Abstract
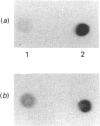
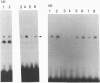
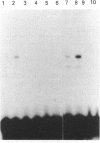
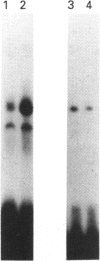
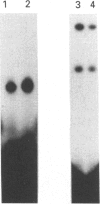
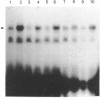
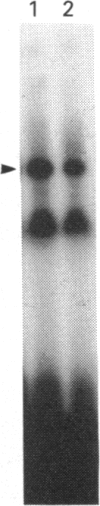
Infection with herpes simplex virus (HSV) results in an increase in the transcription of the endogenous Alu repeated sequence by RNA polymerase III. This effect is also observed in uninfected cells stably transformed with a plasmid expressing the HSV immediate-early protein ICP27 or in cells transfected with the gene encoding this protein. Both uninfected cells expressing ICP27 and cells infected with virus producing functional ICP27 display increased activity of the cellular transcription factor TFIIIC when compared with untreated cells. This increase is not observed, however, in cells infected with a mutant strain of virus which does not produce ICP27. Hence ICP27 induces elevated Alu transcription by activating transcription factor TFIIIC, which is the limiting factor for such transcription. This is the first report of increased activity of a cellular transcription factor during HSV infection, when most cellular gene activity is inhibited.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann M., Falke D., Preuhs J., Schröder H. C., Pfeifer K., Müller W. E. Occurrence of novel small RNAs with concomitant inhibition of host cellular U small nuclear RNA synthesis in Vero cells infected with herpes simplex virus type 1. J Gen Virol. 1986 Dec;67(Pt 12):2587–2594. doi: 10.1099/0022-1317-67-12-2587. [DOI] [PubMed] [Google Scholar]
- Baker R. E., Gabrielsen O., Hall B. D. Effects of tRNATyr point mutations on the binding of yeast RNA polymerase III transcription factor C. J Biol Chem. 1986 Apr 25;261(12):5275–5282. [PubMed] [Google Scholar]
- Ciliberto G., Castagnoli L., Cortese R. Transcription by RNA polymerase III. Curr Top Dev Biol. 1983;18:59–88. doi: 10.1016/s0070-2153(08)60579-7. [DOI] [PubMed] [Google Scholar]
- Ciliberto G., Raugei G., Costanzo F., Dente L., Cortese R. Common and interchangeable elements in the promoters of genes transcribed by RNA polymerase iii. Cell. 1983 Mar;32(3):725–733. doi: 10.1016/0092-8674(83)90058-2. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Dent C. L., McIndoe G. A., Latchman D. S. The constitutively expressed octamer binding protein OTF-1 and a novel octamer binding protein expressed specifically in cervical cells bind to an octamer-related sequence in the human papillomavirus 16 enhancer. Nucleic Acids Res. 1991 Aug 25;19(16):4531–4535. doi: 10.1093/nar/19.16.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. H., Jagadeeswaran P., Wang R. R., Weissman S. M. Structural analysis of templates and RNA polymerase III transcripts of Alu family sequences interspersed among the human beta-like globin genes. Gene. 1981 Mar;13(2):185–196. doi: 10.1016/0378-1119(81)90007-x. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Estridge J. K., Kemp L. M., La Thangue N. B., Mann B. S., Tyms A. S., Latchman D. S. The herpes simplex virus type 1 immediate-early protein ICP27 is obligately required for the accumulation of a cellular protein during viral infection. Virology. 1989 Jan;168(1):67–72. doi: 10.1016/0042-6822(89)90404-2. [DOI] [PubMed] [Google Scholar]
- Everett R. D. The products of herpes simplex virus type 1 (HSV-1) immediate early genes 1, 2 and 3 can activate HSV-1 gene expression in trans. J Gen Virol. 1986 Nov;67(Pt 11):2507–2513. doi: 10.1099/0022-1317-67-11-2507. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor R. B., Feldman L. T., Berk A. J. Transcription of class III genes activated by viral immediate early proteins. Science. 1985 Oct 25;230(4724):447–450. doi: 10.1126/science.2996135. [DOI] [PubMed] [Google Scholar]
- Gottlieb E., Steitz J. A. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 1989 Mar;8(3):851–861. doi: 10.1002/j.1460-2075.1989.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T., Hay J. Properties of herpesvirus-induced "immediate early" polypeptides. Virology. 1980 Jul 15;104(1):230–234. doi: 10.1016/0042-6822(80)90381-5. [DOI] [PubMed] [Google Scholar]
- Hoeffler W. K., Kovelman R., Roeder R. G. Activation of transcription factor IIIC by the adenovirus E1A protein. Cell. 1988 Jun 17;53(6):907–920. doi: 10.1016/s0092-8674(88)90409-6. [DOI] [PubMed] [Google Scholar]
- Hoeffler W. K., Roeder R. G. Enhancement of RNA polymerase III transcription by the E1A gene product of adenovirus. Cell. 1985 Jul;41(3):955–963. doi: 10.1016/s0092-8674(85)80076-3. [DOI] [PubMed] [Google Scholar]
- Howe J. G., Shu M. D. Isolation and characterization of the genes for two small RNAs of herpesvirus papio and their comparison with Epstein-Barr virus-encoded EBER RNAs. J Virol. 1988 Aug;62(8):2790–2798. doi: 10.1128/jvi.62.8.2790-2798.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis S. C. Inhibition of host protein synthesis and degradation of cellular mRNAs during infection by influenza and herpes simplex virus. Mol Cell Biol. 1982 Dec;2(12):1644–1648. doi: 10.1128/mcb.2.12.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang K. L., Latchman D. S. HSV infection induces increased transcription of Alu repeated sequences by RNA polymerase III. FEBS Lett. 1989 Dec 4;258(2):255–258. doi: 10.1016/0014-5793(89)81667-9. [DOI] [PubMed] [Google Scholar]
- Kemp L. M., Brickell P. M., La Thangue N. B., Latchman D. S. Transcriptional induction of cellular gene expression during lytic infection with herpes simplex virus. Biosci Rep. 1986 Nov;6(11):945–951. doi: 10.1007/BF01114970. [DOI] [PubMed] [Google Scholar]
- Kemp L. M., Dent C. L., Latchman D. S. Octamer motif mediates transcriptional repression of HSV immediate-early genes and octamer-containing cellular promoters in neuronal cells. Neuron. 1990 Feb;4(2):215–222. doi: 10.1016/0896-6273(90)90096-x. [DOI] [PubMed] [Google Scholar]
- Kemp L. M., Latchman D. S. Differential regulation of octamer-containing cellular genes by the herpes simplex virus virion protein Vmw65 is mediated by sequence differences in the octamer element. EMBO J. 1988 Dec 20;7(13):4239–4244. doi: 10.1002/j.1460-2075.1988.tb03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp L. M., Latchman D. S. Induction and repression of cellular gene transcription during herpes simplex virus infection are mediated by different viral immediate-early gene products. Eur J Biochem. 1988 Jun 1;174(2):443–449. doi: 10.1111/j.1432-1033.1988.tb14118.x. [DOI] [PubMed] [Google Scholar]
- Kemp L. M., Latchman D. S. The herpes simplex virus type 1 immediate-early protein ICP4 specifically induces increased transcription of the human ubiquitin B gene without affecting the ubiquitin A and C genes. Virology. 1988 Sep;166(1):258–261. doi: 10.1016/0042-6822(88)90170-5. [DOI] [PubMed] [Google Scholar]
- Ladiges W. C., Raff R. F., Brown S., Deeg H. J., Storb R. The canine major histocompatibility complex. Supertypic specificities defined by the primed lymphocyte test (PLT). Immunogenetics. 1984;19(4):359–365. doi: 10.1007/BF00345410. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Martin P. L., Roeder R. G. Transcription of class III genes: formation of preinitiation complexes. Science. 1983 Nov 18;222(4625):740–748. doi: 10.1126/science.6356356. [DOI] [PubMed] [Google Scholar]
- Latchman D. S. Current status review: molecular biology of herpes simplex virus latency. J Exp Pathol (Oxford) 1990 Feb;71(1):133–141. [PMC free article] [PubMed] [Google Scholar]
- Latchman D. S., Estridge J. K., Kemp L. M. Transcriptional induction of the ubiquitin gene during herpes simplex virus infection is dependent upon the viral immediate-early protein ICP4. Nucleic Acids Res. 1987 Sep 25;15(18):7283–7293. doi: 10.1093/nar/15.18.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- MacLean A. R., Brown S. M. A herpes simplex virus type 1 variant which fails to synthesize immediate early polypeptide VmwIE63. J Gen Virol. 1987 May;68(Pt 5):1339–1350. doi: 10.1099/0022-1317-68-5-1339. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Degradation of cellular mRNA during infection by herpes simplex virus. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2370–2374. doi: 10.1073/pnas.74.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Requirement of protein synthesis for the degradation of host mRNA in Friend erythroleukemia cells infected wtih herpes simplex virus type 1. J Virol. 1978 Sep;27(3):619–627. doi: 10.1128/jvi.27.3.619-627.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare P., Goding C. R. Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell. 1988 Feb 12;52(3):435–445. doi: 10.1016/s0092-8674(88)80036-9. [DOI] [PubMed] [Google Scholar]
- Paolella G., Lucero M. A., Murphy M. H., Baralle F. E. The Alu family repeat promoter has a tRNA-like bipartite structure. EMBO J. 1983;2(5):691–696. doi: 10.1002/j.1460-2075.1983.tb01486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Chan W. L., Kemp L. M., La Thangue N. B., Latchman D. S. Isolation of cDNA clones derived from a cellular gene transcriptionally induced by herpes simplex virus. Nucleic Acids Res. 1986 Jul 25;14(14):5629–5640. doi: 10.1093/nar/14.14.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Stable C., Ayres T. M., Shen C. K. Distinctive sequence organization and functional programming of an Alu repeat promoter. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5291–5295. doi: 10.1073/pnas.81.17.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Stable C., Shen C. K. Competitive and cooperative functioning of the anterior and posterior promoter elements of an Alu family repeat. Mol Cell Biol. 1986 Jun;6(6):2041–2052. doi: 10.1128/mcb.6.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979 Jan;29(1):275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. M., Frame M. C., Campbell M. E. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988 Feb 12;52(3):425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- Rice S. A., Knipe D. M. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J Virol. 1990 Apr;64(4):1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice S. A., Su L. S., Knipe D. M. Herpes simplex virus alpha protein ICP27 possesses separable positive and negative regulatory activities. J Virol. 1989 Aug;63(8):3399–3407. doi: 10.1128/jvi.63.8.3399-3407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. H. The origin and evolution of retroposons. Int Rev Cytol. 1985;93:187–279. doi: 10.1016/s0074-7696(08)61375-3. [DOI] [PubMed] [Google Scholar]
- Roizman B., Sears A. E. An inquiry into the mechanisms of herpes simplex virus latency. Annu Rev Microbiol. 1987;41:543–571. doi: 10.1146/annurev.mi.41.100187.002551. [DOI] [PubMed] [Google Scholar]
- Sacks W. R., Greene C. C., Aschman D. P., Schaffer P. A. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985 Sep;55(3):796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H. R., Waldschmidt R., Jahn D., Seifart K. H. Purification of human transcription factor IIIC and its binding to the gene for ribosomal 5S RNA. Nucleic Acids Res. 1989 Jul 11;17(13):5003–5016. doi: 10.1093/nar/17.13.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H. R., Waldschmidt R., Seifart K. H. Human transcription factor IIIC contains a polypeptide of 55 kDa specifically binding to Pol III genes. Nucleic Acids Res. 1990 Aug 25;18(16):4743–4750. doi: 10.1093/nar/18.16.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe N. G., Isenberg D., Williams D. G., Latchman D. S. Elevation of the levels of SmB, B' and D proteins but not that of the La (SS-B) antigen during HSV infections. J Autoimmun. 1989 Oct;2(5):701–708. doi: 10.1016/s0896-8411(89)80008-3. [DOI] [PubMed] [Google Scholar]
- Stenberg R. M., Pizer L. I. Herpes simplex virus-induced changes in cellular and adenovirus RNA metabolism in an adenovirus type 5-transformed human cell line. J Virol. 1982 May;42(2):474–487. doi: 10.1128/jvi.42.2.474-487.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman D. J., Geiduschek E. P. Differential binding of a S. cerevisiae RNA polymerase III transcription factor to two promoter segments of a tRNA gene. EMBO J. 1984 Apr;3(4):847–853. doi: 10.1002/j.1460-2075.1984.tb01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. K., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus. I. Patterns of ribonucleic acid synthesis in productively infected cells. J Virol. 1969 Jul;4(1):36–46. doi: 10.1128/jvi.4.1.36-46.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga S., Dean N., Han M., Berk A. J. Adenovirus stimulation of transcription by RNA polymerase III: evidence for an E1A-dependent increase in transcription factor IIIC concentration. EMBO J. 1986 Feb;5(2):343–354. doi: 10.1002/j.1460-2075.1986.tb04218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]