatic cancer. The current treatment options include surgical gastroenterostomy, endoscopic stenting and more recently EUS-guided gastroenterostomy. Most studies comparing the outcomes of the three procedures focus on technical success, clinical success and safety. Several “occult” outcomes relevant to the patient’s viewpoints and perspective may ultimately impact on cancer-related and overall survival, such as body mass composition, nutritional biomarkers, chemotherapy tolerance and patient-reported quality of life. The aim of this review is to provide an overview of potential key outcomes that should be explored in future comparative research around mGOO treatment options.
Keywords: Malignant gastric outlet obstruction, Endoscopic ultrasound-guided gastroenterostomy, Patient-reported outcomes, Body composition, Nutrition, Quality of life
Core Tip: Gastric outlet obstruction (GOO) is a common complication in pancreatobiliary malignancies, with growing research on its surgical and endoscopic management. However, current studies often overlook the patient's perspective and important clinical outcomes. This publication discusses the need to incorporate body mass composition, nutritional status, chemotherapy tolerance, and quality of life into future GOO research to provide a more comprehensive understanding of patient well-being and treatment effectiveness.
INTRODUCTION
Gastric outlet obstruction (GOO) results from the mechanical blockage of the upper digestive tract at the level of the distal stomach, pylorus, or duodenum (Figure 1)[1]. Most cases result from malignant disease (mGOO), especially pancreatic (PC) or duodenal cancer, and are associated with limited survival[2,3]. GOO is responsible for symptoms like vomiting, inability to tolerate oral nutrition and abdominal pain, hence being associated with severe undernutrition[1]. Moreover, GOO might markedly impact quality of life and it may also compromise the effectiveness of cancer treatment because of significant delays in treatment initiation, or because of intolerance to chemotherapy regimens[4-6]. For patients suffering from GOO, regaining an adequate oral intake and a rapid hospital discharge are, therefore, of the utmost importance[2].
Figure 1.
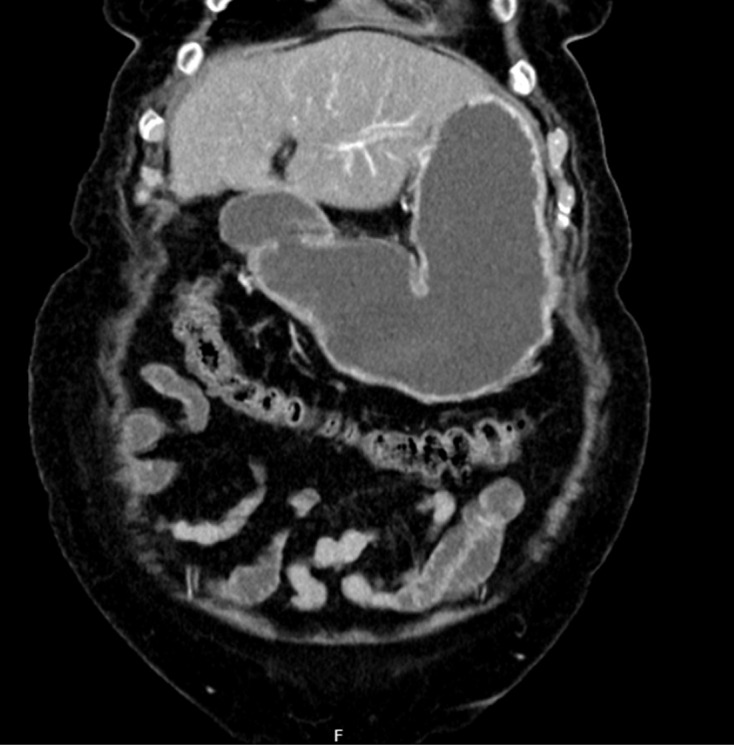
Computerized tomography scan showing gastric outlet obstruction in a patient with pancreatic ductal adenocarcinoma.
Surgical gastroenterostomy (S-GE) and latter endoscopic stenting (ES) have been the standard treatment modalities for mGOO. However, EUS-guided gastroenterostomy (EUS-GE), a technique emerging as an alternative to conventional treatment methods, combines reduced invasiveness and similar efficacy compared to S-GE and offers higher durability compared to ES (Figure 2)[1,6]. Indeed, several systematic reviews have reported that EUS-GE has a lower risk of recurrent obstruction compared to ES and reduced invasiveness with faster refeeding compared to S-GE, with equivalent clinical success[1,7-9].
Figure 2.
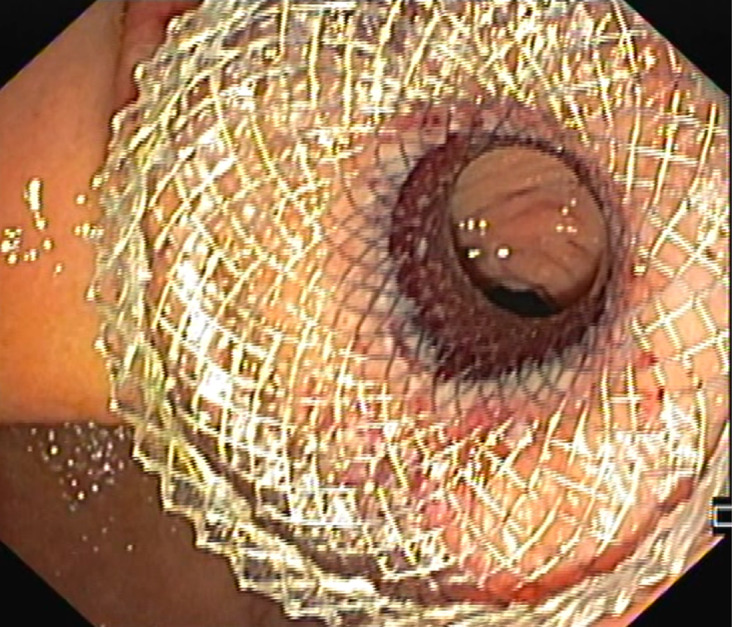
Endoscopic ultrasound-guided gastroenterostomy with lumen-apposing metal stent, performed to bypass a duodenal obstruction from pancreatic cancer.
ADDRESSING RESEARCH GAPS IN MGOO STUDIES
Considering the growing interest in EUS-GE over older alternatives, the identification of the barriers is crucial for its implementation in daily clinical practice. A recent survey from the Pancreas 2000 group aimed to assess worldwide approaches to mGOO and especially explore perceptions around EUS-GE among 290 pancreatologists from 44 countries. The availability of EUS-GE was heterogeneous, and preferences for mGOO treatment varied by specialty, with gastroenterologists favoring enteral stenting and surgeons more inclined towards surgical GJ. Higher annual mGOO cases correlated with increased EUS-GE adoption. Life expectancy and patient frailty were the primary decision factors, with EUS-GE valued for its minimally invasive nature, but hindered by its learning curve[10]. This highlights the need for high-quality literature and standardized algorithms for mGOO management.
Most studies describing the different treatment modalities for GOO are retrospective and focus on mechanical outcomes, such as technical success (stent placement or being able to create a gastroenteric anastomosis), clinical success (ability to eat soft solids without vomiting), and adverse events. Regarding EUS-GE, only one prospective uncontrolled study assessed the impact of this technique on patients quality of life[2]. The perception is that available literature does not consider outcomes that are relevant to the patient’s perspective and their impact on the clinical outcome. The ideal procedure for GOO should optimistically aim to significantly impact on nutritional status, body composition, quality of life and quality of eating experience, furthermore, it should positively effect chemotherapy tolerance, and ultimately cancer-related and overall survival. This review attempts to delve into these aspects, which should be explored in subsequent clinical research.
The literature search was conducted using MEDLINE and Embase electronic databases up to March 2024. No publication date or language restrictions were used. A combination of text words and MeSH terms (nutritional status, body composition, quality of life, feeding and eating disorders, chemotherapy) for the relevant topics were used in combination with cancer, pancreatic cancer and GOO. Cross-references were identified manually through the citation list of selected articles to capture additional sources.
The items detailed in this review are also synthesized, with advantages and disadvantages, in Table 1.
Table 1.
Suggested clinical outcomes to be included in future gastric outlet obstruction research
|
Outcome
|
Instruments/measures
|
Definition
|
Advantages
|
Disadvantages
|
| Nutrition | ||||
| Body composition | Body mass index[20] | Weight (Kg) divided by the square of height (m) | Easy assessment No need for laboratory or instrumental tests |
Affected by hypervolemia[5]; Does not accurately predict lean body mass[64] |
| Bioelectrical impedance vector analysis[34,65] | Body composition measurement (fat, bone, water and muscle) through levels of resistance to electrical current | Noninvasive; Allows detailed knowledge of hydration status and cell mass | Requires extra resources; Equipment cost | |
| Dual-energy X-ray absorptiometry[34,66] | Low-dose radiation technique measuring bone mineral density and body composition, such as fat mass and fat free mass | Noninvasive; Radiation dose lower than CT; Short scan time; Whole-body scan | Availability; Cost of equipment; Training; Exposure to ionizing radiation | |
| CT-based assessment of skeletal muscle mass | Sarcopenia: Loss of skeletal muscle mass[31] Most widely assessed using SMI (calculated by adjusting the total muscle area at the L3 vertebral level to the body height of the patient)[63,67] |
Defined by CT images routinely used in standard care of PC patients; Low cost of using available CT images[63]; Directly correlates with the whole-body skeletal muscle mass[63]; AI-based evaluation may decrease the time for segmentation[68] | Heterogeneity regarding radiological definition of sarcopenia due to varying indices used such as SMI, PMI, SBI[67,69]; Threshold values for sarcopenia vary for different patient populations[63,70]; Training and time for analysis[63] | |
| Biochemical parameters | Prognostic nutrition index[35,38] | Calculated using serum albumin and total lymphocyte count; Reflects nutrition and immune status | Easy to calculate and to follow up; Good predictive ability for prognosis in several cancers | Need for further validation in patients undergoing invasive procedures |
| Neutrophil-to-lymphocyte ratio[35,38] | Ratio between the neutrophil and lymphocyte counts measured in peripheral blood | Easy to calculate and to follow up | Need for further validation in patients undergoing invasive procedures | |
| Albumin-to-globulin ratio[40] | Ratio between albumin and globulin measured in peripheral blood | Easy to calculate and to follow up; Not affected by body fluid balance | Need for further validation in patients undergoing invasive procedures | |
| QoL | EORTC QLQ-C30[49] | The 30 item core cancer questionnaire to assess health-related QoL | Easy to assess and administer | Time-consuming; Accuracy could vary depending on patient’s education and psychiatric medication consumption |
| EORTC QLQ-PAN26[52] | To assess health-related QoL for people with pancreatic ductal adenocarcinoma | Easy to assess and administer; Validated in the palliative and surgical settings | Time-consuming; Accuracy could vary depending on patient’s education and psychiatric medication consumption | |
| EuroQol EQ-5D[48,55] | Five dimensions health-related QoL questionnaire for use in clinical and population health surveys | Easy to assess and administer | Time-consuming; Does not specifically evaluate nutrition or eating ability | |
| The FAACT[50,53] | A patient-reported measure designed to specifically assess anorexia/cachexia-related symptoms | Developed for adult cancer patients, experiencing anorexia/cachexia; Easy to assess and administer | Controversy around optimal cut-off | |
| The Anorexia/Cachexia Subscale (A/CS) of the FAACT questionnaire[50,53] | A specific subscale of FAACT | As FAACT | As FAACT | |
| Chemotherapy tolerance | RDI[59,60] | The ratio of the delivered dose intensity (dose per unit body surface area per unit time [mg/m2 per week]) to the standard or planned dose intensity for a chemotherapy regimen | It may correlate with survival; RDI informs personalized treatment adjustments |
Defining clinically meaningful RDI thresholds (e.g., 80% or 85%) remains challenging; Doesn’t directly account for non-hematologic toxicities; Difficult to calculate a merged RDI for regimens with multiple drugs; Relies on accurate dosing data, not consistently recorded in clinical practice |
| Time to chemotherapy initiation or resumption[2,6,61] | Time from the procedure to chemotherapy initiation or resumption | Time-depending outcome; Detailed evaluation of the impact of the procedure on the systemic therapy | Better to be evaluated in prospective studies | |
AI: Artificial intelligence; CT: Computerized tomography; EORTC QLQ-C30: European Organization for Research and Treatment of Cancer 30 item core cancer questionnaire; EORTC QLQ-PAN26: European Organization for Research and Treatment of Cancer quality of life questionnaire for pancreatic cancer patients; EuroQol EQ-5D: EuroQol 5 dimension questionnaire; FAACT: Functional Assessment of Anorexia/Cachexia Therapy; PMI: Psoas muscle index; QoL: Quality of life; RDI: Relative dose intensity; SBI: Skeletal muscle area/Total body area; SMI: Skeletal muscle index.
NUTRITION
Malnutrition is a widespread complication of periampullary cancers affecting 85% of these patients[11]. Several studies have reported worse outcomes (chemotherapy tolerance, recurrence and survival) for PC patients with impaired nutritional status and systemic inflammatory states[12,13]. Impaired nutrition in PC is complex and its causes include anorexia, elevated energy consumption, malabsorption, chemotherapy side effects, and in some cases GOO, which develops in up to 20% of patients with advanced hepatopancreatobiliary disease[14,15]. Due to the detrimental effects of malnutrition, it is vital to assess and monitor the nutritional status of these patients using objective measures to evaluate the effectiveness of nutritional treatments[5]. It has long been known that such alterations in nutritional status are reflected by changes in the body mass index (BMI) and biochemical parameters of the patient, and therefore these measures can be used in the assessment and follow-up[5].
Body mass index and body composition
BMI, although commonly used as a component of nutritional screening[16], by itself has a limited value as a nutritional marker and may lead to erroneous conclusions on nutritional status[17]. For instance, BMI may be affected by excessive fluid loads such as pleural effusion, ascites and/or edema, therefore body weight measurements should be corrected when such factors exist[5]. In addition, sarcopenic obesity, which is the coexistence of a low muscle mass together with a high BMI or high body fat content, is an increasingly recognized condition; and since fat-free mass represents the distribution volume of many chemotherapy drugs, this condition has been associated with shorter survival in addition to poorer functional status[18,19]. As malnutrition is closely reflected by alterations in body composition[11], the analysis of these alterations should be added to BMI as a more reliable method to diagnose and track the impact of mGOO on nutritional status[20].
Regarding clinical implications, these factors should be considered when interpreting the effectiveness of treatment modalities for mGOO in patients with PC. To our knowledge there are few studies in the literature evaluating nutritional markers and their post-procedural changes, and even less of those evaluating body composition[21-23].
Changes in body composition have been reported to be associated with chemotherapy tolerance[11]. Although data are somewhat conflicting and more research is required, there is evidence that individuals with sarcopenia or sarcopenic obesity, as defined by imaging methods, have a higher probability to experience chemotherapy toxicity and discontinue their chemotherapy regimen[24-28]. Notably, receiving the full dose of chemotherapy is associated with improved treatment efficacy and survival[29]. On the other side, performance status might impact on the choice of the chemotherapy regimen assigned to a patient; that is to say that more aggressive therapies are usually precluded in patients with poor performance status[30]. What is more, sarcopenia which is defined by loss of skeletal muscle mass, is also responsible for decreased strength and worsened quality of life as well as decreased survival[31].
Poor nutritional status may also affect surgical candidacy. It has been shown that decreased skeletal muscle is significantly corelated to worse postoperative survival as well as to higher rates of complications in resectable pancreatic ductal adenocarcinoma patients[32]. Therefore, it is important to preserve the muscle mass of patients before surgery, even while undergoing neoadjuvant therapy. It can be presumed that in patients with GOO, this can be sustained to some extent by providing the best effective oral feeding by enabling gastrointestinal passage, considering that nutritional modification in combination with exercise training can improve sarcopenia[33]. An effective treatment of mGOO may facilitate the possibility of the patient to reach the surgical resection of the tumor by preventing further deterioration of the body composition and enabling effective continuation of neoadjuvant chemotherapy.
Therefore, in our opinion, body composition may be a suitable target for future studies.
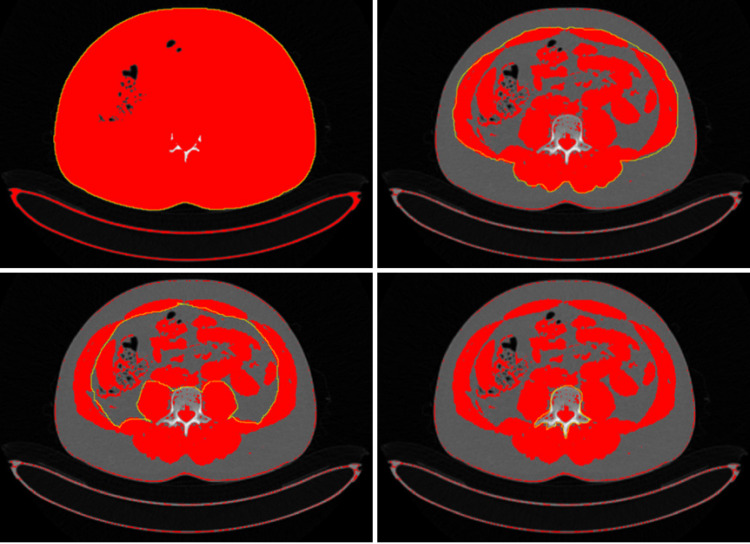
There are various techniques to assess body composition[26] (Table 1). However, performing body composition measurements using computerized tomography (CT) or magnetic resonance imaging (MRI) images, which are routinely obtained as a standard of care in PC management, seems much more feasible. Indeed, CT images have been widely used for this purpose in numerous studies and this method is presently considered the gold standard to measure body composition[18,34]. These methods provide insights into muscle mass and fat distribution using CT images at specific anatomical landmarks, such as the third lumbar vertebra (Figure 3), and involve contouring the boundaries of the skeletal muscles on the CT images and measuring their cross-sectional area using specialized software to ensure accuracy and consistency; the measured muscle area is then normalized for height, typically expressed as cm²/m², to account for variations in body size among individuals.
Figure 3.
Assessment of skeletal muscle mass using a single transverse computed tomography image at the L3 level using NIH image J, a free, public domain software. The data output produced by the software is processed to calculate the skeletal muscle area which is then adjusted to the body height to find the skeletal muscle index[62,63], https://imagej.net/ij/.
Not only the results from CT-scan and MRI are considered the most accurate method of measuring body composition at tissue-organ level, but also, they have significantly impacted the comprehension of the relation of body composition to disease risk and outcome[20].
Biochemical parameters
Although no specific biochemical parameters exist to monitor the nutritional status of the patients undergoing invasive procedures for mGOO, several indices, such as the prognostic nutrition index (PNI), neutrophil-to-lymphocyte ratio (NLR), and albumin-to-globulin ratio (AGR) have been evaluated in previous studies involving PC patients.
PNI is used to evaluate an individual’s nutritional status through the combination of serum albumin and total lymphocyte count. In 2022, a retrospective study showed that beyond operative time and vascular resection, PNI and NLR independently predicted overall survival among patients with oligometastatic PC undergoing resection[35].
Another retrospective study, including 219 consecutive patients with PC (any stage), assessed nutritional status through PNI, inflammation status through NLR, and psoas muscle mass from CT scans as an index of sarcopenia. The authors reported that the survival of patients with normal nutritional status (defined by PNI 45) was significantly longer than that of those who were malnourished (median 8 months vs 16.5 months, P = 0.04, respectively)[36]. Recently, a comprehensive retrospective analysis on 80 PC patients concluded that the assessment of nutritional and immune status using basic diagnostic tools, PNI and immune ratios (neutrophil-to-lymphocyte, monocyte-to-lymphocyte, platelet-to-lymphocyte) calculation should be the standard management of PC patients before surgery in order to improve the prognostication of these patients, as some of these markers (especially PNI) might predict survival time as well as the stage of the disease[12]. Other studies evaluating the above mentioned scores and tools confirmed their prognostic ability in PC[37-39].
In a recent Asian multicenter study, AGR was shown to be an independent prognostic factor in patients with cancer cachexia, especially in advanced disease (this study of 2364 patients with cancer cachexia included 177 patients with hepato-biliary-pancreatic cancers)[40].
As a result, PNI, NLR, and AGR have no specific role in the management and follow-up for patients with PC to date, so they should be explored in future studies as markers of nutritional status in this setting. Notably, all of these scores can be determined using widely available laboratory variables. Their monitoring has the potential to be valuable during follow-up after interventions for GOO resolution, and should allow a more robust evaluation of their impact.
QUALITY OF LIFE/QUALITY OF EATING
Improving quality of life (QoL) is a critical objective for all patients, especially those with an incurable cancer. In fact, besides progression and survival, QoL should be employed as an index for treatment outcome in cancer patients[41]. Malnutrition negatively impacts the QoL and treatment effectiveness of patients with PC. Therefore, the ability to eat and the maintenance of adequate oral intake, should be considered paramount in treatment goals and in the evaluation of the impact of interventions in PC patients.
Regarding interventions for GOO treatment, only a few studies have included the evaluation of QoL as an endpoint[42-47]. In the era of EUS-GE, only two uncontrolled studies have reported changes in QoL scores after intervention, so this is surely an underexplored topic[2,48]. Several QoL evaluation tools have been used in different scenarios[49-53].
The European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Study Group which published in 1993, a 30 item core cancer questionnaire (QLQ—C30) designed to be used in cancer clinical trials[49] and was translated, and validated in numerous languages. This questionnaire was used in the study published by Garcia-Alonso et al[2], and it can be supplemented by additional modules to assess specific patient subgroups, as is the case of QLQ-PAN26 for PC[52]. This specific module was developed combining health professionals’ input and patients’ insight[54]. It is validated in palliative and surgical settings, includes items concerning nutrition and is specific for patients with PC. The main inconvenience is the long (about 12 min) time to complete the questionnaire.
The EQ-5D is a questionnaire developed by the EuroQol group in 1990 to measure health-related QoL and assess patient-reported outcomes. It comprises 5 dimensions: Mobility, self-care, usual activities, pain, and anxiety/depression[55]. This questionnaire was used by Xu et al[48] to evaluate QoL changes after EUS-GE, but it did not include questions regarding nutrition or eating ability.
The Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire is a 39-item scale developed for adult cancer patients experiencing anorexia/cachexia. It has been translated and validated in several languages, and includes five domains: Physical well-being, social/family well-being, emotional well-being, functional well-being, and an anorexia/cachexia subscale. The Anorexia/Cachexia Subscale (A/CS) of the FAACT questionnaire has been recently proposed for the evaluation of cancer cachexia[50,53]. It includes 12 items and responses that are recorded on a 5-point Likert-type scale. Negatively worded items were reverse scored. A cut-off value of ≤ 24 (half of the maximum score) to define anorexia was suggested in a consensus paper of the European society for clinical nutrition and metabolism[56], but a more recent study using two external criteria for validation, indicates that the optimal cut-off values for A/CS of the FAACT questionnaire should be ≤ 37 when assessing anorexia in patients with cancer[50].
Specific indices of QoL should be included as a key endpoint in future studies assessing treatment alternatives for malignant GOO rather than focusing solely on the achievement of a full diet or the reintervention rate[2].
CHEMOTHERAPY TOLERANCE
The response to chemotherapy is dose dependent and there is convincing evidence that outcomes are improved with higher dose intensities as patients experience better overall survival, progression-free survival and disease-free survival when compared to patients receiving lower than planned doses[57,58].
GOO can compromise a patient’s ability to comply with the chemotherapy regimen, warranting a reduction in dose or delay in planned treatment because of its impact on general well-being and nutritional status. Indeed, GOO may delay the start of systemic or maintenance of chemotherapy due to the need for hospitalization and the time for scheduling the procedure for resolving the condition (either surgically or endoscopically).
A significant proportion of patients receive relatively low-dose intensity chemotherapy regimens, which represents a potential reason for treatment failure in the case of curable malignancies and a justification for faster disease progression in the palliative setting[58]. In the treatment of PC, optimizing chemotherapy may have a significant impact on patient survival; however, there is a need to balance regimen tolerability and efficacy through continuous dose intensity adjustments. Relative dose intensity (RDI) is a measure commonly used to describe dose delays or reductions during chemotherapy, and is defined as the ratio between the delivered and planned doses.
Previous reports have shown that a significant RDI reduction could affect therapeutic efficacy and patient survival[59,60], but its role may be confounded by other factors, such as body composition, age, and comorbidities. Body composition, as previously stated, was found to be a major determinant of chemotherapy tolerance and adherence[61].
In clinical practice, for regimens combining different chemotherapy drugs, determining a merged RDI is difficult and is not standardized. To the best of our knowledge, no studies have provided reproducible calculation tools[41], so in clinical practice, simpler measures are preferred to determine patients’ ability to complete a predetermined treatment plan. There is consensus in the literature that dose delays > 7 days from standard regimen (missing doses), a decrease > 15% in chemotherapy dose relative to the standard regimen and the need to stop chemotherapy are significant deviations from the pre-established plans[61].
The impact of GOO on chemotherapy initiation or resumption has been poorly described. In fact, only a few studies describe the effects of GOO treatment modalities on chemotherapy. In the study published by Vanella et al[6], 43 (61.4%) patients were candidates for active oncological treatment, and the median time to chemotherapy (re-)initiation was 19 days after the procedure. The authors also performed a matched comparison with ES (28 patients per group) and found a trend toward shorter time to chemotherapy treatment for patients receiving EUS-GE. Garcia-Alonso et al[2] reported that 71% of patients who had previously received chemotherapy before GOO, were able to resume treatment after EUS-GE. These data show that EUS-GE might result in earlier chemotherapy initiation or resumption prompting the need for its systematic inclusion as an outcome measure when evaluating/comparing the results of GOO treatment modalities.
CONCLUSION
GOO palliation in cancer patients has historically been performed by S-GE or ES. EUS-GE has emerged as an alternative to the more invasive nature of surgery and has been shown to have higher long-term efficacy than ES with a low level of evidence. In this review, we describe the importance of several “missing” outcomes that should be explored in future studies that evaluate mGOO treatment modalities.
Most studies comparing these techniques have focused on technical success and oral feeding resumption (clinical success), but it is known that outcomes such as body composition, nutritional status, quality of life, and the ability to comply with the proposed chemotherapy regimen might have a higher impact on prognosis. SMI is directly related to the total muscle mass of a patient and has been shown to correlate with sarcopenia. For nutritional status evaluation, the PNI and AGR are easy to determine using widely available laboratory parameters and have been shown to correlate with cancer cachexia. The A/CS of the FAACT questionnaire is a validated instrument to evaluate quality of eating and seems easier to apply when compared to QLQ-PAN26 and superior to EQ-5D as the latter instrument includes no questions regarding nutrition or eating ability.
RDI is the best instrument to evaluate the ability to comply with the chemotherapy regimen, but it is difficult to use in clinical practice, especially in regimens that include combinations of several drugs, and the use of simpler measures such as missing doses, dose reductions, and the need to stop chemotherapy are probably good alternatives.
As the final aim of medical interventions in the oncological scenario should be to improve survival, or at least quality-adjusted survival, the inclusion of these essential endpoints in future investigations of mGOO treatment modalities would provide a more comprehensive understanding of what truly matters to the patients.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Machado NC S-Editor: Liu JH L-Editor: Filipodia P-Editor: Zhang L
Contributor Information
Filipe Vilas-Boas, Department of Gastroenterology, São João University Hospital, Porto 4200, Portugal.
Giacomo Emanuele Maria Rizzo, Endoscopy Service, Department of Diagnostic and Therapeutic Services, Scientific Institute for Research, Hospitalization and Healthcare - The Mediterranean Institute for Transplantation and Highly Specialized Therapies, Palermo 90127, Sicilia, Italy.
Charles De Ponthaud, Department of Digestive, Hepato-Biliary and Pancreatic Surgery and Liver Transplantation, AP-HP Pitié-Salpêtriere, Sorbonne Université, Paris 75013, Île-de-France, France.
Stuart Robinson, Department of Hepatobiliary, Pancreatic and Transplant Surgery, Freeman Hospital, Newcastle NE7 7DN, Newcastle upon Tyne, United Kingdom.
Sebastien Gaujoux, Department of Digestive, Hepato-Biliary and Pancreatic Surgery and Liver Transplantation, AP-HP Pitié-Salpêtriere, Sorbonne Université, Paris 75013, Île-de-France, France.
Gabriele Capurso, Division of Pancreato-Biliary Endoscopy and Endosonography, Pancreas Translational and Clinical Research Center, IRCCS San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milano 20132, Italy.
Giuseppe Vanella, Division of Pancreato-Biliary Endoscopy and Endosonography, Pancreas Translational and Clinical Research Center, IRCCS San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milano 20132, Italy. vanella.giuseppe@hsr.it.
Bahadır Bozkırlı, Department of General Surgery, HPB-Unit, Acıbadem Maslak Hospital, Istanbul 34398, Türkiye.
References
- 1.Miller C, Benchaya JA, Martel M, Barkun A, Wyse JM, Ferri L, Chen YI. EUS-guided gastroenterostomy vs. surgical gastrojejunostomy and enteral stenting for malignant gastric outlet obstruction: a meta-analysis. Endosc Int Open. 2023;11:E660–E672. doi: 10.1055/a-2098-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Alonso FJ, Chavarria C, Subtil JC, Aparicio JR, Busto Bea V, Martinez-Moreno B, Vila JJ, Martín-Álvarez V, Sanchez-Delgado L, de la Serna-Higuera C, Perez-Miranda M. Prospective multicenter assessment of the impact of EUS-guided gastroenterostomy on patient quality of life in unresectable malignant gastric outlet obstruction. Gastrointest Endosc. 2023;98:28–35. doi: 10.1016/j.gie.2023.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Krishnamoorthi R, Bomman S, Benias P, Kozarek RA, Peetermans JA, McMullen E, Gjata O, Irani SS. Efficacy and safety of endoscopic duodenal stent versus endoscopic or surgical gastrojejunostomy to treat malignant gastric outlet obstruction: systematic review and meta-analysis. Endosc Int Open. 2022;10:E874–E897. doi: 10.1055/a-1794-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503–509. doi: 10.1016/s0959-8049(97)10090-9. [DOI] [PubMed] [Google Scholar]
- 5.Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Mühlebach S, Muscaritoli M, Oldervoll L, Ravasco P, Solheim T, Strasser F, de van der Schueren M, Preiser JC. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48. doi: 10.1016/j.clnu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Vanella G, Dell'Anna G, Capurso G, Maisonneuve P, Bronswijk M, Crippa S, Tamburrino D, Macchini M, Orsi G, Casadei-Gardini A, Aldrighetti L, Reni M, Falconi M, van der Merwe S, Arcidiacono PG. EUS-guided gastroenterostomy for management of malignant gastric outlet obstruction: a prospective cohort study with matched comparison with enteral stenting. Gastrointest Endosc. 2023;98:337–347.e5. doi: 10.1016/j.gie.2023.04.2072. [DOI] [PubMed] [Google Scholar]
- 7.Bomman S, Ghafoor A, Sanders DJ, Jayaraj M, Chandra S, Krishnamoorthi R. Endoscopic ultrasound-guided gastroenterostomy versus surgical gastrojejunostomy in treatment of malignant gastric outlet obstruction: Systematic review and meta-analysis. Endosc Int Open. 2022;10:E361–E368. doi: 10.1055/a-1783-8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martins RK, Brunaldi VO, Fernandes AL, Otoch JP, Artifon ELA. Palliative therapy for malignant gastric outlet obstruction: how does the endoscopic ultrasound-guided gastroenterostomy compare with surgery and endoscopic stenting? A systematic review and meta-analysis. Ther Adv Gastrointest Endosc. 2023;16:26317745221149626. doi: 10.1177/26317745221149626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teoh AYB, Lakhtakia S, Tarantino I, Perez-Miranda M, Kunda R, Maluf-Filho F, Dhir V, Basha J, Chan SM, Ligresti D, Ma MTW, de la Serna-Higuera C, Yip HC, Ng EKW, Chiu PWY, Itoi T. Endoscopic ultrasonography-guided gastroenterostomy versus uncovered duodenal metal stenting for unresectable malignant gastric outlet obstruction (DRA-GOO): a multicentre randomised controlled trial. Lancet Gastroenterol Hepatol. 2024;9:124–132. doi: 10.1016/S2468-1253(25)00136-0. [DOI] [PubMed] [Google Scholar]
- 10.De Ponthaud C, Bozkirli B, Rizzo GEM, Robinson S, Vilas-Boas F, Capurso G, Gaujoux S, Vanella G. Management of malignant Gastric Outlet Obstruction (mGOO) due to pancreatic cancer in the era of EUS-Gastrojejunostomy: an international practice survey and case vignette study by Pancreas 2000 from the European Pancreatic Club. Surg Endosc. 2024;38:3231–3240. doi: 10.1007/s00464-024-10803-0. [DOI] [PubMed] [Google Scholar]
- 11.Kowalska M, Kamocki Z. Body composition of patients suffering from pancreatic cancer. Pol Przegl Chir. 2022;95:1–5. doi: 10.5604/01.3001.0015.8570. [DOI] [PubMed] [Google Scholar]
- 12.Jabłońska B, Pawlicki K, Mrowiec S. Associations between Nutritional and Immune Status and Clinicopathologic Factors in Patients with Pancreatic Cancer: A Comprehensive Analysis. Cancers (Basel) 2021;13 doi: 10.3390/cancers13205041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawa K, Sho M, Akahori T, Nagai M, Nakamura K, Takagi T, Tanaka T, Nishiofuku H, Ohbayashi C, Kichikawa K, Ikeda N. Significance of the inflammation-based prognostic score in recurrent pancreatic cancer. Pancreatology. 2019;19:722–728. doi: 10.1016/j.pan.2019.05.461. [DOI] [PubMed] [Google Scholar]
- 14.Carrato A, Cerezo L, Feliu J, Macarulla T, Martín-Pérez E, Vera R, Álvarez J, Botella-Carretero JI. Clinical nutrition as part of the treatment pathway of pancreatic cancer patients: an expert consensus. Clin Transl Oncol. 2022;24:112–126. doi: 10.1007/s12094-021-02674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manuel-Vázquez A, Latorre-Fragua R, Ramiro-Pérez C, López-Marcano A, la Plaza-Llamas R, Ramia JM. Laparoscopic gastrojejunostomy for gastric outlet obstruction in patients with unresectable hepatopancreatobiliary cancers: A personal series and systematic review of the literature. World J Gastroenterol. 2018;24:1978–1988. doi: 10.3748/wjg.v24.i18.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondrup J, Rasmussen HH, Hamberg O, Stanga Z Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–336. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 17.Hull HR, Thornton J, Wang J, Pierson RN Jr, Kaleem Z, Pi-Sunyer X, Heymsfield S, Albu J, Fernandez JR, Vanitallie TB, Gallagher D. Fat-free mass index: changes and race/ethnic differences in adulthood. Int J Obes (Lond) 2011;35:121–127. doi: 10.1038/ijo.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lobo DN, Gianotti L, Adiamah A, Barazzoni R, Deutz NEP, Dhatariya K, Greenhaff PL, Hiesmayr M, Hjort Jakobsen D, Klek S, Krznaric Z, Ljungqvist O, McMillan DC, Rollins KE, Panisic Sekeljic M, Skipworth RJE, Stanga Z, Stockley A, Stockley R, Weimann A. Perioperative nutrition: Recommendations from the ESPEN expert group. Clin Nutr. 2020;39:3211–3227. doi: 10.1016/j.clnu.2020.03.038. [DOI] [PubMed] [Google Scholar]
- 19.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 20.Holmes CJ, Racette SB. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients. 2021;13 doi: 10.3390/nu13082493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JH, Song HY, Yun SC, Yoo MW, Ryu MH, Kim JH, Kim do H, Lee JH, Zhou WZ, Yook JH, Jung HY. Gastroduodenal stent placement versus surgical gastrojejunostomy for the palliation of gastric outlet obstructions in patients with unresectable gastric cancer: a propensity score-matched analysis. Eur Radiol. 2016;26:2436–2445. doi: 10.1007/s00330-015-4106-4. [DOI] [PubMed] [Google Scholar]
- 22.Wang C, Lin S, Zhang X, Yang C, Li W. Laparoscopic Gastrojejunostomy with Conversion Therapy in Gastric Outlet Obstruction Caused by Incurable Advanced Gastric Cancer. Cancer Manag Res. 2021;13:6847–6857. doi: 10.2147/CMAR.S322569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Hao J, Wu D, Lang H. Retrospective evaluation of endoscopic stenting of combined malignant common bile duct and gastric outlet-duodenum obstructions. Exp Ther Med. 2014;8:1173–1177. doi: 10.3892/etm.2014.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, Latko E, Taieb J. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66:583–589. doi: 10.1080/01635581.2014.894103. [DOI] [PubMed] [Google Scholar]
- 25.Kurita Y, Kobayashi N, Tokuhisa M, Goto A, Kubota K, Endo I, Nakajima A, Ichikawa Y. Sarcopenia is a reliable prognostic factor in patients with advanced pancreatic cancer receiving FOLFIRINOX chemotherapy. Pancreatology. 2019;19:127–135. doi: 10.1016/j.pan.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Ogushi K, Chuma M, Numata K, Nozaki A, Moriya S, Uojima H, Kondo M, Morimoto M, Maeda S. Impact of psoas muscle index assessed by a simple measurement method on tolerability and duration of continued treatment with sorafenib in hepatocellular carcinoma patients. Eur J Gastroenterol Hepatol. 2022;34:774–781. doi: 10.1097/MEG.0000000000002346. [DOI] [PubMed] [Google Scholar]
- 27.Uojima H, Chuma M, Tanaka Y, Hidaka H, Nakazawa T, Iwabuchi S, Kobayashi S, Hattori N, Ogushi K, Morimoto M, Kagawa T, Tanaka K, Kako M, Koizumi W. Skeletal Muscle Mass Influences Tolerability and Prognosis in Hepatocellular Carcinoma Patients Treated with Lenvatinib. Liver Cancer. 2020;9:193–206. doi: 10.1159/000504604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youn S, Chen A, Ha V, Chambers C, Eurich DT, McCall M, Sawyer MB. An exploratory study of body composition as a predictor of dose-limiting toxicity in metastatic pancreatic cancer treated with gemcitabine plus nab-paclitaxel. Clin Nutr. 2021;40:4888–4892. doi: 10.1016/j.clnu.2021.06.026. [DOI] [PubMed] [Google Scholar]
- 29.Bland KA, Zadravec K, Landry T, Weller S, Meyers L, Campbell KL. Impact of exercise on chemotherapy completion rate: A systematic review of the evidence and recommendations for future exercise oncology research. Crit Rev Oncol Hematol. 2019;136:79–85. doi: 10.1016/j.critrevonc.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Springfeld C, Jäger D, Büchler MW, Strobel O, Hackert T, Palmer DH, Neoptolemos JP. Chemotherapy for pancreatic cancer. Presse Med. 2019;48:e159–e174. doi: 10.1016/j.lpm.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 31.Basile D, Corvaja C, Caccialanza R, Aprile G. Sarcopenia: looking to muscle mass to better manage pancreatic cancer patients. Curr Opin Support Palliat Care. 2019;13:279–285. doi: 10.1097/SPC.0000000000000455. [DOI] [PubMed] [Google Scholar]
- 32.Ozola Zalite I, Zykus R, Francisco Gonzalez M, Saygili F, Pukitis A, Gaujoux S, Charnley RM, Lyadov V. Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: a systematic review. Pancreatology. 2015;15:19–24. doi: 10.1016/j.pan.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Beetz NL, Maier C, Shnayien S, Trippel TD, Gehle P, Fehrenbach U, Geisel D. Artificial intelligence-based analysis of body composition in Marfan: skeletal muscle density and psoas muscle index predict aortic enlargement. J Cachexia Sarcopenia Muscle. 2021;12:993–999. doi: 10.1002/jcsm.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bossi P, Delrio P, Mascheroni A, Zanetti M. The Spectrum of Malnutrition/Cachexia/Sarcopenia in Oncology According to Different Cancer Types and Settings: A Narrative Review. Nutrients. 2021;13 doi: 10.3390/nu13061980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frigerio I, Malleo G, de Pastena M, Deiro G, Surci N, Scopelliti F, Esposito A, Regi P, Giardino A, Allegrini V, Bassi C, Girelli R, Salvia R, Butturini G. Prognostic Factors After Pancreatectomy for Pancreatic Cancer Initially Metastatic to the Liver. Ann Surg Oncol. 2022;29:8503–8510. doi: 10.1245/s10434-022-12385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yıldırım İ, Kaya T, İşsever K, Genç AC, Karacan A, Önmez A, Hacıbekiroğlu İ. Psoas muscle mass, nutritional status, inflammation, and their relationship with prognosis in patients with pancreatic adenocarcinoma. Nutr Hosp. 2021;38:1009–1015. doi: 10.20960/nh.03573. [DOI] [PubMed] [Google Scholar]
- 37.Geng Y, Qi Q, Sun M, Chen H, Wang P, Chen Z. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol. 2015;41:1508–1514. doi: 10.1016/j.ejso.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 38.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 39.Miyoshi K, Shimoda M, Udo R, Oshiro Y, Suzuki S. Urinary titin N-terminal fragment concentration is an indicator of preoperative sarcopenia and nutritional status in patients with gastrointestinal tract and hepatobiliary pancreatic malignancies. Nutrition. 2020;79-80:110957. doi: 10.1016/j.nut.2020.110957. [DOI] [PubMed] [Google Scholar]
- 40.Xie HL, Zhang Q, Ruan GT, Ge YZ, Hu CL, Song MM, Song CH, Zhang X, Zhang XW, Li XR, Zhang KP, Liu T, Yang M, Tang M, Xu HX, Shi HP. Evaluation and Validation of the Prognostic Value of Serum Albumin to Globulin Ratio in Patients With Cancer Cachexia: Results From a Large Multicenter Collaboration. Front Oncol. 2021;11:707705. doi: 10.3389/fonc.2021.707705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JC, Kim JW, Ahn S, Kim HW, Lee J, Kim YH, Paik KH, Kim J, Hwang JH. Optimal dose reduction of FOLFIRINOX for preserving tumour response in advanced pancreatic cancer: Using cumulative relative dose intensity. Eur J Cancer. 2017;76:125–133. doi: 10.1016/j.ejca.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Fujitani K, Ando M, Sakamaki K, Terashima M, Kawabata R, Ito Y, Yoshikawa T, Kondo M, Kodera Y, Yoshida K. Multicentre observational study of quality of life after surgical palliation of malignant gastric outlet obstruction for gastric cancer. BJS Open. 2017;1:165–174. doi: 10.1002/bjs5.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeurnink SM, Steyerberg EW, Vleggaar FP, van Eijck CH, van Hooft JE, Schwartz MP, Kuipers EJ, Siersema PD Dutch SUSTENT Study Group. Predictors of survival in patients with malignant gastric outlet obstruction: a patient-oriented decision approach for palliative treatment. Dig Liver Dis. 2011;43:548–552. doi: 10.1016/j.dld.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 44.Mehta S, Hindmarsh A, Cheong E, Cockburn J, Saada J, Tighe R, Lewis MP, Rhodes M. Prospective randomized trial of laparoscopic gastrojejunostomy versus duodenal stenting for malignant gastric outflow obstruction. Surg Endosc. 2006;20:239–242. doi: 10.1007/s00464-005-0130-9. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt C, Gerdes H, Hawkins W, Zucker E, Zhou Q, Riedel E, Jaques D, Markowitz A, Coit D, Schattner M. A prospective observational study examining quality of life in patients with malignant gastric outlet obstruction. Am J Surg. 2009;198:92–99. doi: 10.1016/j.amjsurg.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 46.van den Berg MW, Haijtink S, Fockens P, Vleggaar FP, Dijkgraaf MG, Siersema PD, van Hooft JE. First data on the Evolution duodenal stent for palliation of malignant gastric outlet obstruction (DUOLUTION study): a prospective multicenter study. Endoscopy. 2013;45:174–181. doi: 10.1055/s-0032-1326077. [DOI] [PubMed] [Google Scholar]
- 47.van Hooft JE, van Montfoort ML, Jeurnink SM, Bruno MJ, Dijkgraaf MG, Siersema PD, Fockens P. Safety and efficacy of a new non-foreshortening nitinol stent in malignant gastric outlet obstruction (DUONITI study): a prospective, multicenter study. Endoscopy. 2011;43:671–675. doi: 10.1055/s-0030-1256383. [DOI] [PubMed] [Google Scholar]
- 48.Xu G, Shen Y, Lv Y, Zhou X, Li W, Wang Y, Hassan S, Wang L, Zou X. Safety and efficacy of endoscopic ultrasound-guided gastroenterostomy using double balloon occlusion methods: a clinical retrospective study in 36 patients with malignant gastric outlet obstruction. Endosc Int Open. 2020;8:E1690–E1697. doi: 10.1055/a-1221-9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 50.Blauwhoff-Buskermolen S, Ruijgrok C, Ostelo RW, de Vet HCW, Verheul HMW, de van der Schueren MAE, Langius JAE. The assessment of anorexia in patients with cancer: cut-off values for the FAACT-A/CS and the VAS for appetite. Support Care Cancer. 2016;24:661–666. doi: 10.1007/s00520-015-2826-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmülling C, Neugebauer E, Troidl H. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216–222. doi: 10.1002/bjs.1800820229. [DOI] [PubMed] [Google Scholar]
- 52.Fitzsimmons D, Johnson CD, George S, Payne S, Sandberg AA, Bassi C, Beger HG, Birk D, Büchler MW, Dervenis C, Fernandez Cruz L, Friess H, Grahm AL, Jeekel J, Laugier R, Meyer D, Singer MW, Tihanyi T. Development of a disease specific quality of life (QoL) questionnaire module to supplement the EORTC core cancer QoL questionnaire, the QLQ-C30 in patients with pancreatic cancer. EORTC Study Group on Quality of Life. Eur J Cancer. 1999;35:939–941. doi: 10.1016/s0959-8049(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 53.Ribaudo JM, Cella D, Hahn EA, Lloyd SR, Tchekmedyian NS, Von Roenn J, Leslie WT. Re-validation and shortening of the Functional Assessment of Anorexia/Cachexia Therapy (FAACT) questionnaire. Qual Life Res. 2000;9:1137–1146. doi: 10.1023/a:1016670403148. [DOI] [PubMed] [Google Scholar]
- 54.Fitzsimmons D, George S, Payne S, Johnson CD. Differences in perception of quality of life issues between health professionals and patients with pancreatic cancer. Psychooncology. 1999;8:135–143. doi: 10.1002/(SICI)1099-1611(199903/04)8:2<135::AID-PON348>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 55.EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 56.Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G, Boirie Y, Bosaeus I, Cederholm T, Costelli P, Fearon KC, Laviano A, Maggio M, Rossi Fanelli F, Schneider SM, Schols A, Sieber CC. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) "cachexia-anorexia in chronic wasting diseases" and "nutrition in geriatrics". Clin Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Morita Y, Sakaguchi T, Kitajima R, Furuhashi S, Kiuchi R, Takeda M, Hiraide T, Shibasaki Y, Kikuchi H, Konno H, Takeuchi H. Body weight loss after surgery affects the continuity of adjuvant chemotherapy for pancreatic cancer. BMC Cancer. 2019;19:416. doi: 10.1186/s12885-019-5621-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nielson CM, Bylsma LC, Fryzek JP, Saad HA, Crawford J. Relative Dose Intensity of Chemotherapy and Survival in Patients with Advanced Stage Solid Tumor Cancer: A Systematic Review and Meta-Analysis. Oncologist. 2021;26:e1609–e1618. doi: 10.1002/onco.13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Havrilesky LJ, Reiner M, Morrow PK, Watson H, Crawford J. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol. 2015;93:203–210. doi: 10.1016/j.critrevonc.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 60.Lian L, Shen XM, Huang TA, Li DP, Li XM, Han SG, Xu XF, Ma CT, Zhou C. Efficacy of relative dose intensity of nab-paclitaxel for the short-term outcomes, survival, and quality of life in patients with advanced pancreatic cancer: a retrospective study. Transl Cancer Res. 2022;11:2310–2320. doi: 10.21037/tcr-22-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crawford J, Denduluri N, Patt D, Jiao X, Morrow PK, Garcia J, Barron R, Lyman GH. Relative dose intensity of first-line chemotherapy and overall survival in patients with advanced non-small-cell lung cancer. Support Care Cancer. 2020;28:925–932. doi: 10.1007/s00520-019-04875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Corrigendum. JPEN J Parenter Enteral Nutr. 2016;40:742–743. doi: 10.1177/0148607116648918. [DOI] [PubMed] [Google Scholar]
- 63.Gomez-Perez SL, Haus JM, Sheean P, Patel B, Mar W, Chaudhry V, McKeever L, Braunschweig C. Measuring Abdominal Circumference and Skeletal Muscle From a Single Cross-Sectional Computed Tomography Image: A Step-by-Step Guide for Clinicians Using National Institutes of Health ImageJ. JPEN J Parenter Enteral Nutr. 2016;40:308–318. doi: 10.1177/0148607115604149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 65.Grundmann O, Yoon SL, Williams JJ. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients--a comprehensive review. Eur J Clin Nutr. 2015;69:1290–1297. doi: 10.1038/ejcn.2015.126. [DOI] [PubMed] [Google Scholar]
- 66.Marra M, Sammarco R, De Lorenzo A, Iellamo F, Siervo M, Pietrobelli A, Donini LM, Santarpia L, Cataldi M, Pasanisi F, Contaldo F. Assessment of Body Composition in Health and Disease Using Bioelectrical Impedance Analysis (BIA) and Dual Energy X-Ray Absorptiometry (DXA): A Critical Overview. Contrast Media Mol Imaging. 2019;2019:3548284. doi: 10.1155/2019/3548284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ratnayake CB, Loveday BP, Shrikhande SV, Windsor JA, Pandanaboyana S. Impact of preoperative sarcopenia on postoperative outcomes following pancreatic resection: A systematic review and meta-analysis. Pancreatology. 2018;18:996–1004. doi: 10.1016/j.pan.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Vogele D, Otto S, Sollmann N, Haggenmüller B, Wolf D, Beer M, Schmidt SA. Sarcopenia - Definition, Radiological Diagnosis, Clinical Significance. Rofo. 2023;195:393–405. doi: 10.1055/a-1990-0201. [DOI] [PubMed] [Google Scholar]
- 69.Thormann M, Hinnerichs M, Barajas Ordonez F, Saalfeld S, Perrakis A, Croner R, Omari J, Pech M, Zamsheva M, Meyer HJ, Wienke A, Surov A. Sarcopenia is an Independent Prognostic Factor in Patients With Pancreatic Cancer - a Meta-analysis. Acad Radiol. 2023;30:1552–1561. doi: 10.1016/j.acra.2022.10.025. [DOI] [PubMed] [Google Scholar]
- 70.Yang L, Liao X, Xie Z, Li H. Prognostic value of pretreatment skeletal muscle index in pancreatic carcinoma patients: A meta-analysis. Medicine (Baltimore) 2023;102:e33663. doi: 10.1097/MD.0000000000033663. [DOI] [PMC free article] [PubMed] [Google Scholar]