Abstract
Background:
The safety and efficacy of neoadjuvant immunochemotherapy (nICT) for locally advanced gastric cancer (LAGC) remain controversial.
Methods:
Patients with LAGC who received either nICT or neoadjuvant chemotherapy (nCT) at 3 tertiary referral teaching hospitals in China between January 2016 and October 2022 were analyzed. After propensity-score matching (PSM), comparing the radiological response, pathological response rate, perioperative outcomes, and early recurrence between the two groups.
Results:
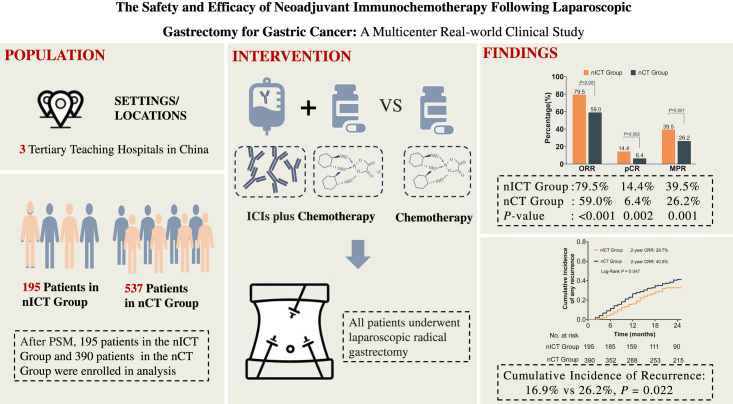
After PSM, 585 patients were included, with 195 and 390 patients comprising the nICT and nCT groups, respectively. The nICT group exhibited a higher objective response rate (79.5% vs. 59.0%; P<0.001), pathological complete response rate (14.36% vs. 6.41%; P=0.002) and major pathological response rate (39.49% vs. 26.15%; P=0.001) compared with the nCT group. The incidence of surgical complications (17.44% vs. 16.15%, P=0.694) and the proportion of perioperative textbook outcomes (80.0% vs. 81.0%; P=0.767) were similar in both groups. The nICT group had a significantly lower proportion of early recurrence than the nCT group (29.7% vs. 40.8%; P=0.047). Furthermore, the multivariable logistic analysis revealed that immunotherapy was an independent protective factor against early recurrence [odds ratio 0.62 (95% CI 0.41–0.92); P=0.018]. No significant difference was found in neoadjuvant therapy drug toxicity between the two groups (51.79% vs. 45.38%; P=0.143).
Conclusions:
Compared with nCT, nICT is safe and effective, which significantly enhanced objective and pathological response rates and reduced the risk for early recurrence among patients with LAGC.
Trial registration:
Clinical Trials.gov.
Keywords: efficacy, gastric cancer, laparoscopic gastrectomy, neoadjuvant immunochemotherapy, safety
Introduction
Highlights
The safety and efficacy of the perioperative use of immune checkpoint inhibitors (ICIs) in locally advanced gastric cancer (LAGC) remain controversial.
This multicenter clinical study found that neoadjuvant immunochemotherapy (nICT) is safe and effective, which significantly enhanced objective and pathological response rates, and reduced the risk for early recurrence among patients with LAGC.
These findings highlight the promising potential and wide-ranging application prospects for nICT.
Gastric cancer (GC) is a significant global health issue, ranking fifth among the most common malignant tumour and the fourth leading cause of cancer-related deaths worldwide1. Surgery remains the primary treatment option for GC2,3. In China, the proportion of locally advanced GC (LAGC) is accounting for ~80%4. Unfortunately, some patients with LAGC cannot undergo R0 resection at the time of initial diagnosis and are at a higher risk for postoperative recurrence, and have poor prognosis5, thus posing significant challenges in the management of GC.
In recent years, perioperative treatment has been widely promoted, leading to an increase in the R0 resection rate and surgical efficacy for LAGC. Several large clinical studies, including MAGIC, PRODIGY and RESOLVE, have demonstrated the advantages of neoadjuvant chemotherapy (nCT)6–9. These advantages include reduced preoperative tumour stage, improved R0 resection rate, enhanced patient tolerance to treatment, and prolonged overall survival (OS)10–12. Despite the benefits of nCT, traditional approaches rely primarily on chemotherapeutic agents to achieve an objective response rate (ORR) of ~50%13. Therefore, there is an urgent need for new therapeutic options to improve ORR in the neoadjuvant treatment of LAGC.
Immunotherapy has emerged as a promising treatment option in recent years, and its effectiveness in advanced GC has been demonstrated in numerous studies14–17. The use of immunotherapy in the perioperative treatment of LAGC is being explored, such as Keynote-585 and MATTERHORN, are investigating the safety and efficacy of neoadjuvant immunochemotherapy (nICT) compared with nCT in patients with LAGC18,19. However, real-world studies are urgently needed to provide guidance for the clinical application of neoadjuvant immunotherapy in patients with LAGC.
Laparoscopic gastrectomy (LG) has become the standard procedure for patients with LAGC in many experienced centres. It has been shown to have short- and long-term outcomes comparable to open gastrectomy (OG) while offering advantages such as reduced intraoperative bleeding, smaller incisions, and faster postoperative recovery20–23. Several previous studies have reported that nCT followed by LG does not significantly increase postoperative complications and is considered to be safe and feasible compared with OG24–28. In fact, the mechanism of immunotherapy is complex, and the potential impact of nICT on LG complications is not well established. Although previous studies have demonstrated that nCT followed by LG does not significantly increase postoperative complications, it remains unclear whether the addition of immunotherapy to the treatment regimen has any effect on the perioperative safety and prognosis of LG. Further research is required to investigate the potential complications and outcomes associated with nICT followed by LG in patients with LAGC.
As such, this study aimed to compare the safety and efficacy of nICT and nCT for LAGC using a strict propensity-score matching (PSM) approach based on data from multiple high-volume centres in China to provide valuable information for healthcare professionals to design optimal treatment protocols in clinical practice in cases for which uncertainties exist.
Methods
Patients
Clinicopathological data of patients with LAGC who underwent neoadjuvant therapy were collected from multiple high-volume centres in China between January 2016 and October 2022. The eligibility criteria were as follows: pathologically confirmed adenocarcinoma; clinical staging at cT2N+M0 or cT3-4bNanyM0 based on the Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC), 8th Edition guidelines; receiving nCT or nICT; undergoing LG after neoadjuvant therapy; and availability of complete clinicopathological data. Individuals with a history of other malignancies, an inability to undergo R0 resection, and those who underwent other anti-tumour treatments, such as preoperative radiotherapy or targeted therapy, were excluded. The study was approved by the Ethics Committees of the three participating research centres (2023KY160, 2023LWB046 and 2023YD079RS-01) and conformed to the Declaration of Helsinki guidelines. Informed consent was obtained from all enroled patients before perioperative treatment. This multicenter real-world clinical study has been reported in line with the Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery (STROCSS) criteria29. The study was registered at Clinical Trials.gov.
PSM analysis
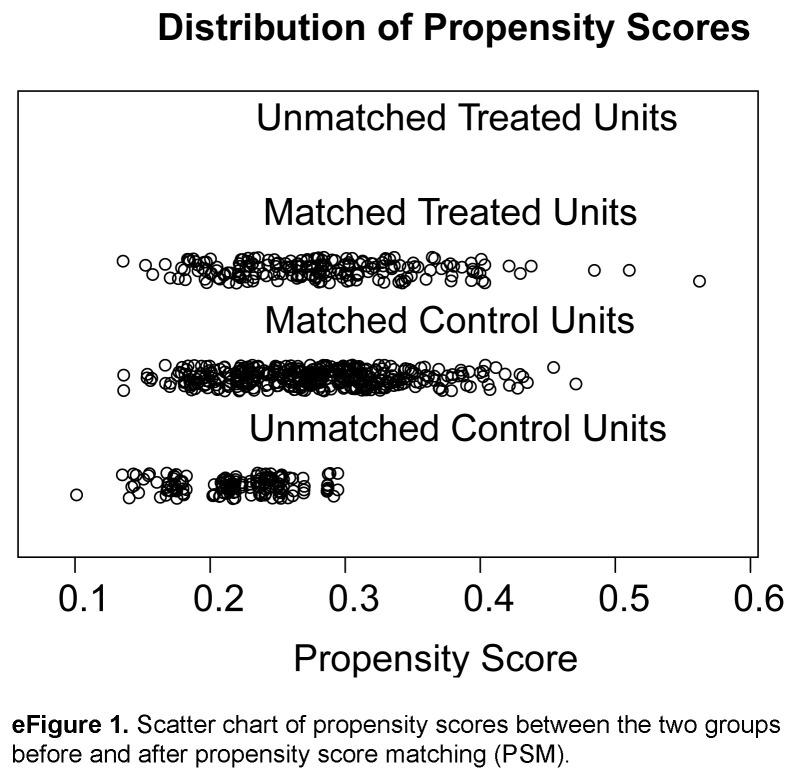
To minimize the effect of potential confounders in this retrospective study, PSM was used to remove potential preoperative influencing factors30. To enhance the comparability between the two groups, the research team held a consensus meeting to identify baseline characteristics that could affect surgical complications and outcomes. A total of 32 covariates (eTable 1, Supplemental Digital Content 1, http://links.lww.com/JS9/C421), including details of patient variables and tumour characteristics, were identified. Eleven factors [sex, age, BMI, history of laparotomy, age-corrected Charlson Comorbidity Index (aCCI), American Society of Anesthesiologists (ASA) class, tumour size, cT staging, cN staging, cTNM staging, and surgical type] were exactly matched using a logistic regression model by a biostatistician blinded to the outcomes. Greedy nearest neighbour matching (ratio 1:2 and matched without replacement) with a caliper width of 0.1 standard deviation of the estimated logit was performed. All data were compared between the nICT and nCT groups (n=195 and n=390, respectively) after PSM (Table 1 and eFigure 1, Supplemental Digital Content 8, http://links.lww.com/JS9/C422). In this study, an absolute standardized difference of<0.10 was considered to indicate a relatively small imbalance.
Table 1.
Baseline characteristics before and after propensity-score matching.
| Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|
| n (%)/mean (SD) | n (%)/mean (SD) | ||||||
| Variable | nICT group (n=195) | nCT group (n=537) | P | nICT group (n=195) | nCT group (n =390) | P | SMD |
| Age (year) | 62.13 (9.95) | 61.71 (10.10) | 0.611 | 62.13 (9.95) | 62.27 (9.73) | 0.872 | 0.014 |
| Sex | 0.690 | 0.810 | 0.030 | ||||
| Male | 147 (75.39) | 397 (73.93) | 147 (75.39) | 299 (76.67) | |||
| Female | 48 (24.61) | 140 (26.07) | 48 (24.61) | 91 (23.33) | |||
| BMI (kg/m2) | 22.01 (3.18) | 21.61 (3.04) | 0.127 | 22.01 (3.18) | 21.93 (3.08) | 0.756 | 0.027 |
| History of abdominal surgery | 0.983 | 0.969 | 0.014 | ||||
| Yes | 34 (17.44) | 94 (17.50) | 34 (17.44) | 66 (16.92) | |||
| No | 161 (82.56) | 443 (82.50) | 161 (82.56) | 324 (83.08) | |||
| aCCI score | 0.345 | 0.608 | 0.053 | ||||
| <5 | 77 (39.49) | 192 (35.75) | 77 (39.49) | 144 (36.92) | |||
| ≥5 | 118 (60.51) | 345 (64.25) | 118 (60.51) | 246 (63.08) | |||
| ASA Grade | 0.954 | 0.876 | 0.045 | ||||
| I | 14 (7.18) | 36 (6.70) | 14 (7.18) | 25 (6.41) | |||
| II | 152 (77.45) | 424 (78.96) | 152 (77.45) | 311 (79.74) | |||
| III | 29 (14.87) | 77 (14.34) | 29 (14.87) | 54 (13.85) | |||
| Tumour size (cm) | 0.037 | 0.578 | 0.056 | ||||
| <5 | 96 (49.23) | 218 (40.60) | 96 (49.23) | 181 (46.41) | |||
| ≥5 | 99 (50.77) | 319 (59.40) | 99 (50.77) | 209 (53.59) | |||
| cT | 0.468 | 0.938 | 0.032 | ||||
| T3 | 28 (14.36) | 67 (12.48) | 28 (14.36) | 56 (14.36) | |||
| T4a | 144 (78.85) | 389 (72.44) | 144 (78.85) | 284 (72.82) | |||
| T4b | 23 (11.79) | 81 (15.08) | 23 (11.79) | 50 (12.82) | |||
| cN | 0.271 | 1.000 | 0.012 | ||||
| N0 | 10 (5.13) | 40 (7.45) | 10 (5.13) | 19 (4.87) | |||
| N+ | 185 (94.87) | 497 (92.55) | 185 (94.87) | 371 (95.13) | |||
| Clinical stage TNM | 0.509 | 0.931 | 0.033 | ||||
| II | 23 (11.79) | 65 (12.11) | 23 (11.79) | 44 (11.28) | |||
| III | 149 (76.42) | 391 (72.81) | 149 (76.42) | 296 (75.90) | |||
| IVA | 23 (11.79) | 81 (15.08) | 23 (11.79) | 50 (12.82) | |||
| Surgical type | 0.068 | 0.793 | 0.033 | ||||
| Distal | 38 (19.49) | 75 (13.97) | 38 (19.49) | 71 (18.21) | |||
| Total | 157 (80.51) | 462 (86.03) | 157 (80.51) | 319 (81.79) | |||
aCCI, age-adjusted Charlson Comorbidity Index; ASA, American Society of Anesthesiologists; nCT, neoadjuvant chemotherapy; nICT, neoadjuvant chemotherapy combined with immunotherapy; PSM, propensity-score matching; SMD, standardized mean difference.
Therapeutic regimens for nICT and nCT
Neoadjuvant chemotherapy regimens were primarily categorized into two- and three-agent regimens. The two-agent regimens included: SOX (S-1 + oxaliplatin), CapeOx (capecitabine + oxaliplatin), AS (S-1 + nab-paclitaxel), FOLFOX (oxaliplatin + fluorouracil) and DS (S-1 + docetaxel). The three-agent regimens included: FLOT (docetaxel + oxaliplatin + fluorouracil), DOS (docetaxel + oxaliplatin + S-1) and POF (paclitaxel + oxaliplatin + fluorouracil). The immune checkpoint inhibitors (ICIs) used for neoadjuvant immunotherapy in this study included sintilimab, nivolumab, and camrelizumab. Dosages were calculated based on drug monographs, guidelines, and patient body surface area. The number of patients treated with each regimen is shown in eTable 2, Supplemental Digital Content 2, http://links.lww.com/JS9/C421. The patients underwent LG within 4–6 weeks after completing neoadjuvant therapy.
Laparoscopic surgery
All patients included in the present study underwent LG after the completion of neoadjuvant therapy, with experienced surgeons performing all procedures. For tumours located in the gastric antrum or the lower part of the gastric body, laparoscopic distal gastrectomy (LDG) and Billroth II gastrojejunostomy were performed; for tumours located in the upper part of the gastric body, gastric fundus, and cardia, laparoscopic total gastrectomy (LTG) and Roux-en-Y esophagojejunostomy were performed. Combined resection was performed in patients with local invasion of adjacent structures to achieve intraoperative R0 resection.
Definitions
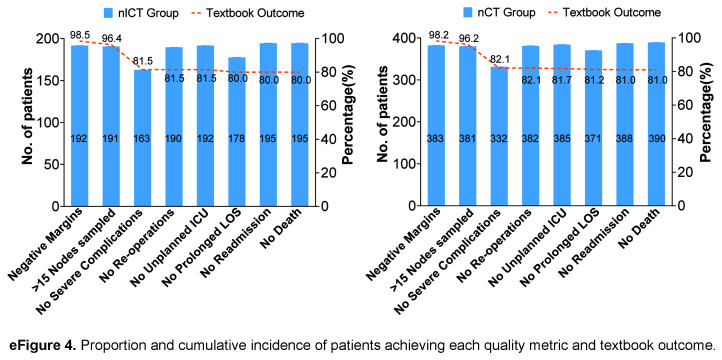
The study’s median follow-up duration was 38 months. The primary end point was pathological complete response (pCR), and the secondary end points were major pathological remission (MPR) and safety. Preoperative treatment response was assessed according to RECIST version 1.1 criteria31. The ORR was defined as the sum of the percentages of patients achieving complete response (CR) and partial response (PR). The disease control rate (DCR) was calculated by adding the rate of stable disease (SD) to ORR. Postoperative complications were defined as those occurring during hospitalization after surgery and graded using the Clavien–Dindo classification system32,33. Severe complications were defined as Clavien–Dindo greater than or equal to II grade. Tumour regression grading (TRG) was evaluated according to the Becker criteria34. The pCR was defined as the absence of residual cancer cells in both the primary site of the surgical specimen and the resected lymph nodes. Textbook outcome (TO) encompasses a comprehensive set of 8 quality metrics that evaluate various aspects of the surgical management of patients with GC undergoing gastrectomy35,36. These metrics included negative tumour margins, greater than 15 cleared lymph nodes, no severe complications, no unplanned reoperations, no unplanned ICU admissions, length of hospital stay no more than 21 days, no readmission within 30 days of discharge, and no mortality within 30 days of surgery. Early recurrence was defined as the patient experiencing recurrence within 2 years after surgery37. The recurrence-free survival (RFS) was defined as the period from the date of surgery to the first documented recurrence or metastasis. Overall survival (OS) was defined as the period from the date of surgery to the date of death or final follow-up.
Statistical analysis
Statistical analyses were performed using SPSS version 26.0 (IBM Corporation) and R version 4.3.1. To address baseline bias, PSM was performed at a 1:2 ratio. Categorical variables were assessed using the chi-squared test or Fisher’s exact test, whereas continuous variables were analyzed using the Student’s t-test or Mann–Whitney U test. Differences in the perioperative TO between the two groups were visualized using histograms and line graphs created using GraphPad Prism version 8.0 (GraphPad Inc.). The cumulative risk for tumour recurrence was analyzed using the log-rank test. Univariable and multivariable logistic regression analyses were performed to identify factors influencing recurrence. Variables with a value of P less than 0.05 in the univariate analysis were subsequently included in a multivariate logistic regression. Stepwise backward variable removal was applied to the multivariate model. Differences with P less than 0.05 were considered to be statistically significant.
Results
Baseline characteristics
Based on the inclusion and exclusion criteria, 732 patients from three high-volume centres were enroled, with 195 and 537 patients comprising the nICT and nCT groups, respectively. Before PSM, there was a significant difference in the distribution of patients between the two groups in terms of tumour size (P=0.036), and after 1:2 PSM, a total of 585 patients were incorporated into the analysis, including 195 patients in nICT group and 390 patients in nCT group (Fig. 1). There were no statistically significant differences in clinical baseline characteristics, including sex, age, BMI, history of laparotomy, aCCI, ASA class, tumour size, cT, cN, cTNM staging, and surgical type, between the two groups [standardized mean difference (SMD)<0.1 (all P > 0.05)] (Table 1).
Figure 1.
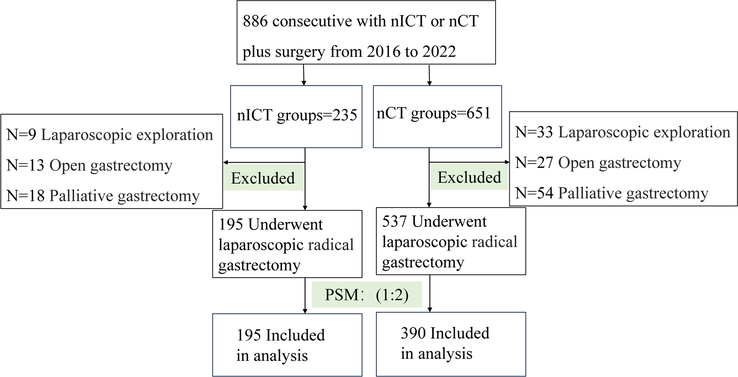
Flow-diagram illustrating the patient selection process. nCT, neoadjuvant chemotherapy; nICT, neoadjuvant chemotherapy combined with immunotherapy; PSM, propensity-score matching.
Neoadjuvant therapy and radiological response
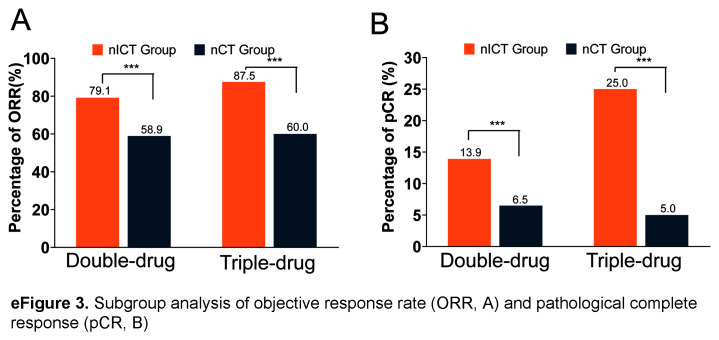
After PSM, there were no significant differences in neoadjuvant treatment cycles and chemotherapy regimens between the two groups (eTable 3, Supplemental Digital Content 3, http://links.lww.com/JS9/C421). According to the RECIST v1.1 criteria, patients in the nICT group exhibited had a higher CR rate (11.8% vs. 6.2%; P<0.001), PR rate (67.7% vs. 52.8%; P<0.001), ORR (79.5% vs. 59.0%; P<0.001), and DCR (99.5% vs. 98.5%; P=0.434) compared to the nCT group (eFigure 2, Supplemental Digital Content 9, http://links.lww.com/JS9/C424). Subgroup analysis indicated that the nICT group exhibited a higher ORR compared to the nCT group (Double-drug: 79.1% vs. 58.9%, P<0.05; Triple-drug: 87.5% vs. 60.0%, P<0.05; eFigure 3A, Supplemental Digital Content 10, http://links.lww.com/JS9/C423), regardless of the number of drugs used.
Surgical and pathology findings
The surgical and pathological characteristics of the two groups are summarized in Table 2. All patients underwent LG with D2 or D2+ lymph node dissection, and none required conversion to open surgery. Combined organ resection was performed in 17 (8.72%) and 25 patients (6.41%) in the nICT and nCT groups, respectively. A detailed list of the combined organs is presented in eTable 4, Supplemental Digital Content 4, http://links.lww.com/JS9/C421. LTG with Roux-en-Y gastrointestinal reconstruction was performed in 476 patients [nICT group, 157 patients (80.51%); nCT group, 319 patients (81.79%)], and LDG with Billroth II anastomosis was performed in 109 patients [nICT group, 38 patients (19.49%); nCT group, 71 patients (18.21%)]. Postoperative pathology indicated positive margins in 3 cases (1.54%) in the nICT group and 7 (1.79%) in the nCT group, and there was no significant difference between the 2 groups in terms of tumour site, degree of lymph node eradication, and length of the surgical incision (8.89±2.10 vs. 8.95±2.11; P=0.729).
Table 2.
Operative findings and pathological characteristics.
| nICT group (n=195) | nCT Group (n=390) | ||
|---|---|---|---|
| Variable | n (%)/mean (SD) | n (%)/mean (SD) | P |
| Reconstruction approach | 0.793 | ||
| Billroth II | 38 (19.49) | 71 (18.21) | |
| Roux-en-Y | 157 (80.51) | 319 (81.79) | |
| Lymph nodes dissection range | 0.777 | ||
| D2 | 187 (95.90) | 372 (95.38) | |
| D2+ | 8 (4.10) | 18 (4.62) | |
| Surgical radicalness | |||
| R0 | 192 (98.46) | 383 (98.21) | 0.822 |
| R1 | 3 (1.54) | 7 (1.79) | |
| Combined resection | 0.308 | ||
| Yes | 17 (8.72) | 25 (6.41) | |
| No | 17 8 (91.28) | 365 (93.59) | |
| Incision length (cm) | 8.89 (2.10) | 8.95 (2.11) | 0.729 |
| Tumour location | 0.217 | ||
| Upper | 91 (46.67) | 187 (47.95) | |
| Middle | 59 (30.26) | 90 (23.08) | |
| Lower | 42 (51.24) | 107 (27.44) | |
| Mix | 3 (1.53) | 6 (1.53) | |
| Lauren type | 0.378 | ||
| Intestinal | 93 (47.69) | 201 (51.54) | |
| Diffused | 73 (37.44) | 146 (37.43) | |
| Mixed | 29 (14.87) | 43 (11.03) | |
| Differentiation | 0.237 | ||
| Well/moderate | 76 (38.97) | 172 (44.11) | |
| Poor/undifferentiated | 119 (61.03) | 218 (55.89) | |
| Nerve invasion | 0.726 | ||
| Yes | 99 (50.77) | 204 (52.31) | |
| No | 96 (49.23) | 186 (47.69) | |
| Vascular invasion | 0.262 | ||
| Yes | 91 (46.67) | 163 (41.79) | |
| No | 104 (53.33) | 227 (58.21) | |
| No. lymph nodes dissected | 43.97 (17.89) | 39.54 (15.01) | 0.003 |
| Positive lymph nodes | 4.45 (7.63) | 4.71 (7.45) | 0.686 |
| ypT stage | 0.013 | ||
| ypT0 | 30 (15.38) | 28 (7.18) | |
| ypT1 | 25 (12.82) | 37 (9/49) | |
| ypT2 | 21 (10.77) | 46 (11.79) | |
| ypT3 | 56 (28.72) | 139 (35.64) | |
| ypT4 | 63 (32.31) | 140 (35.90) | |
| ypN stage | 0.161 | ||
| ypN0 | 85 (43.59) | 140 (35.90) | |
| ypN1 | 38 (19.49) | 87 (22.31) | |
| ypN2 | 34 (17.43) | 61 (15.64) | |
| ypN3 | 38 (19.49) | 102 (26.15) | |
| ypTNM stage | 0.008 | ||
| ypT0N0M0 | 28 (14.36) | 25 (6.41) | |
| I | 33 (16.92) | 56 (14.36) | |
| II | 52 (26.67) | 127 (32.56) | |
| III | 82 (42.05) | 182 (46.67) | |
| Tumour regression grade | 0.002 | ||
| 1a | 30 (15.38) | 27 (6.92) | |
| 1b | 47 (24.10) | 75 (19.23) | |
| 2 | 63 (32.31) | 142 (36.41) | |
| 3 | 55 (28.21) | 146 (37.44) | |
| pCR | 28 (14.36) | 25 (6.41) | 0.002 |
| MPR | 77 (39.49) | 102 (26.15) | 0.001 |
MPR, major pathological response; nCT, neoadjuvant chemotherapy; nICT, neoadjuvant chemotherapy combined with immunotherapy; pCR, pathological complete response.
Pathological results revealed that tumours in the nICT group had earlier ypT and ypTNM staging, and more lymph nodes were dissected than those in the nCT group (43.97±17.89 vs. 39.54±15.01; P=0.003). Furthermore, the tumours in the nICT group exhibited a greater extent of regression, with higher pCR rate (14.36% vs. 6.41%; P=0.002) and MPR rate (39.49% vs. 26.15%; P=0.001), while there was no significant difference in the distribution of patients between the two groups in terms of the differentiation, Lauren type, choroidal embolus, nerve invasion, pN staging and number of positive lymph nodes. Subgroup analysis showed that the nICT group exhibited a higher pCR rate compared to the nCT group (Double-drug: 13.9% vs 6.5%, P<0.05; Triple-drug: 25.0% vs 5.0%, P<0.05; eFigure 3B, Supplemental Digital Content 10, http://links.lww.com/JS9/C423).
Postoperative complications and 30-day perioperative outcomes
Thirty-four (17.44%) patients in the nICT group and 63 (16.15%) in the nCT group experienced postoperative complications, and there was no statistically significant difference in the incidence of total postoperative complications between the two groups (P=0.694). The most common postoperative complications included pulmonary infections (20 vs. 46 patients), abdominal infections (8 vs. 14 patients), and anastomotic fistulas (3 vs. 5 patients). According to the Clavien–Dindo grading of surgical complications, the severity of complications was similar between the two groups (16.41% vs. 14.87%; P=0.627). The detailed distribution of complications is shown in eTable 5, Supplemental Digital Content 5, http://links.lww.com/JS9/C421.
The percentage of 30-day perioperative TO was similar in both groups (80.0% vs. 81.0%; P=0.767) (eFigure 4, Supplemental Digital Content 11, http://links.lww.com/JS9/C425). There was no significant difference in the mean duration of surgery (214.75±49.03 vs. 211.65±44.71 min; P=0.554), intraoperative bleeding (68.59±106.78 vs 66.03±68.04 ml; P=0.831), perioperative transfusion (9.23% vs 6.15%; P=0.174), and use of antibiotics (25.64% vs 21.79%; P=0.298), between the two groups, in which it should be noted that the percentage of intraoperative haemorrhage (>200 ml) in the nICT group was less than that in the nCT group (4.10% vs. 9.23%; P=0.027). Postoperative recovery was comparable between the two groups, with time to first aerofluxus (2.61±0.81 vs. 2.64±0.71; P=0.695), time to first defecation (3.60±0.61 vs. 3.64±0.54; P=0.409), time to first fluid intake (4.43±2.18 vs. 4.41±2.56; P=0.927), and time to first semifluid intake (6.68±2.76 vs. 6.41±2.98; P=0.124) were not significantly different. None of the patients in either group died within 30 days, and the proportion of postoperative ICU admissions was similar (7.18% vs. 5.90%; P=0.548). The postoperative hospitalization time (9.11±5.07 vs. 9.00±7.43 days; P =.849), total cost (8456.74±3024.46 vs. 8523.71±4763.70 USD; P=0.877) neoadjuvant therapy to surgery time (107.48±58.64 vs. 101.96±41.68 days; P=0.190), the proportion of postoperative adjuvant therapy (90.26% vs. 90.26%; P=1.000), and the duration of postoperative adjuvant therapy (32.63±10.27 vs. 33.06±24.77 days; P=0.827) were comparable, and the differences were not statistically significant (eTable 6, Supplemental Digital Content 6, http://links.lww.com/JS9/C421).
Survival
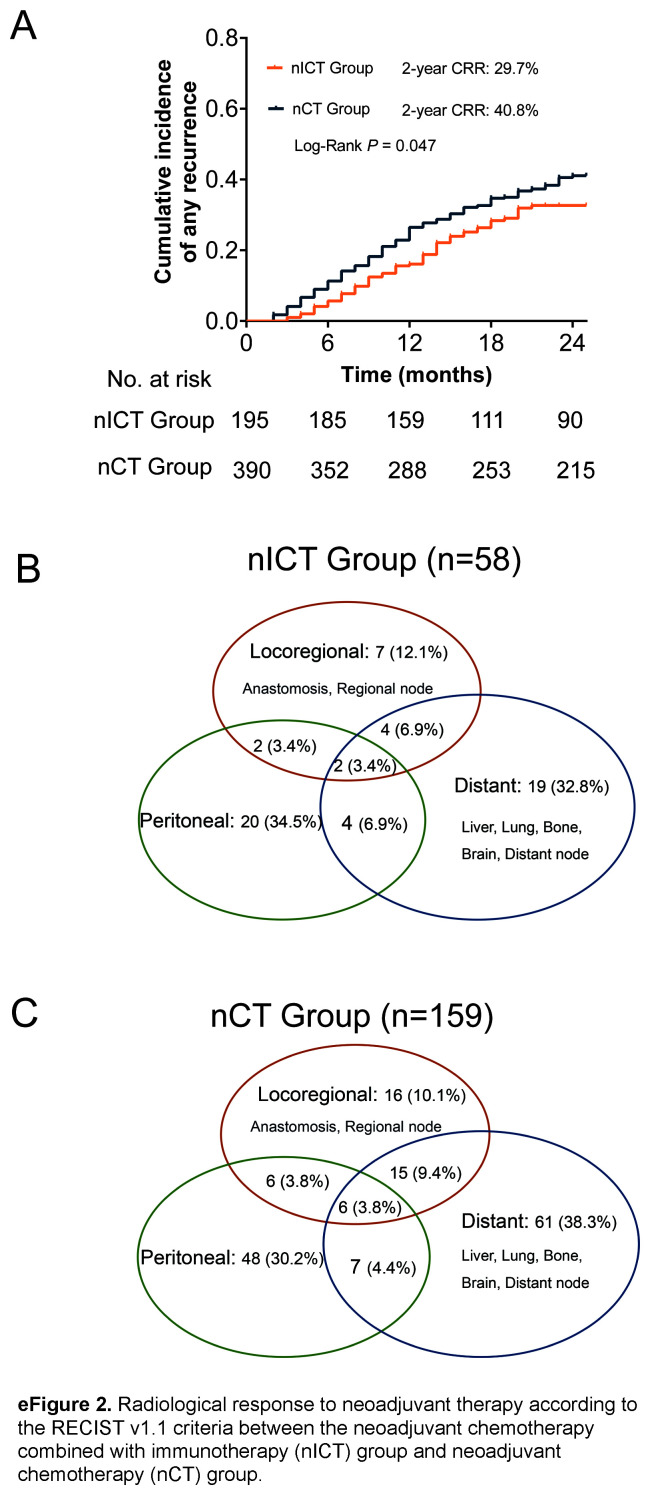
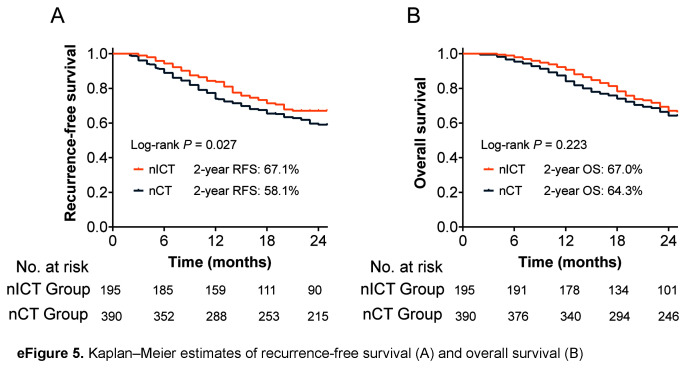
Regarding follow-up data, 58 patients in the nICT group and 159 in the nCT group experienced recurrence within 2 years, and the proportion of early recurrence in the nICT group was significantly lower than that in the nCT group (29.7% vs. 40.8%; P=0.047) (Fig. 2A). The pattern of distant metastasis, peritoneal metastasis, and local recurrence were similar between the two groups, as shown in Figs. 2B and C. Univariable and multivariable logistic regression analysis identified immunotherapy as an independent protective factor for early recurrence [OR 0.62 (95% CI 0.41–0.92)]; P=0.018] (eTable 7, Supplemental Digital Content 7, http://links.lww.com/JS9/C421). The RFS and OS curves obtained using the Kaplan–Meier method are shown in eFigure 5, Supplemental Digital Content 12, http://links.lww.com/JS9/C426. Compared to the nCT group, nICT group had a higher 2-year RFS (67.1% vs. 58.1%, P=0.027) and 2-year OS (67.0% vs. 64.3%, P=0.223).
Figure 2.
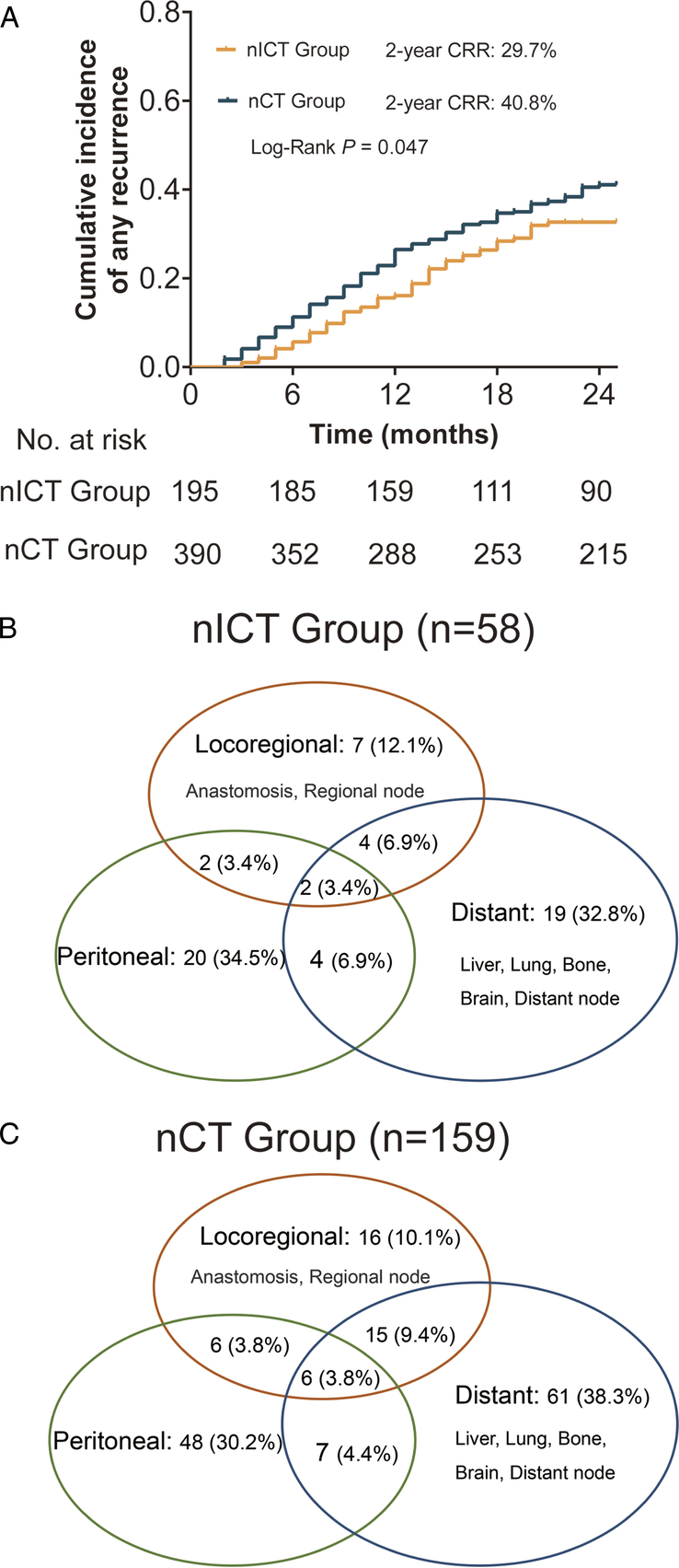
Kaplan–Meier curves for cumulative incidence of any recurrence for the neoadjuvant chemotherapy combined with immunotherapy (nICT) group versus the neoadjuvant chemotherapy (nCT) group (A). Venn diagram of recurrence patterns in the nICT (B), and nCT (C) groups.
Safety
In this study, the incidence of neoadjuvant therapy-related adverse events (nTRAEs) was slightly higher in the nICT group than in the nCT group (51.79% vs. 45.38%; P=0.143). However, the rate of grade 3/4 nTRAEs was comparable between the two groups (12.03% vs. 11.53%; P=0.786), and the difference was not statistically significant. The most common nTRAEs in both groups were hematopoietic events, such as leukopenia, neutropenia, and thrombocytopenia. In the immunotherapy group, hyper- or hypothyroidism was common (Table 3). None of the patients in either group died of nTRAEs during neoadjuvant therapy.
Table 3.
Neoadjuvant therapy-related adverse events.
| nICT group (n=195) | nCT group (n=390) | ||||
|---|---|---|---|---|---|
| Neoadjuvant therapy-related adverse events (nTRAEs) | Any grade | Grade≥3 | Any grade | Grade≥3 | P |
| Tumour perforation | 2 | 2 | 3 | 3 | |
| Tumour haemorrhage | 2 | 2 | 4 | 4 | |
| Bone marrow suppression | 70 | 21 | 145 | 40 | |
| White blood cell count decrease | 60 | 18 | 121 | 35 | |
| Neutrophil decrease | 55 | 18 | 120 | 35 | |
| Platelet decrease | 26 | 9 | 39 | 15 | |
| Transaminase elevation | 9 | 0 | 21 | 0 | |
| Rash | 4 | 0 | 7 | 0 | |
| Nausea or vomiting or diarrhoea | 25 | 0 | 44 | 0 | |
| Immune-related adverse events | |||||
| Hyperthyroidism | 7 | 0 | 0 | 0 | |
| Hypothyroidism | 8 | 0 | 0 | 0 | |
| Hypoadrenocorticism | 2 | 0 | 0 | 0 | |
| Reactive capillary endothelial proliferation | 4 | 0 | 0 | 0 | |
| Pneumonitis | 1 | 0 | 0 | 0 | |
| Myocarditis | 1 | 0 | 0 | 0 | |
| Overall nTRAEs rate [n (%)] | 101 (51.79) | 177 (45.38) | 0.143 | ||
| Severe nTRAEs ratea [n (%)] | 24 (12.03) | 45 (11.53) | 0.786 | ||
nCT, Neoadjuvant chemotherapy; nICT, Neoadjuvant chemotherapy combined with immunotherapy.
nTRAEs Grade≥3 was regarded as severe adverse events.
Discussion
In the past decade, there have been significant changes in treatment approaches to GCs, particularly with the emergence of ICIs for the treatment of unresectable, recurrent, and metastatic GC14,38. As a result, immunotherapy has also been considered for the perioperative treatment of LAGC. Nevertheless, the safety and effectiveness of LG in the treatment of GC after nICT remains unclear.
Neoadjuvant therapy has the potential to reduce the tumour burden, downgrade the preoperative tumour stage, and even achieve pCR6,7. However, it is important to note that the proportion of patients who benefit from nCT alone is limited. Recent studies have confirmed that nICT can significantly improve pCR rates and short-term outcomes in colorectal, lung, oesophageal, and triple-negative breast cancers39–42. nICT has the same effect in GC, and a prospective phase II clinical study reported that patients who received nICT (CapeOx combined with sintilimab) had higher pCR (19.4%) and MRP rates (47.2%)43. In the present study, the radiological evaluation revealed that patients in the nICT group exhibited a higher ORR than those in the nCT group (79.5% vs. 59.0%; P<0.001). Furthermore, postoperative pathology revealed that the nICT group, compared with the nCT group, had significantly pCR (14.36% vs. 6.41%; P=0.002) and MPR (39.49% vs. 26.15%; P=0.001) improved, which could potentially result in a survival benefit for patients with GC.
Many large randomized controlled clinical trials have demonstrated the safety and efficacy of LG compared with OG; as a result, the procedure has gained widespread acceptance and is now available in experienced centres worldwide20,22,38. Despite the potential risks of neoadjuvant therapy, such as myelosuppression, malnutrition, impaired immune function, and tissue oedema, which may contribute to increased postoperative complications, several previous studies have successfully demonstrated the safety and feasibility of LG following nCT compared to OG24,25,27. However, it remains unclear whether the benefits of laparoscopic surgery persist after immunochemotherapy. Sihag et al. 44 reported the safety and feasibility of esophagectomy after nICT for locally advanced oesophageal cancer. Similarly, the Checkmate-816 study demonstrated that nICT did not compromise the safety of surgery for resectable lung cancer40. Several small-sample retrospective studies have investigated the safety of gastrectomy after nICT. Su et al. 45 observed that the nICT and nCT groups exhibited comparable operative times, postoperative recoveries, and complication rates. Similarly, Wang et al. 46 reported that the complication rates for laparoscopic and open surgery after nICT were 33.3% and 31.2%, respectively (P=1.000). In our study, we found that the overall rates of surgical complications in the nICT and nCT groups were 17.44% and 16.15%, respectively (P=0.694). These findings are consistent with previous studies investigating nCT combined with LG, and there was no significant difference compared with LG alone20,24. Notably, the most common postoperative complication in both groups was pulmonary infection, which may be attributed to the higher proportion of total gastrectomy performed in this study (80.51% in the nICT group and 81.79% in the nCT group). Collectively, our study supports the hypothesis that LG is safe and feasible for LAGC after nICT.
In this study, the 30-day perioperative TO were similar in the two groups (80.0% vs. 81.0%; P=0.767). We observed a significant increase in the number of lymph node dissections in the nICT group compared to the nCT group. However, there was no significant prolongation of the operative duration or increase in intraoperative bleeding, and the two groups were comparable in terms of perioperative transfusion and antibiotic use. Interestingly, the proportion of patients with intraoperative haemorrhage (blood loss > 200 ml) was lower in the nICT group than in the nCT group (4.10% vs. 9.23%; P=0.027). This may be attributed to the fact that some of the patients in the nCT group had minimal tumour regression, which increased the difficulty of lymph node dissection and the risk of perigastric vascular injuries during the operation, thereby increasing the likelihood of haemorrhage. In the present study, no significant differences were observed between the nICT and nCT groups in terms of postoperative recovery, postoperative hospitalization days, postoperative ICU admission, readmission within 30 days, unplanned reoperation, or total cost. Previous studies have indicated that postoperative complications in GC can affect the completion of subsequent comprehensive anti-tumour therapy, and higher complication rates can lead to shorter long-term survival in patients47,48. However, in our study, we found that nICT did not result in delayed surgery, and the rate and duration of postoperative adjuvant chemotherapy were comparable to those in the nCT group.
In this multicenter study, we also observed that the early recurrence rate in the nICT group was significantly lower than that in the nCT group (29.7% vs. 40.8%; P=0.047). Furthermore, both univariable and multivariable logistic regression analyses identified immunotherapy as an independent protective factor for early recurrence (≤ 24 months) [OR 0.62 (95% CI 0.41–0.92); P=0.018]. Survival analysis indicates a higher 2-year RFS (67.1% vs. 58.1%, P=0.027) and 2-year OS (67.0% vs. 64.3%, P=0.223) in the nICT group compared to the nCT group, although the difference of OS did not achieve statistical significance. These findings suggest that LG for LAGC after nICT may afford favourable survival benefits. However, the long-term prognosis needs to be confirmed through long-term follow-up.
However, whether neoadjuvant immunotherapy yields effectiveness with an increase in nTRAEs is noteworthy. The Checkmate-649 and Keynote-859 studies demonstrated that the occurrence of TRAEs in chemotherapy combined with immunotherapy for advanced GC is comparable to that of chemotherapy alone49,50. In this study, there was no statistically significant difference in the incidence of total nTRAEs (51.79% vs. 45.38%; P=0.143) or grade 3/4 nTRAEs (12.03% vs. 11.53%; P=0.786) between the two groups. Some patients in the nICT group experienced immune-related adverse events, specifically related to thyroid and adrenocorticotropic hormones, which is consistent with findings reported in previous studies43. However, all of these adverse events were successfully managed according to safety guidelines, and no patient had to discontinue immunotherapy or delay surgery because of these immune-related adverse events. Although neoadjuvant immunotherapy has demonstrated promising results in the treatment of GC, it is important to acknowledge the complex mechanism of immunotherapy that poses challenges in terms of potentially serious immunotherapy-related toxicity in clinical practice.
This study, however, did have limitations that should be acknowledged, the first of which was its retrospective design, although it is noteworthy that the clinical data used were obtained from three independent, high-volume centres. Additionally, rigorous PSM was conducted to minimize disparities between the two groups to ensure reliable and generalizable results. Second, patients were not screened based on programmed death-ligand-1 expression and microsatellite instability status before immunotherapy, which is consistent with the fact that most clinical studies investigating ICIs are still conducted in the overall population, suggesting that there is still a need to explore biomarkers related to ICIs and their efficacy. Finally, although nICT can significantly reduce the risk for early recurrence, long-term follow-up is necessary to confirm the outcomes of nICT. We also anticipate that the results of subsequent phase III clinical studies will confirm the real effect of ICIs in neoadjuvant therapy for GC, which will represent a significant breakthrough in the field of immunotherapy and have the potential to further improve the prognosis of patients with GC.
Conclusions
In conclusion, this observational study, which was rigorously adjusted for confounding factors, provides evidence suggesting that nICT is safe and effective, which significantly enhanced objective and pathological response rates and reduced the risk for early recurrence without increasing surgical complications and drug toxicity among patients with LAGC. These findings highlight the promising potential and wide-ranging application prospects for nICT.
Ethical approval
This study passed the Institutional Review Board (IRB) of the FJMUUH (IRB number: 2023KY160).
Consent for publication
This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal.
Sources of funding
This study was supported by the Construction Funds for “High-level Hospitals and Clinical Specialties” of Fujian Province (No. [2021]76) and Fujian Research and Training Grants for Young and Middle-aged Leaders in Healthcare for Jun Lu. (No. [2023] 26).
Author contribution
Study concept or design: S.Y.Q. Z.Q., L.J. C.L.S. and H.C.M. Data collection, data analysis or interpretation: L.C.B., C.Q.X., L.M.Q., Z.W.M., Z.Y.B., W.C.M., L.G.T., and Z.Z.Q. writing the paper: S.Y.Q., Z.Q., L.C.B., L.J., C.L.S. and H.C.M. Statistical analysis: S.Y.Q., Z.Q., W.D., L.G.T., and L.J. Administrative, technical, or material support: S.Y.Q., Z.Q., C.Q.Y., L.G.T., W.D., L.J.X., X.J.W., L.P. and Z.C.H. Supervision: S.Y.Q., Z.Q., L.C.B. and H.C.M.
Conflicts of interest disclosure
All authors have no conflict of interest and no potential benefits.
Research registration unique identifying number (UIN)
Name of the registry: Clinical Trials.gov.
Unique Identifying number or registration ID: NCT06235164.
Hyperlink to your specific registration (must be publicly accessible and will be checked): ClinicalTrials.gov PRS: Record Summary NCT06235164.
Guarantor
Chang-Ming Huang, Yu-Qin Sun, Qing Zhong,
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Provenance and peer review
Not applicable.
Supplementary Material
Acknowledgements
The authors thank those who have devoted a lot to this study, including nurses, pathologists, further-study surgeons, statisticians, reviewers and editors. The authors thank Editage (www.editage.cn) for English language editing. They were not financially compensated for their contributions.
Footnotes
Y.-Q.S., Q.Z., and C.-B.L. contributed equally to this work and should be considered co-first authors.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.lww.com/international-journal-of-surgery.
Published online 23 April 2024
Contributor Information
Yu-Qin Sun, Email: sunyuqin2017@163.com.
Qing Zhong, Email: zhongqingys@foxmail.com.
Chen-Bin Lv, Email: 1032356893@qq.com.
Ji-Yun Zhu, Email: crazymather@163.com.
Guang-Tan Lin, Email: 313448388@qq.com.
Zhi-Quan Zhang, Email: zhangzhiquan997@163.com.
Dong Wu, Email: 1979138986@qq.com.
Cai-Ming Weng, Email: wengcm123@gmail.com.
Qiu-Xian Chen, Email: 4626033@qq.com.
Ming-Qiao Lian, Email: 619937472@qq.com.
Wei-Ming Zeng, Email: 1174177199@qq.com.
Yong-Bin Zhang, Email: 1643002474@qq.com.
Qi-Yue Chen, Email: 690934662@qq.com.
Jian-Xian Lin, Email: linjian379@163.com.
Jian-Wei Xie, Email: 364531721@qq.com.
Ping Li, Email: 24627878@qq.com.
Chao-Hui Zheng, Email: wwkzch@163.com.
Jun Lu, Email: 78379048@qq.com.
Li-Sheng Cai, Email: 1272110762@qq.com.
Chang-Ming Huang, Email: hcmlr2002@163.com.
References
- 1. Sung H, Ferlay J, Siegel RL. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Wang FH, Zhang XT, Li YF, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (London, England) 2021;41:747–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ajani JA, D’Amico TA, Bentrem DJ, et al. Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Comprehens Cancer Netw JNCCN 2022;20:167–192. [DOI] [PubMed] [Google Scholar]
- 4. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–132. [DOI] [PubMed] [Google Scholar]
- 5. Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet (London, England) 2020;396:635–648. [DOI] [PubMed] [Google Scholar]
- 6. Smyth EC, Fassan M, Cunningham D, et al. Effect of pathologic tumor response and nodal status on survival in the medical research council adjuvant gastric infusional chemotherapy trial. J Clin Oncol 2016;34:2721–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kang YK, Yook JH, Park YK, et al. PRODIGY: A Phase III Study of Neoadjuvant Docetaxel, Oxaliplatin, and S-1 Plus Surgery and Adjuvant S-1 Versus Surgery and Adjuvant S-1 for Resectable Advanced Gastric Cancer. J Clin Oncol 2021;39:2903–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang X, Liang H, Li Z, et al. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol 2021;22:1081–1092. [DOI] [PubMed] [Google Scholar]
- 9. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Eng J Med 2006;355:11–20. [DOI] [PubMed] [Google Scholar]
- 10. Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet (London, England) 2019;393:1948–1957. [DOI] [PubMed] [Google Scholar]
- 11. van der Wielen N, Straatman J, Daams F, et al. Open versus minimally invasive total gastrectomy after neoadjuvant chemotherapy: results of a European randomized trial. Gastric Cancer 2021;24:258–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715–1721. [DOI] [PubMed] [Google Scholar]
- 13. Coccolini F, Nardi M, Montori G, et al. Neoadjuvant chemotherapy in advanced gastric and esophago-gastric cancer. Meta-analysis of randomized trials. Int J Surg (London, England) 2018;51:120–127. [DOI] [PubMed] [Google Scholar]
- 14. Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:234–247. [DOI] [PubMed] [Google Scholar]
- 15. Shah MA, Kennedy EB, Alarcon-Rozas AE, et al. Immunotherapy and targeted therapy for advanced gastroesophageal cancer: ASCO Guideline. J Clin Oncol 2023;41:1470–1491. [DOI] [PubMed] [Google Scholar]
- 16. Shitara K, Van Cutsem E, Bang YJ, et al. Efficacy and Safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol 2020;6:1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoon HH, Jin Z, Kour O, et al. Association of PD-L1 expression and other variables with benefit from immune checkpoint inhibition in advanced gastroesophageal cancer: systematic review and meta-analysis of 17 phase 3 randomized clinical trials. JAMA Oncol 2022;8:1456–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Janjigian YY, Van Cutsem E, Muro K, et al. MATTERHORN: phase III study of durvalumab plus FLOT chemotherapy in resectable gastric/gastroesophageal junction cancer. Future Oncol 2022;18:2465–2473. [DOI] [PubMed] [Google Scholar]
- 19. Bang YJ, Van Cutsem E, Fuchs CS, et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol 2019;15:943–952. [DOI] [PubMed] [Google Scholar]
- 20. Yu J, Huang C, Sun Y, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: The CLASS-01 Randomized Clinical Trial. JAMA 2019;321:1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang C, Liu H, Hu Y, et al. Laparoscopic vs open distal gastrectomy for locally advanced gastric cancer: five-year outcomes from the CLASS-01 randomized clinical trial. JAMA Surg 2022;157:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim HH, Han SU, Kim MC, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage i gastric cancer: The KLASS-01 Randomized Clinical Trial. JAMA Oncol 2019;5:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsekrekos A, Vossen LE, Lundell L, et al. Improved survival after laparoscopic compared to open gastrectomy for advanced gastric cancer: a Swedish population-based cohort study. Gastric Cancer 2023;26:467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Z, Shan F, Ying X, et al. Assessment of laparoscopic distal gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: a randomized clinical trial. JAMA Surg 2019;154:1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhong H, Liu X, Tian Y, et al. Comparison of short- and long-term outcomes between laparoscopic and open gastrectomy for locally advanced gastric cancer following neoadjuvant chemotherapy: a propensity score matching analysis. Surg Endosc 2023;37:5902–5915. [DOI] [PubMed] [Google Scholar]
- 26. Huang ZN, Su Y, Qiu WW, et al. Assessment of indocyanine green tracer-guided lymphadenectomy in laparoscopic gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: results from a multicenter analysis based on propensity matching. Gastric Cancer 2021;24:1355–1364. [DOI] [PubMed] [Google Scholar]
- 27. Xing J, Wang Y, Shan F, et al. Comparison of totally laparoscopic and laparoscopic assisted gastrectomy after neoadjuvant chemotherapy in locally advanced gastric cancer. Eur J Surg Oncol 2021;47:2023–2030. [DOI] [PubMed] [Google Scholar]
- 28. Al-Batran SE, Homann N, Pauligk C, et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: The AIO-FLOT3 Trial. JAMA Oncol 2017;3:1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 30. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford, England : 1990) 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 32. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992;111:518–526. [PubMed] [Google Scholar]
- 34. Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521–1530. [DOI] [PubMed] [Google Scholar]
- 35. Levy J, Gupta V, Amirazodi E, et al. Textbook outcome and survival in patients with gastric cancer: an analysis of the Population Registry of Esophageal and Stomach Tumours in Ontario (PRESTO). Ann Surg 2022;275:140–148. [DOI] [PubMed] [Google Scholar]
- 36. Levy J, Gupta V, Amirazodi E, et al. Gastrectomy case volume and textbook outcome: an analysis of the Population Registry of Esophageal and Stomach Tumours of Ontario (PRESTO). Gastric Cancer 2020;23:391–402. [DOI] [PubMed] [Google Scholar]
- 37. Kang WM, Meng QB, Yu JC, et al. Factors associated with early recurrence after curative surgery for gastric cancer. World J Gastroenterol 2015;21:5934–5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin 2021;71:264–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kanani A, Veen T, Søreide K. Neoadjuvant immunotherapy in primary and metastatic colorectal cancer. Br J Surg 2021;108:1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Eng J Med 2022;386:1973–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ge F, Huo Z, Cai X, et al. Evaluation of clinical and safety outcomes of neoadjuvant immunotherapy combined with chemotherapy for patients with resectable esophageal cancer: a systematic review and meta-analysis. JAMA Netw Open 2022;5:e2239778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Loibl S, Schneeweiss A, Huober J, et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann Oncol 2022;33:1149–1158. [DOI] [PubMed] [Google Scholar]
- 43. Jiang H, Yu X, Li N, et al. Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: early results of a phase 2 study. J Immunother Cancer 2022;10:e003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sihag S, Ku GY, Tan KS, et al. Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J Thorac Cardiovasc Surg 2021;161:836–843.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Su J, Guo W, Chen Z, et al. Safety and short-term outcomes of laparoscopic surgery for advanced gastric cancer after neoadjuvant immunotherapy: a retrospective cohort study. Front Immunol 2022;13:1078196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang Y, Lei X, Shan F, et al. Safety and short-term outcomes of gastrectomy after preoperative chemotherapy plus immunotherapy versus preoperative chemotherapy: a retrospective cohort study. BMC Cancer 2022;22:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tokunaga M, Kurokawa Y, Machida R, et al. Impact of postoperative complications on survival outcomes in patients with gastric cancer: exploratory analysis of a randomized controlled JCOG1001 trial. Gastric Cancer 2021;24:214–223. [DOI] [PubMed] [Google Scholar]
- 48. Knight SR, Shaw CA, Pius R, et al. Global variation in postoperative mortality and complications after cancer surgery: a multicentre, prospective cohort study in 82 countries. Lancet (London, England) 2021;397:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (London, England) 2021;398:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tabernero J, Bang YJ, Van Cutsem E, et al. KEYNOTE-859: a Phase III study of pembrolizumab plus chemotherapy in gastric/gastroesophageal junction adenocarcinoma. Future Oncol 2021;17:2847–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.