Abstract
Background:
Whether health inequalities of disease burden and medical utilization exist by ethnicity in Asian breast cancer (BC) patients remains unclear. The authors aim to measure ethnic disparities in disease burden and utilization among Mongolian and Han female BC patients in China.
Materials and methods:
Based on data extracted from Inner Mongolia Regional Health Information Platform, a retrospective cohort study was established during 2012–2021. Disease burden including incidence, 5-year prevalence, mortality, survival rate, and medical cost were analyzed and compared between Han and Mongolian patients.
Results:
A total of 34 878 female patients [mean (SD) age, 52.34 (10.93) years] were included among 18.19 million Chinese, and 4315 (12.03%) participants were Mongolian. Age-standardized rates of incidence are 32.68 (95% CI: 20.39–44.98) per 100 000. Higher age-specific incidence and 5-year prevalence were observed in Mongolian than in Han. The cost of BC annually per capita was significantly lower for Mongolian than Han [$1948.43 (590.11–4 776.42) vs. $2227.35 (686.65–5929.59), P<0.001]. Mongolian females showed higher all-cause mortality [30.92 (95% CI: 28.15–33.89) vs. 27.78 (95% CI: 26.77–28.83) per 1000, P=0.036] and BC-specific mortality [18.78 (95% CI: 16.64–21.13) vs. 15.22 (95% CI: 14.47–16.00) per 1000, P=0.002] than Han females. After adjusting covariates, Mongolian were associated with increased all-cause mortality [HR, 1.21, (95% CI: 1.09–1.34); P<0.001] and BC-specific mortality [HR, 1.31, (95% CI: 1.14–1.49); P<0.001].
Conclusion:
The findings of this cohort study highlight a higher level of disease burden with unmet medical demand in Mongolian patients, suggesting that more practical efforts should be made for the minority. Further research is needed to explore the concrete mechanisms of the disparities as well as eliminate health disproportion.
Keywords: breast cancer, ethnic disparities, medical utilization, cohort study
Introduction
Highlights
This study first reported the disease burden and healthcare utilization of female breast cancer in Inner Mongolia based on large-scale population-based data including 18.19 million adults. The largest Mongolian population, accounting for over 50% worldwide, was observed, which addressed the research gap of ethnic disparities within Asia.
Mongolian had higher ASR of incidence (P<0.001) and 5-year prevalence (P<0.001), all-cause mortality (P=0.036), and breast cancer-specific mortality (P=0.002).
Mongolian patients had a worse medical utilization, with a lower cost of breast cancer annually per capita ($1948.43 vs. $2227.35, P<0.001).
Mongolian patients had a worse prognosis than Han patients with significantly increased all-cause mortality (HR=1.21; P<0.001) and breast-specific mortality (HR=1.31; P<0.001).
Breast cancer (BC) is the most commonly diagnosed cancer and the leading cause of cancer death among females worldwide1. In China, BC was the first cause of cancer incidence among women1, accounting for 18.4% of the global BC cases2. The disease burden of BC is increasing both worldwide and in China3,4. However, disparities exist in disease burden among different groups of people5, in which race/ethnicity plays a crucial part. African Americans have been focused on by most studies about race/ethnicity for female breast cancer (FBC), where the disparities in disease burden6–9, inequalities in medical utilization10–12, and the possible factors such as socioeconomic status8,9,13, healthcare system10,12, genomic biomarker13–15, geographic region16,17, comorbidity11,18,19, and cultural background8,9 were reported. In contrast, Asians were included in only a few studies and often classified into ‘Others’20,21. Some studies reported the disease burden of FBC in Asian Americans and the disparities between them and other ethnic groups16,22–24 but it is inappropriate to extrapolate the conclusions to Asians because of the evident differences between Asian Americans and Asians25. Even in the group of Asian Americans, there is a call for further differentiation of the ethnicity due to the heterogeneity that has been reported26. Moreover, significant disparities in the disease burden of FBC have been observed within Asian countries at the national level27,28, indicating the necessity of further study within Asian ethnic groups.
In sum, few studies of ethnic disparities have been conducted in China, which contributed to the most BC patients (18.4% in 2020) in the world29. Inner Mongolian is the third largest province in China, and it has a population of 4.25 million Mongolian, accounting for over 70% of the Mongolian population in China and 50% of the world. Mongolian are one of the largest ethnic minorities in East Asia with more than 10 million population worldwide, of which 6.3 million are Chinese residents30. Therefore, exploring ethnic differences between Mongolian and Han helps people better understand the health equity status of the minority group not only in China but also worldwide. Inner Mongolia Regional Health Information Platform (IMRHIP) collects and administrates healthcare information of residents in Inner Mongolia to assist in addressing cancer health disparities across ethnicities31. This study was conducted to outline and analyze ethnic disparities of disease burden and medical utilization of BC based on IMRHIP.
Methods
Data sources
Data were obtained from IMRHIP, which integrated and collected data from National Basic Medical Insurance (NBMI), National Cancer Registry (NCR), and Cause-of-death Reporting System (CDRS) since 2012 with provincial coverage of 95% by 2020. The NBMI includes two databases, the Urban Employee Basic Medical Insurance database covering urban employees and the Urban Resident Basic Medical Insurance database covering unemployed urban and rural residents. It provides demographic characteristics of insured participants, records of inpatient and outpatient services, and cost information. The NCR collects, organizes, and analyzes tumor incidence, death, and survival data, which offers variables such as date of onset, age at diagnosis, disease diagnoses, tumor characteristics, etc. The CDRS collects data from national disease surveillance sites, National Health Commission, and some provinces and cities through an information management system. We obtained variables including the direct and underlying cause of death, International Classification of Disease code of the disease, date of death, etc. In sum, the advantages of IMRHIP include broad coverage, great interconnectivity between databases, accessibility to longitudinal and complete visit records, and rich variable information. More details can be checked elsewhere31.
The information about the Surveillance Cohort of All Cancers Species has been shared on the China Cohort Consortium (No. CCC2023050801). Patient information including demographics, disease diagnoses, tumor characteristics, cause of death, treatments, and expenses of inpatient and outpatient services were recorded and linked by encrypted ID numbers among databases. The patients were followed up until death or the end of the study, whichever came first. All records were deidentified to protect patient’s privacy and informed consent from participants was approved for exemption, approved by the Medical Ethics Committee of Inner Mongolia Autonomous Region Center for Disease Control and Prevention (NMCDCIRB2021001).
Study population
Patients diagnosed with BC between 1 January 2012, and 9 March 2021, in NBMI were identified based on diagnostic text and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code of BC (C50). Natural language processing was applied to standardize the diagnostic text and codes, which were conducted independently by two researchers and reviewed by a third one. Patients without ID numbers (10, 0.03%) and male patients (630, 1.77%) were excluded. According to ethnicity, Han patients and Mongolian patients were analyzed and compared as two cohort groups.
Measurements
Age at diagnosis was categorized into seven groups: 18–30, 31–40, 41–50, 51–60, 61–70, 71–80, and ≥816,9,13 due to the nonlinear association between age and outcomes. The tumor stage was defined as ‘early’, ‘advanced’, and ‘unknown’ according to ‘5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5)’32. If the patient is operable, the tumor stage would be defined as ‘early’. If the patient is diagnosed with inoperable locally advanced breast cancer (LABC) or metastatic breast cancer (MBC), the stage would be ‘advanced’. Based on the comorbidities diagnosed before and after the diagnosis of BC, the Charlson Comorbidity Index (CCI) score was calculated and categorized as 0, 1, 2, and ≥333,34. For medical insurance, there were two types: employee basic medical insurance and resident basic medical insurance. Alcohol consumption and smoking rates were obtained from the 2018 Adult Chronic Disease and Nutrition Monitoring Survey in Inner Mongolia31. GDP level of residence was based on gross domestic product (GDP) per capita in 202035. Education level was based on the average years of schooling in 2020 obtained from the national population census36. GDP level, education level, alcohol consumption rate, and smoking rate were stratified into two levels (high/low) by prefecture-level cities according to the median. Treatments for BC included surgery, chemotherapy, endocrine therapy, targeted therapy, and radiotherapy37.
Statistical analysis
First, incidence and 5-year prevalence were calculated. We set a window of two years (2012 and 2013) to exclude prevalent cases when processing the data from the NBMI, which was one of the considerable data sources of new cases38. Thus the incidence rates for each of the 7 years from 2014 to 2020 were calculated. The numerator of incidence was the number of new cases each year, and the numerator of 5-year prevalence was the number of BC patients alive during the past 5 years for each of the 5 years from 2017 to 202131. The denominator for incidence and 5-year prevalence was the total number of residents each year in the NBMI. Subgroup analyses were conducted according to the GDP level of the residence, medical insurance, and education level.
Second, health utilization was measured by costs annually per capita and per-episode (US $1.00=6.47 RMB on 15 July 2020). Medical costs on overall services and specifically for BC were calculated, and inpatient and outpatient costs were differentiated. The number of inpatient and outpatient visits per capita overall and specifically for BC were also recorded.
Third, 5-year, 3-year, 2-year, and 1-year survival rates and mortality rates were calculated. The 5-year survival rate, for example, was defined as the proportion of patients who survive more than 5 years after diagnosis among patients diagnosed that year. For survival rates, subgroup analyses were conducted according to the GDP level of the residence, age at diagnosis, medical insurance, and education level. Both the all-cause mortality rate and BC-specific mortality rate were measured, and they were equal to the number of deaths divided by the total number of observations. A stratified Cox model was performed for all-cause mortality as the proportional hazard assumption was not satisfied by stage. The Fine and Gray competing risk model was performed for BC-specific mortality to estimate hazard ratios (HR) and 95% CI. Age and stage at diagnosis, GDP level of residence, alcohol consumption rate, smoking rate, CCI, medical insurance, education level, and all treatments were adjusted.
Mean±standard error (SD), median (interquartile range, IQR), and frequency (proportion) were presented for normal continuous variables, non-normal continuous variables, and categorical variables, respectively. Age-standardized rates (ASR) were determined using the WHO world standardized rates, 2000–202539. The average estimates in the subgroups were calculated by combining the estimates each year using a random effects meta-analysis based on the Clopper–Pearson method40. The 95% CIs were estimated by the Poisson distribution40. χ 2 test, t test, and Kruskal–Wallis test were performed to compare the difference between variables of interest. All statistical analyses were performed using R version 4.1.2, and a two-sided P<0.05 was considered statistically significant. Data were analyzed and reported according to the Strengthening The Reporting of Observational Studies in Epidemiology (STROBE, Supplemental Digital Content 1, http://links.lww.com/JS9/C384) reporting guideline41 and the Strengthening The Reporting Of Cohort Studies in Surgery (STROCSS, Supplemental Digital Content 2, http://links.lww.com/JS9/C385) criteria42.
Results
Demographic characteristic
Within a total of 18.19 million participants in the cohort (eTable 1, Supplemental Digital Content 3, http://links.lww.com/JS9/C386), 35 508 BC patients were identified with a median follow-up of 3.16 years during 2012–2021, and 34 878 (98.23%) females were included in the study, contributing to 121 550.60 person-years. Four thousand three hundred fifteen (12.03%) females were Mongolian, who were more likely to be diagnosed 2 years younger compared with Han (50.20 vs. 52.67 years, P<0.001), had a higher proportion of resident basic medical insurance participants (30.52 vs. 14.93%, P<0.001), and more distributed in residences with lower GDP level (71.91 vs. 55.97%, P<0.001) and lower education level (67.72 vs. 43.89%, P<0.001) (Table 1).
Table 1.
Baseline characteristics of breast cancer patients during 2012–2021 in Inner Mongolia, grouped by ethnic groupsa.
| Overall | Han | Mongolian | P | |
|---|---|---|---|---|
| Total: no. (%) | 34 878 (98.23) | 29 151 (81.27) | 4 315 (12.03) | – |
| Age at diagnosis: x (s) | 52.34±10.93 | 52.67±10.93 | 50.20±10.48 | <0.001 |
| Follow-up: median (IQR) | 3.15 (1.54–5.09) | 3.15 (1.54–5.08) | 3.15 (1.46–4.98) | 0.132 |
| Stage | ||||
| Early | 13 852 (39.72) | 11 574 (39.70) | 1 723 (39.93) | 0.149 |
| Advanced | 12 246 (35.11) | 10 278 (35.26) | 1 447 (33.53) | |
| Unknown | 8 780 (25.17) | 72 99 (25.04) | 1 145 (26.44) | |
| Charlson comorbidity index scoreb | ||||
| 0 | 12 728 (40.81) | 11 753 (40.32) | 1 969 (45.63) | <0.001 |
| 1 | 1 610 (4.62) | 13 11 (4.50) | 218 (5.05) | |
| 2 | 2 491 (7.14) | 19 66 (6.74) | 406 (9.41) | |
| ≥3 | 16 544 (47.43) | 14 121 (48.44) | 1 722 (39.91) | |
| GDP level of residencec | ||||
| Low | 20 349 (58.52) | 16 315 (55.97) | 3 103 (71.91) | <0.001 |
| High | 14 430 (41.48) | 12 776 (43.83) | 1 188 (27.53) | |
| Medical insuranced | ||||
| Employee | 28 987 (83.11) | 24 810 (85.11) | 2 998 (69.48) | <0.001 |
| Resident | 5 891 (16.89) | 4 351 (14.93) | 1 317 (30.52) | |
| Education levele | ||||
| Low | 16 217 (46.63) | 12 770 (43.89) | 2 905 (67.72) | <0.001 |
| High | 18 558 (53.37) | 16 328 (56.11) | 1 385 (32.28) | |
GDP, gross domestic product.
There were missing data for GDP level of residence (99, 0.28%) and education level (103, 0.30%). For ethnic group, 10 patients (0.03%) were categorized into ‘others’.
Charlson comorbidity index score was calculated and categorized as 0, 1, 2, and ≥3.
GDP level of residence was stratified into two levels (high/low) by cities according to the median (66 377 RMB=$10 259.20).
There were two types of medical insurance: employee basic medical insurance and resident basic medical insurance.
Education level was stratified into two levels (high/low) by cities according to the average years of schooling in 2020.
Incidence and prevalence
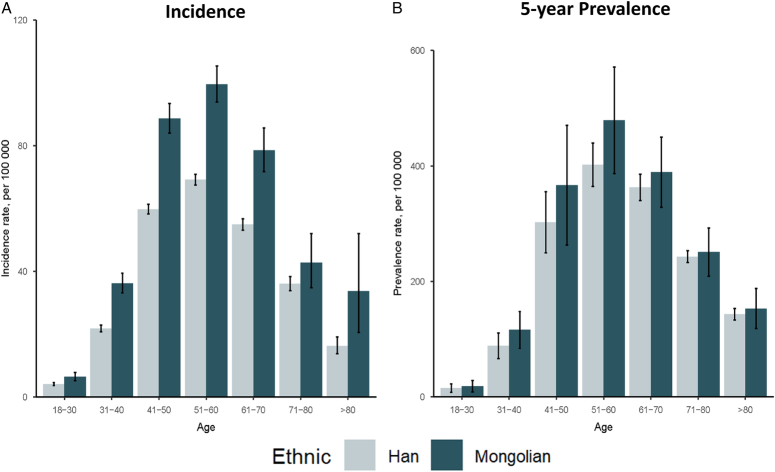
The ASR of incidence for FBC during 2014–2020 was 32.68 (95% CI: 20.39–44.98) per 100 000. The ASR of 5-year prevalence was 167.48 (95% CI: 124.30–210.67) per 100 000. The age-specific incidence and prevalence showed unimodal distributions for both ethnic groups with the same age of peak (Fig. 1). The ASR of incidence in Mongolian patients was significantly higher than in Han [44.77 (95% CI: 17.77–71.77) vs. 34.28 (95% CI: 10.61–57.95) per 100 000, P<0.001], and the incidence in 2019 and 2020 were also significantly higher in Mongolian patients (P=0.049 and P<0.001, respectively) (eTable 2, Supplemental Digital Content 3, http://links.lww.com/JS9/C386). Additionally, Mongolian showed significantly higher 5-year prevalence rates in all age groups [173.00 (95% CI: 84.72–261.28) vs. 152.35 (95% CI: 86.38–218.32) per 100 000, P<0.001] (eTable 3, Supplemental Digital Content 3, http://links.lww.com/JS9/C386).
Figure 1.
Incidence rates and 5-year prevalence rates in Inner Mongolia among breast cancer patients, per 100 000, grouped by ethnic group and age group. All results are the rates of disease burden grouped by ethnic group and age group, and the error lines in each bar chart show 95% CI. (A) Incidence rates; (B) 5-year prevalence rates. Note: Incidence rate (per 100 000) was calculated as number of new cases of breast cancer each year among population at risk each year. 5-year prevalence rate (per 100 000) was calculated as the number of people alive who diagnosed during the past 5 years among the population each year.
Healthcare utilization
Only 66.04% of female patients recorded treatments for BC, with a lower proportion of Mongolian receiving treatments than Han (59.86 vs. 66.93%, P<0.001) (Table 2). The overall cost was $3333.29 (1388.73–7130.97) and the cost of BC was $2147.14 (660.51–5698.42) annually per capita.
Table 2.
Cost of breast cancer patients in Inner Mongolian during 2012–2021, grouped by ethnic groups, median (IQR).
| Characteristics | Han | Mongolian | P |
|---|---|---|---|
| Patients with treatment of breast cancer (%) | 66.93 | 59.86 | <0.001 |
| Annual per-capita cost (dollars) | 3 404.00 (1 432.66–7 259.04) | 3 023.61 (1 198.06–6 494.60) | <0.001 |
| Annual per-capita cost on breast cancer (dollars) | 2 227.35 (686.65–5 929.59) | 1 948.43 (590.11–4 776.42) | <0.001 |
| Inpatient | |||
| Annual per-capita cost (dollars) | 2 797.87 (1 116.85–6 059.44) | 2 561.00 (979.88–5 474.45) | 0.162 |
| Annual per-capita cost on breast cancer (dollars) | 2 353.01 (778.00–6 000.94) | 2 021.41 (672.78–4 736.29) | <0.001 |
| Per-episode cost (dollars) | 1 050.72 (581.64–1 932.21) | 1 049.81 (576.59–1 891.22) | 0.008 |
| Per-episode cost on breast cancer (dollars) | 1 057.80 (582.47–1 937.89) | 1 057.66 (599.64–1 901.87) | <0.001 |
| Visit | 5.00 (2.00–10.00) | 6.00 (2.00–10.00) | <0.001 |
| Visit on breast cancer | 4.00 (2.00–9.00) | 4.00 (2.00–9.00) | 0.796 |
| Length of stay (d) | 5.00 (3.00–11.00) | 5.00 (3.00–10.00) | <0.001 |
| Length of stay on breast cancer (d) | 5.00 (3.00–11.00) | 5.00 (3.00–9.00) | <0.001 |
| Outpatient | |||
| Annual per-capita cost (dollars) | 368.05 (146.50–991.89) | 307.34 (138.24–841.78) | <0.001 |
| Annual per-capita cost for breast cancer (dollars) | 150.15 (40.82–537.74) | 181.29 (51.68–482.05) | 0.119 |
| Per-episode cost (dollars) | 14.84 (4.64 51.00) | 12.36 (4.59–40.34) | <0.001 |
| Per-episode cost for breast cancer (dollars) | 84.40 (20.09–322.76) | 106.57 (40.07–259.69) | <0.001 |
| Visit | 29.00 (8.00–67.00) | 28.00 (8.00–65.75) | 0.619 |
| Visit for breast cancer | 3.00 (1.00–7.00) | 4.00 (2.00–9.00) | <0.001 |
The RMB-to-USD exchange rate is based on the 15 July 2020, exchange rate (1.00 USD=6.47 RMB).
The cost of BC annually per capita was significantly lower for Mongolian than Han in FBC [$1948.43 (590.11–4776.42) vs. $2227.35 (686.65–5929.59), P<0.001]. For inpatient cost, Mongolian patients recorded significantly lower annual per-capita cost for BC [$2021.41 (672.78–4736.29) vs. $2353.01 (778.00–6000.94), P<0.001]. For the outpatient cost, Mongolian recorded lower annual per-capita cost [$307.34 (138.24–841.78) vs. $368.05 (146.50–991.89), P<0.001] and per-episode cost [$12.36 (4.59–40.34) vs. $14.84 (4.64–51.00), P<0.001] but a higher per-episode cost for BC [$106.57 (40.07–259.69) vs. $84.40 (20.09–322.76), P<0.001] and more visits for BC [4.00 (2.00–9.00) vs. 3.00 (1.00–7.00) days, P<0.001] (Table 2).
Survival and prognosis
The all-cause mortality was 28.14 per 1000 person-years (95% CI: 27.21–29.10), while BC-specific mortality was 15.66 per 1000 person-years. Mongolian females showed higher all-cause mortality [30.92 (95% CI: 28.15–33.89) vs. 27.78 (95% CI: 26.77–28.83) per 1000, P=0.036] and BC-specific mortality [18.78 (95% CI: 16.64–21.13) vs. 15.22 (95% CI: 14.47–16.00) per 1000, P=0.002] than Han females (Table 3). No significant differences were observed in 5-year survival rates (Table 3 and eTable 4, Supplemental Digital Content 3, http://links.lww.com/JS9/C386) due to the small sample size (eTable 5, Supplemental Digital Content 3, http://links.lww.com/JS9/C386).
Table 3.
Prognosis and survival rates in Inner Mongolia during 2012–2021 among breast cancer patients, grouped by ethnic groups.
| Characteristics | Han | Mongolian | P |
|---|---|---|---|
| No. of death | |||
| All-cause | 2 822 (9.68) | 456 (10.57) | 0.071 |
| Breast cancer-specific | 1 546 (5.30) | 277 (6.42) | 0.003 |
| Mortality, per 1 000 person-years | |||
| All-cause | 27.78 (26.77–28.83) | 30.92 (28.15–33.89) | 0.036 |
| Breast cancer-specific | 15.22 (14.47–16.00) | 18.78 (16.64–21.13) | 0.002 |
| 5-year survival rate (2012–2015 combined), % | 89.23 (87.10–91.37) | 89.58 (87.22–91.95) | 0.574 |
| 3-year survival rate (2012–2017 combined), % | 92.66 (91.69–93.62) | 93.37 (91.86–94.88) | 0.800 |
| 2-year survival rate (2012–2018 combined), % | 94.59 (93.78–95.39) | 94.69 (93.5–95.88) | 0.340 |
| 1-year survival rate (2012–2019 combined), % | 96.68 (96.15–97.21) | 97.03 (96.22–97.84) | 0.315 |
Taking 5-year survival rate as an example, 5-year survival rate was defined as the proportion of patients alive more than 5 years after diagnosis among patients diagnosed that year.
After adjusting covariates, Mongolian were associated with increased all-cause mortality [HR, 1.21, (95% CI: 1.09–1.34); P<0.001] and BC-specific mortality [HR, 1.31, (95% CI: 1.14–1.49); P<0.001]. Older age at diagnosis and resident basic medical insurance purchasers were related to higher risks of mortality. Lower GDP level of residence was related to higher all-cause mortality [HR, 1.23, (95% CI: 1.08–1.41); P=0.002], and lower education level was associated with lower BC-specific mortality [HR, 0.83, (95% CI: 0.72–0.96); P=0.013] (Table 4).
Table 4.
Hazard ratios for risk of all-cause mortality and breast cancer-specific mortality in Inner Mongolia among breast cancer patients during 2012–2021.
| All-cause mortalitya | Breast cancer-specific mortalityb | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Death | Person-year | HR (95% CI)a | P | Death | Person-year | HR (95% CI) | P |
| Age at diagnosis | ||||||||
| 18–30 | 36 | 84 | 1.00 [Reference] | 25 | 53 | 1.00 [Reference] | ||
| 31–40 | 227 | 587 | 1.09 (0.77–1.56) | 0.617 | 154 | 392 | 1.06 (0.70–1.61) | 0.790 |
| 41–50 | 878 | 2 084 | 1.32 (0.95–1.85) | 0.102 | 533 | 1 165 | 1.15 (0.77–1.71) | 0.500 |
| 51–60 | 1 075 | 2 349 | 1.82 (1.30–2.54) | <0.001 | 611 | 1 239 | 1.47 (0.98–2.19) | 0.060 |
| 61–70 | 732 | 1 599 | 2.56 (1.83–3.59) | <0.001 | 361 | 682 | 1.76 (1.17–2.64) | 0.006 |
| 71–80 | 367 | 751 | 4.96 (3.52–7.00) | <0.001 | 165 | 270 | 2.94 (1.93–4.49) | <0.001 |
| >80 | 97 | 154 | 12.83 (8.74–18.84) | <0.001 | 50 | 71 | 7.87 (4.83–12.81) | <0.001 |
| Ethnic | ||||||||
| Han | 2 819 | 6 305 | 1.00 [Reference] | 1 546 | 3 140 | 1.00 [Reference] | ||
| Mongolian | 455 | 962 | 1.21 (1.09–1.34) | <0.001 | 277 | 549 | 1.31 (1.14–1.49) | <0.001 |
| Others | 138 | 340 | 1.04 (0.88–1.24) | 0.632 | 76 | 184 | 1.05 (0.83–1.32) | 0.710 |
| GDP level of residence | ||||||||
| High | 1 289 | 3 030 | 1.00 [Reference] | 720 | 1 629 | 1.00 [Reference] | ||
| Low | 2 123 | 4 577 | 1.23 (1.07–1.42) | 0.004 | 1 179 | 2 244 | 1.22 (0.98–1.52) | 0.076 |
| Medical insurance | ||||||||
| Employee | 2 517 | 5 414 | 1.00 [Reference] | 1 386 | 2 771 | 1.00 [Reference] | ||
| Resident | 895 | 2 193 | 1.43 (1.32–1.55) | <0.001 | 513 | 1 102 | 1.39 (1.24–1.55) | <0.001 |
| Education level | ||||||||
| High | 1 820 | 4 385 | 1.00 [Reference] | 1 030 | 2 357 | 1.00 [Reference] | ||
| Low | 1 592 | 3 222 | 1.00 (0.90–1.11) | 0.961 | 869 | 1 516 | 0.83 (0.72–0.96) | 0.013 |
GDP, gross domestic product.
Stratified Cox model adjusted for age at diagnosis, ethnicity, GDP level of residence, medical insurance, education level, alcohol consumption rate level, smoking rate level, Charlson comorbidity index, surgery, chemotherapy, endocrine therapy, targeted therapy, radiotherapy, stage (strata) (n=34 775, excluded population=103).
Fine and Gray competing risk model, adjusted for age at diagnosis, ethnicity, GDP level of residence, medical insurance, education level, alcohol consumption rate level, smoking rate level, Charlson comorbidity index, surgery, chemotherapy, endocrine therapy, targeted therapy, radiotherapy, stage (strata) (n=34 775, excluded population=103).
Discussion
We described the disease burden, medical utilization, and ethnic disparities in both aspects of BC based on IMRHIP data from 2012 to 2021. Mongolian showed higher incidence and prevalence with lower costs, and they were associated with increased all-cause and BC-specific mortality, which indicates health inequalities in the minority.
A moderate level of incidence of FBC was observed in Inner Mongolia compared to other countries and regions. The ASR of incidence of FBC (32.68 per 100 000) was close to the previous result in China29 (39.1 per 100 000 in 2020) and other regions in Asia3,43, and much lower than European and American countries1,44, which was consistent with prior studies4,5,29. There may be several explanations. First, the incidence might be under-reported due to the lower compliance of BC screening. It is reported that screening attendance is lower among Asian women regardless of nativity or acculturation45–47, and the self-perception of low risk45, use of alternative medicine48, cultural custom45 are possible reasons. Second, population-related genetic predispositions may cause disparities in incidence. With similar prevalence and genetically inherited susceptibility of BRCA1/2 mutations49–51, BRCA1 was more common among western women while BRCA2 was more common among Asian women49,52, with BRCA1 related to higher risk than the other49,53. Also, Chinese women were reported to have a higher level of alpha-fetoprotein as a protective factor54. Lastly, higher socioeconomic status, educational level, and the accompanying lifestyle (e.g. more alcohol consumption and late marriage) in western developed countries may contribute to a higher incidence rate5,45,46,54.
Higher incidence and prevalence in Mongolian may be associated with the following factors: (1) Endocrine diseases such as obesity, and diabetes have been reported to increase the risk by increasing available estrogens55. Higher proportions of obesity and overweight population, as well as diabetes patients, were observed in Mongolian than in Han56,57. Furthermore, lifestyle (tobacco, alcohol, and diet) may increase the risk of obesity58, and Mongolian tended to consume red meat, more alcohol, and tobacco30,59. (2) Although no specific gene has been found, population genetic susceptibility may also play a part. Gene polymorphisms exist in BRCA1/2 between Mongolian and Han female patients with sporadic BC60,61, which might provide a clue. (3) Allergy-related diseases such as allergic rhinitis were also found higher in Mongolian than in Han62. A review suggested a higher risk of BC among patients with a history of allergic rhinitis63. In sum, Mongolian patients are faced with a higher disease burden of BC, appealing to more attention and inventions on these underserved minorities to eliminate ethnic disparities.
Another novel finding is the ethnic disparities in medical utilization. The cost of BC and the proportion of patients who received treatment were lower for Mongolian patients. Although few studies have described the ethnic disparities in cost in Asian areas, studies about African Americans showed that minorities were often disadvantaged in medical utilization10, which is consistent with our result. Potential reasons are as follows: for one thing, Mongolian showed a lower health literacy as fewer Mongolian females were willing to attend screening than Han females30. Our results also showed that Mongolian had a lower education level, which might have influenced their awareness of the disease and treatment12,18,64,65. For another, differences in medical insurance policies can lead to a gap in costs18,65. Traditional Mongolian medicine accounts for a majority of treatment strategies among Mongolian patients, in parallel with Western medicine and traditional Chinese medicine30, but it is normally uncovered by health insurance66. Additionally, Mongolian had a higher proportion of participants covered by resident basic medical insurance, which generally offers a lower reimbursement ratio compared to other types of medical insurance in this study. These unfavorable situations may discourage Mongolian from seeking more medical care. Last but not least, our result displayed that Mongolian patients were more distributed in the residences of lower GDP levels, suggesting a disadvantaged economic position. These factors hindered healthcare-seeking behaviors and aggravated the inequalities of healthcare utilization. Although gaps remain in determining the cause of increased or decreased cost and higher or lower financial burden65, the above discussion indicated Mongolian patients might be underserved with a higher burden.
Besides, the medical cost in this study was reported to be lower than that of western countries, which was consistent with prior studies67–69. The cost of BC was $2147.14 annually per capita in Inner Mongolia, which was lower than that of the United States ($9567 for patients under 65, from 2008 to 2012)70, Italy ($12 524, from 2007 to 2011)71, and Belgium ($2249, from 1997 to 2004)72. Taking the long span of the data into account, this probably resulted from the lower accessibility to newly expensive drugs and lower pricing in China. Take trastuzumab as an example. It was approved by the US Food and Drug Administration (FDA) in 1998 and the European Medicines Agency (EMA) in 200073. However, in China, trastuzumab was not included in the health insurance list until 201774. Observational studies73,75 indicated that between 2000 and 2015 in patients with HER2þ metastatic BC, nearly 12% in America did not receive trastuzumab or other HER2-targeted agent in their treatment, while in China, 27.1% of those did not receive trastuzumab at any time after diagnosis and 49.2% did not receive trastuzumab in the first-line setting76. As for the pricing, according to the 2022 Average Sale Price (ASP) and Chinese national drug prices, the cost of trastuzumab per mg in America ranged from $6.91–$10.37 but ranged from $1.55–$2.33 in China77. In sum, the lower drug accessibility and pricing represented by trastuzumab78 is an important factor in China’s lower medical expenses68,79.
Furthermore, we first demonstrated that Mongolian patients had a worse prognosis than Han patients with significantly higher all-cause mortality and breast-specific mortality. One of the main reasons contributing to the worse outcome is the delay in diagnosis and treatment9,47,48,64. Mongolian had a lower screening participation rate30, which is not conducive to timely intervention of the disease. Second, considering important prognosis indicators of BC, Mongolian patients manifested higher TNM stage, larger tumor size, lower estrogen receptor (ER), and progesterone receptor (PR) positivity, and a higher rate of axillary nodal metastasis80. Third, Mongolian were reported to have a 6–8 years younger average death age than Han81,82, and have a shorter life expectancy since 194983,84. This could partly explain the higher mortality in Mongolian. Fourth, low economic status9,14,85 and medical utilization12 implied difficulties in accessing medical resources, leading to worse survival. Our results showed residences with lower economic levels where Mongolian were more distributed were associated with higher all-cause mortality, which was consistent with prior studies. Also, many drugs were not covered by insurance in China, causing a high burden and limiting access to first-line treatment for disadvantaged people50. Last but not least, the aforementioned disadvantaged lifestyle accompanied by a higher prevalence of comorbidities30 could generate a higher risk of cancer death18,86.
In conclusion, our result reflected a potential unmet medical demand with a worse prognosis for Mongolian patients31, calling for stakeholders to take action to reduce the inequalities.
To our knowledge, this is the first study to reveal the ethnic disparities in the disease burden of BC based on 34 878 patients from 18.19 million general population in China. Several limitations should be taken into account when considering our findings. First, case ascertainment was restricted by the unavailability of laboratory data and imaging information obtained from medical insurance, but previous studies using NBMI to identify cancer cases have verified sensitivities and Positive predictive value (PPVs) above 90%87,88. Second, larger sample sizes were required since the small sample size of patients with more than 5 years of follow-up resulted in the low powers of survival analyses. Moreover, the above disparities were only observed in Inner Mongolia, which means that external verification in other populations is needed. Nonetheless, it is important to note that the population of this study is currently the largest Mongolian patient population. Finally, variables of interest could be more comprehensive. BMI, tumor grade, and subtype were not available in our study, which may influence the further interpretation. However, the disparities in disease burden and medical utilization still hold substantial importance89.
Conclusion
We revealed the ethnic disparities in the disease burden of BC based on large-scale population-based data in Inner Mongolia. Higher incidence and prevalence with worse prognosis and lower healthcare utilization were found in Mongolian than in Han. Mongolian BC patients with a higher level of disease burden might be faced with unmet medical demand, requiring more efforts on these minorities. To improve the health literacy of ethnic minorities, reform the health insurance system, and further implement the screening program among disadvantaged populations are feasible directions. Still, further research is needed to explore the concrete mechanisms of the disparities as well as eliminate health disproportion.
Ethical approval and consent to participate
Approved by the Medical Ethics Committee of the Inner Mongolia Center for Disease Control and Prevention (NMCDCIRB2021001), informed consent from participants was approved for exemption.
Consent
Approved by the Medical Ethics Committee of the Inner Mongolia Center for Disease Control and Prevention (NMCDCIRB2021001), informed consent from participants was approved for exemption.
Sources of funding
This work was supported by the Natural Science Foundation of China (No. 82173616 and No. 72342015).
Author contribution
J.C., L.Q., M.Q., Y.Z., Y.Y., Y.X., and S.W.: concept and design; J.C., L.Q., Y.X., Y.Z., H.Z., and S.W.: drafting the manuscript; J.C., L.Q., Y.Z., W.K., H.Z., Y.K., Y.J., Y.R., L.X., G.H., Y.Y., J.R., X.Y., S.D., X.Y., Y.S., Y.Y., Q.W., J.H., Y.W., G.L., M.W., X.Z., and S.W.: statistical analysis; Y.X. and S.W.: administrative, technical, or material support; Y.X. and S.W.: supervision. All authors contributed in acquisition, analysis, or interpretation of data and critical revision of manuscript for important intellectual content.
Conflicts of interest disclosure
Dr Shengfeng Wang reports grants from Natural Science Foundation of China (No. 82173616 and No. 72342015) during the conduct of the study. Dr. Shengfeng Wang confirmed that the funders did not play a role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication. No potential conflicts of interest were disclosed for the remaining authors.
Research registration unique identifying number (UIN)
ChiCTR1800018217.
Guarantor
Shengfeng Wang, PhD, Department of Epidemiology and Biostatistics, School of Public Health, Peking University, 38 Xueyuan Road, Haidian District, Beijing, 100191, China (shengfeng1984@126.com, 86-010-82801525).
Availability of data and materials
Yunfeng Xi and Shengfeng Wang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The datasets used and analyzed during the study are available from Yunfeng Xi on reasonable request.
Provenance and peer review
Not applicable.
Assistance with the study
Not applicable.
Presentation
Not applicable.
Supplementary Material
Acknowledgements
Not applicable.
Footnotes
Jieying Chen, BA, Liying Qiao, and Meng Qi contributed equally to this work.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Published online 11 April 2024
Contributor Information
Jieying Chen, Email: 2110120136@stu.pku.edu.cn;13790183901@163.com.
Liying Qiao, Email: qiaoliyingdoll@163.com.
Meng Qi, Email: qimeng-lisa@163.com.
Yunjing Zhang, Email: 936561567@pku.edu.cn.
Ying Yan, Email: yanying_pku@163.com.
Weiwei Kang, Email: kx_260233@hotmail.com.
Huziwei Zhou, Email: zhzw@pku.edu.cn.
Yuelin Yu, Email: yyl26my@163.com.
Yalei Ke, Email: keyalei@pku.edu.cn.
Yuling Jiang, Email: 1810306105@pku.edu.cn.
Yingting Rao, Email: raoyingting@pku.edu.cn.
Lu Xu, Email: luxu@bjmu.edu.cn.
Guohua He, Email: heguohua@hotmail.com.
Jing Ren, Email: 475010108@qq.com.
Xue Yan, Email: yanxue0331@163.com.
Siwei Deng, Email: a597575996@163.com.
Xinyu Yang, Email: 1910306221@pku.edu.cn.
Yutong Song, Email: 1910306227@pku.edu.cn.
Yingzi Yang, Email: yingzi0595@gmail.com.
Qiaorui Wen, Email: wqr119@163.com.
Jing Han, Email: 15247095649@163.com.
Yiwei Wu, Email: w13948782139@163.com.
Guozhen Liu, Email: liu_guozhen@outlook.com.
Mingyuan Wang, Email: buctwang@163.com.
Xiaoyu Zhang, Email: 2010306133@stu.pku.edu.cn.
Yunfeng Xi, Email: xiyunfeng210@163.com.
Shengfeng Wang, Email: shengfeng1984@126.com.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Cao W, Chen H-D, Yu Y-W, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J 2021;134:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu N, Yang D-W, Wu Y-X, et al. Burden, trends, and risk factors for breast cancer in China from 1990 to 2019 and its predictions until 2034: an up-to-date overview and comparison with those in Japan and South Korea. BMC Cancer 2022;22:826–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang P, Zhou C, Zhao K, et al. Associations of air pollution and greenness with global burden of breast cancer: an ecological study. Environm Sci Pollut Res 2023;30:103921–103931. [DOI] [PubMed] [Google Scholar]
- 5. Jiang D, Niu Z, Tan X, et al. The mortalities of female-specific cancers in China and other countries with distinct socioeconomic statuses: a longitudinal study. J Adv Res 2023;49:127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giaquinto AN, Sung H, Miller KD, et al. Breast cancer statistics, 2022. CA Cancer J Clin 2022;72:524–541. [DOI] [PubMed] [Google Scholar]
- 7. Ellington TD, Henley SJ, Wilson RJ, et al. Trends in breast cancer mortality by race/ethnicity, age, and US census region, United States─1999‐2020. Cancer 2022;129:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jatoi I, Sung H, Jemal A. The emergence of the racial disparity in U.S. Breast-Cancer Mortality. N Engl J Med 2022;386:2349–2352. [DOI] [PubMed] [Google Scholar]
- 9. Lemos LLP, Souza MC, Guerra AA, et al. Racial disparities in breast cancer survival after treatment initiation in Brazil: a nationwide cohort study. The Lancet Global Health 2024;12:e292–e305. [DOI] [PubMed] [Google Scholar]
- 10. Sheckter CC, Matros E. Discussion: racial disparities in the cost of unplanned hospitalizations after breast reconstruction. Plast Reconst Surg 2023;152:291–292. [DOI] [PubMed] [Google Scholar]
- 11. Nasser JS, Fahmy JN, Song Y, et al. Regional implicit racial bias and rates of breast reconstruction, complications, and cost among US patients with breast cancer. JAMA Netw Open 2023;6:e2325487–e2325499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Çeli̇k Y, Çeli̇k SŞ, Sariköse S, et al. Evaluation of financial toxicity and associated factors in female patients with breast cancer: a systematic review and meta-analysis. Support Care Cancer 2023;31:691–702. [DOI] [PubMed] [Google Scholar]
- 13. Hoskins KF, Calip GS, Huang H-C, et al. Association of social determinants and tumor biology with racial disparity in survival from early-stage, hormone-dependent breast cancer. JAMA Oncol 2023;9:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iyer HS, Zeinomar N, Omilian AR, et al. Neighborhood disadvantage, African genetic ancestry, cancer subtype, and mortality among breast cancer survivors. JAMA Netw Open 2023;6:e2331295–e2331310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lipsyc-Sharf M, Ballman KV, Campbell JD, et al. Age, body mass index, tumor subtype, and racial and ethnic disparities in breast cancer survival. JAMA Netw Open 2023;6:e2339584–e2339597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sung H, Wiese D, Jatoi I, et al. State variation in racial and ethnic disparities in incidence of triple-negative breast cancer among US women. JAMA Oncol 2023;9:700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reeder-Hayes KE, Jackson BE, Baggett CD, et al. Race, geography, and risk of breast cancer treatment delays: a population-based study 2004–2015. Cancer 2023;129:925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schermerhorn MC, Grunvald MW, O’Donoghue CM, et al. Factors mediating racial/ethnic disparities in delayed treatment of breast cancer. Ann Surg Oncol 2022;29:7652–7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stabellini N, Dmukauskas M, Bittencourt MS, et al. Social determinants of health and racial disparities in cardiac events in breast cancer. J Natl Comprehens Cancer Netw 2023;21:705–14.e17. [DOI] [PubMed] [Google Scholar]
- 20. Shoemaker ML, White MC, Wu M, et al. Differences in breast cancer incidence among young women aged 20-49 years by stage and tumor characteristics, age, race, and ethnicity, 2004 -2013. Breast Cancer Res Treat 2018;169:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ellington TD, Miller JW, Henley SJ, et al. Trends in breast cancer incidence, by race, ethnicity, and age among women aged ≥20 years - United States, 1999-2018. MMWR Morb Mortal Wkly Rep 2022;71:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen T, Kharazmi E, Fallah M. Race and ethnicity–adjusted age recommendation for initiating breast cancer screening. JAMA Netw Open 2023;6:e238893–e238900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gomez SL, Noone A-M, Lichtensztajn DY, et al. Cancer incidence trends among Asian American populations in the United States, 1990-2008. J Natl Cancer Inst, 105:1096–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCracken M, Olsen M, Chen MS, Jr, et al. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin 2007;57:190–205. [DOI] [PubMed] [Google Scholar]
- 25. Gomez SL, Yao S, Kushi LH, et al. Is breast cancer in Asian and Asian American women a different disease? JNCI 2019;111:1243–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eden CM, Johnson J, Syrnioti G, et al. The Landmark Series: the breast cancer burden of the Asian American population and the need for disaggregated data. Ann Surg Oncol 2023;30:2121–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Iqbal J, Ginsburg O, Rochon PA, et al. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 2015;313:165–173. [DOI] [PubMed] [Google Scholar]
- 28. Ly D, Forman D, Ferlay J, et al. An international comparison of male and female breast cancer incidence rates. Int J Cancer 2013;132:1918–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lei S, Zheng R, Zhang S, et al. Global patterns of breast cancer incidence and mortality: A population-based cancer registry data analysis from 2000 to 2020. Cancer Commun (Lond) 2021;41:1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu Y, Qiao L, Han J, et al. Integrated database-based Screening Cohort for Asian Nomadic descendants in China (Scan-China): insights on prospective ethnicity-focused cancer screening. Epidemiol Health 2023;45:e2023048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou HZ, Qiao LY, Zhang YJ, et al. Association of ethnicity, sex, and age with cancer diagnoses and health care utilization among children in Inner Mongolia, China. JAMA Netw Open 2022;5:e2231182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020;31:1623–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 34. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 35. Bureau IMARS [Internet] . Annual data of gross regional product - city data in Inner Mongolia Autonomous Region Inner Mongolia: Inner Mongolia Autonomous Region Statistics Bureau; 2022. Accessed December 02, 2022. http://tj.nmg.gov.cn/datashow/easyquery/easyquery.htm?cn=B0103
- 36. Bureau IMARS [Internet] . Bulletin of the 7th National Census of Inner Mongolia Autonomous (No. 5)--- Education status of the population Inner Mongolia: Inner Mongolia Autonomous Region Statistics Bureau; 2021. Accessed March 04, 2024. https://www.nmg.gov.cn/tjsj/sjfb/tjsj/tjgb/202105/t20210526_1596841.html?slb=true
- 37. Chinese Society of Clinical Oncology . Breast cancer guidelines of Chinese Society of Clinical Oncology. People’s Medical Publishing House; 2021. [Google Scholar]
- 38. Tian H, Yang W, Hu Y, et al. Estimating cancer incidence based on claims data from medical insurance systems in two areas lacking cancer registries in China. EClinicalMedicine 2020;20:100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ahmad OB, Boschi-Pinto C, Lopez AD, et al. Age Standardization of rates: a new WHO standard. Global Programme on Evidence for Health Policy Discussion Paper Series 2000;31:1–14. [Google Scholar]
- 40. Zhang C, Feng J, Wang S, et al. Incidence of and trends in hip fracture among adults in urban China: A nationwide retrospective cohort study. PLoS Med 2020;17:e1003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 42. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional a and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 43. Aryannejad A, Saeedi Moghaddam S, Mashinchi B, et al. National and subnational burden of female and male breast cancer and risk factors in Iran from 1990 to 2019: results from the Global Burden of Disease study 2019. Breast Cancer Res 2023;25:47–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taylor C, McGale P, Probert J, et al. Breast cancer mortality in 500 000 women with early invasive breast cancer diagnosed in England, 1993-2015: population based observational cohort study. BMJ 2023;381:e074684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gao Y, Heller SL. Health disparity and breast cancer outcomes in Asian women. Radiographics 2022;42:1912–1924. [DOI] [PubMed] [Google Scholar]
- 46. Wang W, Wang Y, Qi X, et al. Spatial pattern and environmental drivers of breast cancer incidence in Chinese women. Environm Sci Pollution Res 2023;30:82506–82516. [DOI] [PubMed] [Google Scholar]
- 47. Sivasubramaniam PG, Zhang B-L, Zhang Q, et al. Breast cancer disparities: a multicenter comparison of tumor diagnosis, characteristics, and surgical treatment in China and the U.S. Oncologist 2015;20:1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Petrova D, Garrido D, Špacírová Z, et al. Duration of the patient interval in breast cancer and factors associated with longer delays in low-and middle-income countries: A systematic review with meta-analysis. Psycho-Oncol 2022;32:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kwong A, Shin VY, Ho JCW, et al. Comprehensive spectrum of BRCA1 and BRCA2 deleterious mutations in breast cancer in Asian countries. J Med Genet 2016;53:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fan L, Strasser-Weippl K, Li J-J, et al. Breast cancer in China. Lancet Oncol 2014;15:e279–e289. [DOI] [PubMed] [Google Scholar]
- 51. Wang H, MacInnis RJ, Li S. Family history and breast cancer risk for Asian women: a systematic review and meta-analysis. BMC Med 2023;21:239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Song Q-K, Li J, Huang R, et al. Age of diagnosis of breast cancer in China: almost 10 years earlier than in the United States and the European Union. Asian Pac J Cancer Prev 2014;15:10021–10025. [DOI] [PubMed] [Google Scholar]
- 53. Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017;317:2402–2416. [DOI] [PubMed] [Google Scholar]
- 54. Lambe M, Trichopoulos D, Hsieh C-C, et al. Ethnic differences in breast cancer risk: a possible role for pregnancy levels of alpha-fetoprotein? Epidemiology 2003;14:85–89. [DOI] [PubMed] [Google Scholar]
- 55. Siiteri PK. Adipose tissue as a source of hormones. Am J Clin Nutr 1987;45:277–282. [DOI] [PubMed] [Google Scholar]
- 56. Tong X, Wang X, Wang D, et al. Prevalence and ethnic pattern of overweight and obesity among middle-aged and elderly adults in China. Eur J Prev Cardiol, 26:1785–1789. [DOI] [PubMed] [Google Scholar]
- 57. Li N, Wang H, Yan Z, et al. Ethnic disparities in the clustering of risk factors for cardiovascular disease among the Kazakh, Uygur, Mongolian and Han populations of Xi njiang: a cross-sectional study. BMC Public Health 2012;12:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Scala M, Bosetti C, Bagnardi V, et al. Dose-response relationships between cigarette smoking and breast cancer risk: a systematic review and meta-analysis. J Epidemiol 2023;33:640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jia L, Lu H, Wu J, et al. Association between diet quality and obesity indicators among the working-age adults in Inner Mongolia, Northern China: a cross-sectional study. BMC Public Health 2020;20:1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jinzhu MA, Zhao T, Liu M, et al. Association between polymorphism of the BRCA2 Gene rs206115 Loci and Sporadic Breast Cancer. J China Med Univ 2019;48:149–152. [Google Scholar]
- 61. Ma J, Liu M, Zhang X, et al. Correlation anslysis of sporadic breast cancer and BRCA1 gene plymorph isms in the Han Nationality and the Mongol Nationality of Inner Mongol ia Region. Zhonghua Yi Xue Za Zhi 2015;95:3746–3749. [PubMed] [Google Scholar]
- 62. Ma TT, Zhuang Y, Shi HY, et al. Epidemiology of allergic rhinitis in children in grassland of Inner Mongolia. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi, 54:571–575. [DOI] [PubMed] [Google Scholar]
- 63. Sadeghi F, Shirkhoda M. Allergy-related diseases and risk of breast cancer: the role of skewed immune system on this association. Allergy Rhinol (Providence) 2019;10:2152656719860820–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li J, Zhou J, Wang H, et al. Trends in disparities and transitions of treatment in patients with early breast cancer in China and the US, 2011 to 2021. JAMA Netw Open 2023;6:e2321388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ehsan AN, Wu CA, Minasian A, et al. Financial toxicity among patients with breast cancer worldwide. JAMA Netw Open 2023;6:e2255388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li M, Fan Y, McNeil EB, et al. Traditional Mongolian, Traditional Chinese, and Western Medicine Hospitals: System Review and Patient Survey on Expectations and Perceptions of Quality of Healthcare in Inner Mongolia, China. Evid Based Complement Alternat Med 2018;2018:2698461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fang CY, Frosch ZAK. Understanding and addressing cancer care costs in the United States. JAMA Netw Open 2021;4:e2127964–6. [DOI] [PubMed] [Google Scholar]
- 68. Diaby V, Sun L, Legood R, et al. Global treatment costs of breast cancer by stage: a systematic review. PLoS ONE 2018;13:e0207993–8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McGarvey N, Gitlin M, Fadli E, et al. Out-of-pocket cost by cancer stage at diagnosis in commercially insured patients in the United States. J Med Econ 2023;26:1318–1329. [DOI] [PubMed] [Google Scholar]
- 70. Zheng Z, Yabroff KR, Guy GP, et al. Annual medical expenditure and productivity loss among colorectal, female breast, and prostate cancer survivors in the United States. J Natl Cancer Inst 2015;108:382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Capri S, Russo A. Cost of breast cancer based on real-world data: a cancer registry study in Italy. BMC Health Serv Res 2017;17:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Broekx S, Hond ED, Torfs R, et al. The costs of breast cancer prior to and following diagnosis. Eur J Health Econ 2010;12:311–317. [DOI] [PubMed] [Google Scholar]
- 73. Blackwell K, Gligorov J, Jacobs I, et al. The global need for a trastuzumab biosimilar for patients with HER2-positive breast cancer. Clin Breast Cancer 2018;18:95–113. [DOI] [PubMed] [Google Scholar]
- 74.https://www.gov.cn/xinwen/2017-07/20/content_5211868.htm General Office of the State Council of the People’s Republic of China [Internet]. 36 kinds of high-priced just-in-need drugs have been included in the medical insurance; 2017. Accessed March 12, 2024.
- 75. Figueroa-Magalhães MC, Jelovac D, Connolly RM, et al. Treatment of HER2-positive breast cancer. The Breast 2014;23:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li J, Wang S, Wang Y, et al. Disparities of trastuzumab use in resource-limited or resource-abundant regions and its survival benefit on HER2 positive breast cancer: a real-world study from China. Oncologist 2017;22:1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tang Z, Xu X, Gao J, et al. Economic evaluation of margetuximab vs. trastuzumab for pretreated ERBB2-positive advanced breast cancer in the US and China. Front Public Health 2022;10:942767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schneider PP, Ramaekers BL, Pouwels X, et al. Direct medical costs of advanced breast cancer treatment: a real-world study in the southeast of the Netherlands. Value Health 2021;24:668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Taylor D. The reality of economics for oncologists. The Breast 2017;33:183–190. [DOI] [PubMed] [Google Scholar]
- 80. Song YL. The Contrast Analysis of Related Factors between Mongolian and Han Female with Breast Cancer (Master Thesis). Inner Mongolia University For Nationalities; 2013. Chinese. [Google Scholar]
- 81. Han RQ, Zhang XM, Han Y. Comparison of death related data in population in Yakeshi, Inner Mongolia, 2000 and 2010. Dis Surveill 2012;27:235–238; Chinese. [Google Scholar]
- 82. Lei CY, Wang GD, Tang JS, et al. Comparison of death causes in population in Hulunbeier in Inner Mongolia between 2000 and 2011. Dis Surveill 2012;27:898–902; Chinese. [Google Scholar]
- 83. Xu HY. Average life expectancy of Mongolian residents in Damao Banner, Inner Mongolia. Popul Res 1985;9:49–51; Chinese. [Google Scholar]
- 84. Shen BH. The Development of Mongolian and Other Minority Population in the region. J Inner Mongol Univ 1987;3:84–93; Chinese. [Google Scholar]
- 85. Binkley JM, Gabram S, Finley J, et al. Racial disparity in breast cancer survivorship: themes from a series of four national healthcare provider live virtual forums. J Cancer Survivorship 2023;17:1008–1016. [DOI] [PubMed] [Google Scholar]
- 86. Nicot-Cartsonis MS, Digbeu BDE, Raji MA, et al. Disparities in late-stage breast and colorectal cancer diagnosis among Hispanic, Non-Hispanic White, and Non-Hispanic Black patients: a retrospective cohort study of texas medicare beneficiaries. J Racial Ethnic Health Disparities 2022;10:3168–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Tian H, Xu R, Li F, et al. Identification of cancer patients using claims data from health insurance systems: a real-world comparative study. Chin J Cancer Res 2019;31:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shi C, Liu M, Liu Z, et al. Using health insurance reimbursement data to identify incident cancer cases. J Clin Epidemiol 2019;114:141–149. [DOI] [PubMed] [Google Scholar]
- 89. Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J 2019;40:3889–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Yunfeng Xi and Shengfeng Wang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The datasets used and analyzed during the study are available from Yunfeng Xi on reasonable request.