Abstract
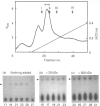
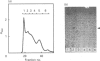
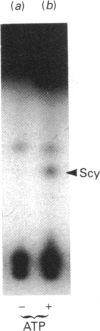
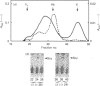
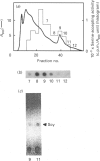
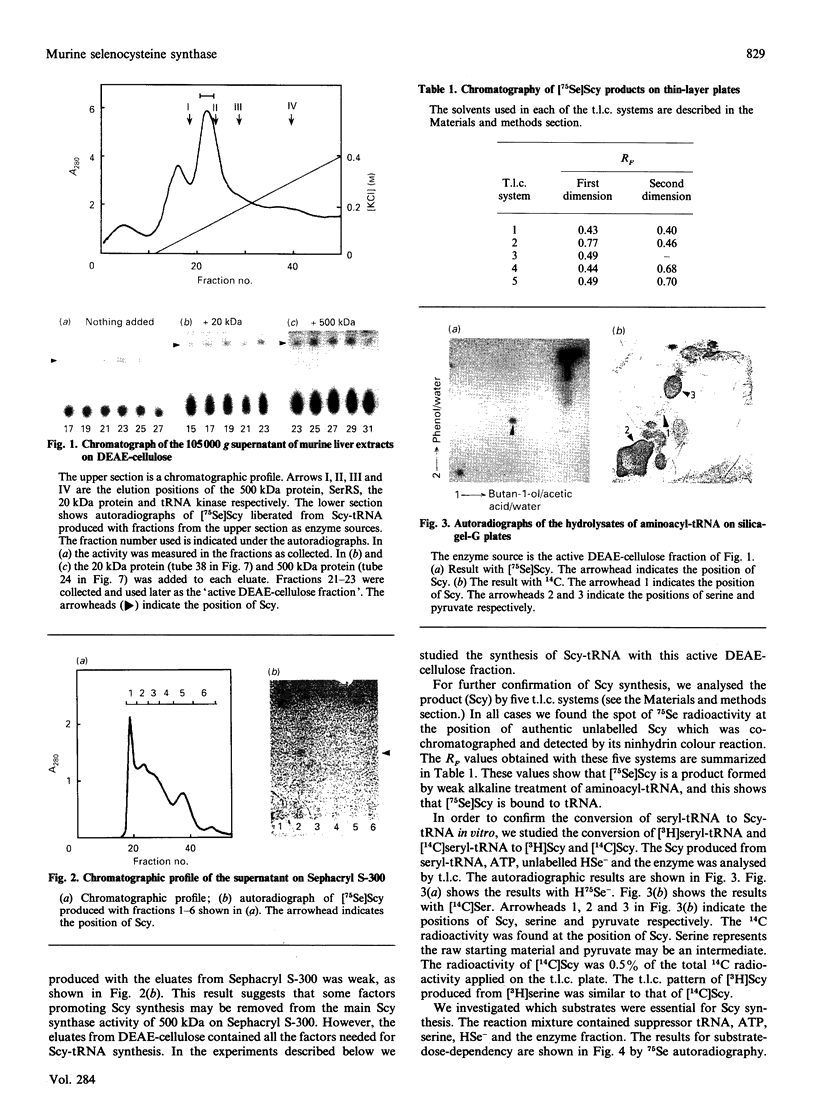
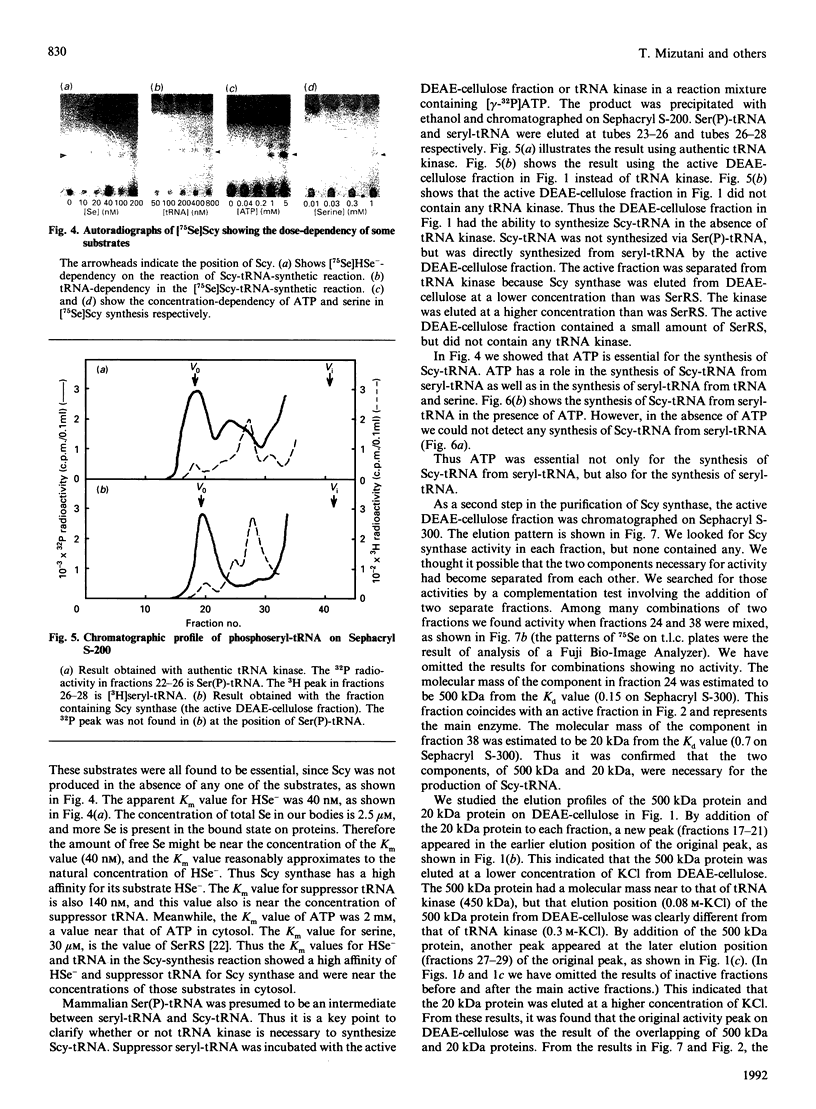
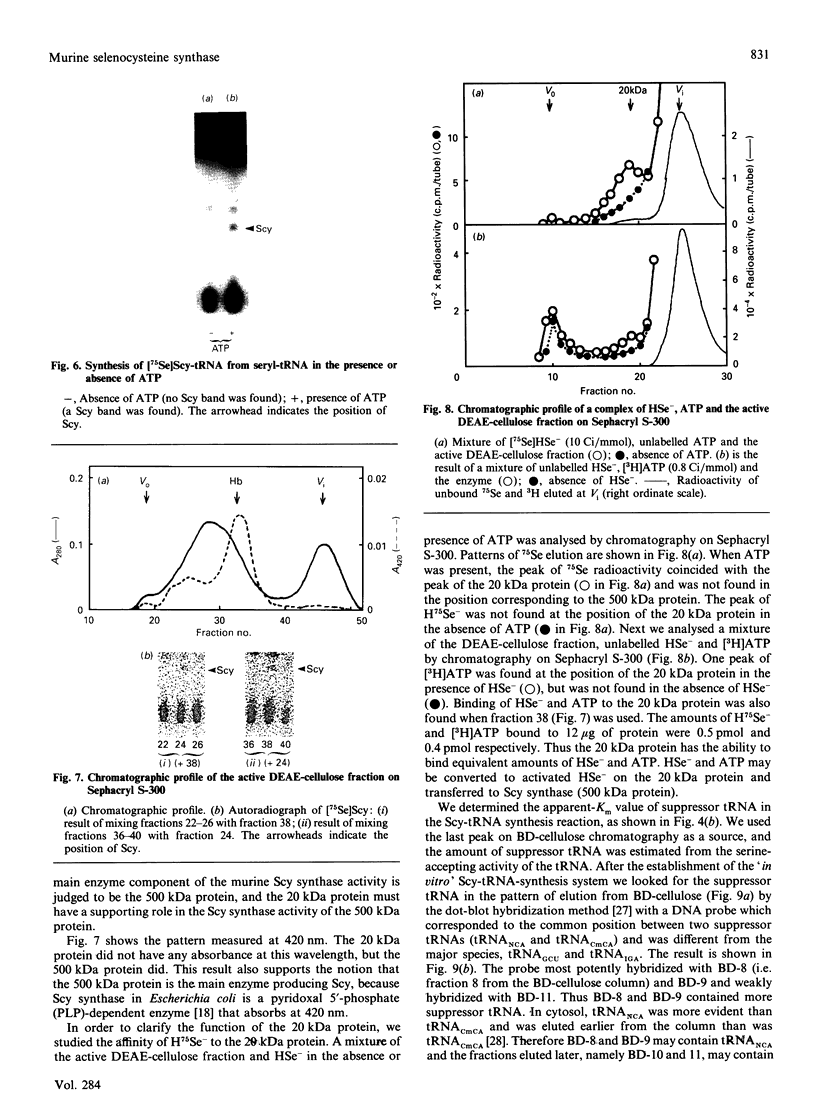
Selenocysteine (Scy) was synthesized on natural opal suppressor tRNA(Ser) by conversion from seryl-tRNA. We studied the mechanisms of the synthesis of mammalian Scy-tRNA using hydro[75Se]selenide (H75Se-). We found Scy synthase activity in the 105,000 g supernatant of a murine liver extract. The supernatant was chromatographed on DEAE-cellulose, and the activity was eluted at 0.12 M-KCl. The reaction mixture for synthesis of Scy-tRNA contained suppressor tRNA, serine, ATP, seryl-tRNA synthetase (SerRS), HSe- and the enzyme to synthesize Scy-tRNA. These are all essential for the synthesis of Scy-tRNA. Scy in the tRNA product was confirmed by five t.l.c. systems. The conversion from seryl-tRNA to Scy-tRNA was also confirmed with the use of [14C]- and [3H]-serine. The apparent Km values for the substrates serine, tRNA, ATP and HSe- were 30 microM, 140 nM, 2 mM and 40 nM respectively. The active eluates from DEAE-cellulose contained no tRNA kinase. This result showed that Scy-tRNA was not synthesized through phosphoseryl-tRNA. ATP was necessary when Scy-tRNA was synthesized from seryl-tRNA and HSe-. Therefore ATP is used for not only the synthesis of seryl-tRNA but also for the synthesis of Scy-tRNA from seryl-tRNA. The active fraction from DEAE-cellulose was chromatographed on Sephacryl S-300, but the activity disappeared. However, the activity was recovered by mixing the eluates corresponding to proteins of 500 kDa and 20 kDa. In order to examine the binding of HSe- to proteins, a mixture of the active fraction, H75Se- and ATP was analysed by chromatography on Sephacryl S-300. The 75Se radioactivity was found at the position of a 20 kDa protein in the presence of ATP. Thus the 20 kDa protein plays a role in binding HSe- in the presence of ATP. The 500 kDa protein must have a role in the synthesis of Scy-tRNA. There are two natural suppressor serine tRNAs, tRNA(NCA) and tRNA(CmCA), in cell cytosol. The present paper shows that the suppressor tRNA fraction, eluted later on benzoylated DEAE-(BD-)cellulose, is a better substrate with which to synthesize Scy-tRNA. Thus we consider that murine Scy-tRNA is synthesized from a suppressor seryl-tRNA on the 500 kDa protein with the activated HSe-, which is synthesized with ATP on the 20 kDa protein. This mammalian mechanism used to synthesize Scy is similar to that seen in Escherichia coli.
Full text
PDF

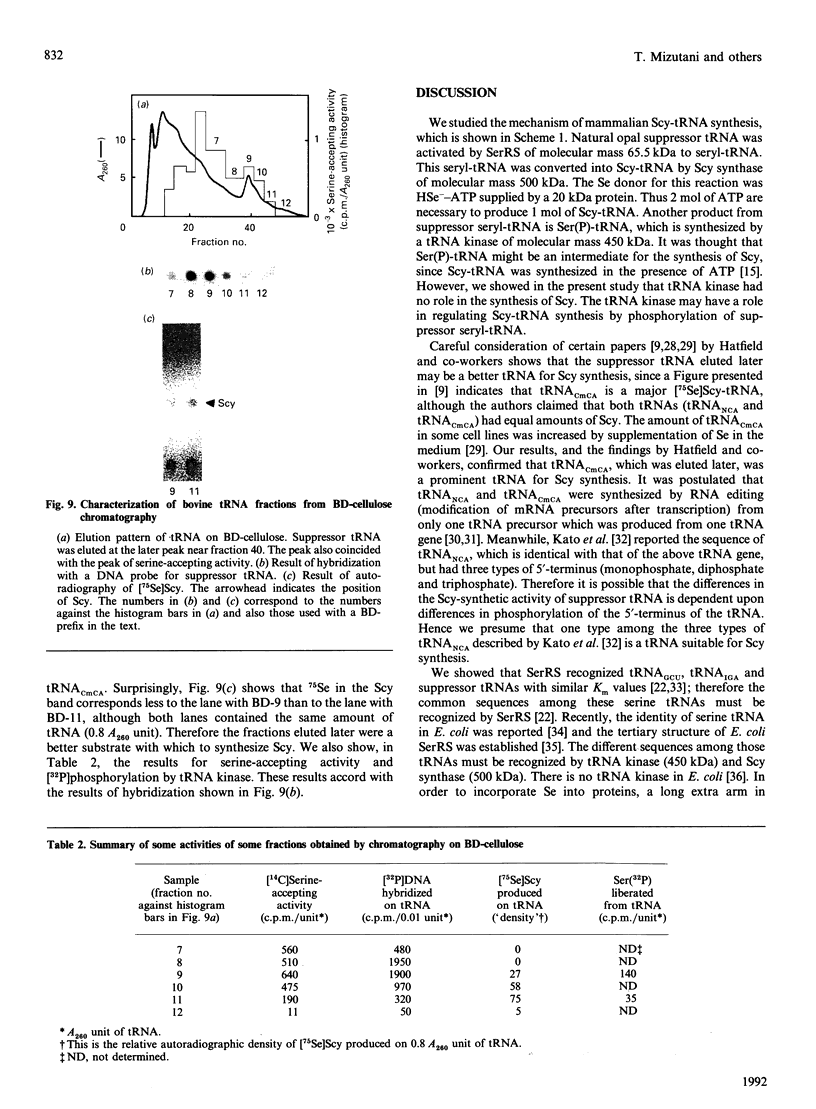
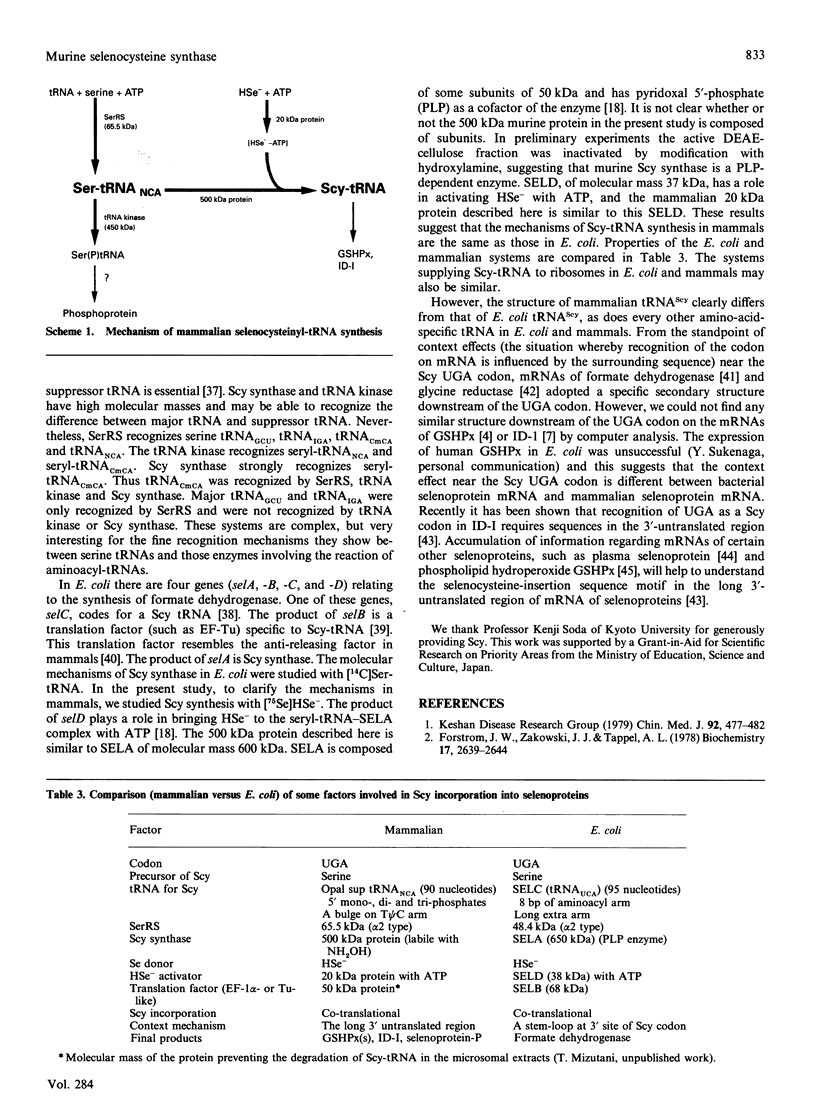

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur J. R., Nicol F., Grant E., Beckett G. J. The effects of selenium deficiency on hepatic type-I iodothyronine deiodinase and protein disulphide-isomerase assessed by activity measurements and affinity labelling. Biochem J. 1991 Feb 15;274(Pt 1):297–300. doi: 10.1042/bj2740297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C., Heider J., Böck A. Mutagenesis of selC, the gene for the selenocysteine-inserting tRNA-species in E. coli: effects on in vivo function. Nucleic Acids Res. 1990 Dec 11;18(23):6761–6766. doi: 10.1093/nar/18.23.6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. J., Banu L., Chen Y. Y., Mandel S. J., Kieffer J. D., Harney J. W., Larsen P. R. Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3' untranslated region. Nature. 1991 Sep 19;353(6341):273–276. doi: 10.1038/353273a0. [DOI] [PubMed] [Google Scholar]
- Berry M. J., Banu L., Larsen P. R. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature. 1991 Jan 31;349(6308):438–440. doi: 10.1038/349438a0. [DOI] [PubMed] [Google Scholar]
- Chambers I., Frampton J., Goldfarb P., Affara N., McBain W., Harrison P. R. The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the 'termination' codon, TGA. EMBO J. 1986 Jun;5(6):1221–1227. doi: 10.1002/j.1460-2075.1986.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack S., Berthet-Colominas C., Härtlein M., Nassar N., Leberman R. A second class of synthetase structure revealed by X-ray analysis of Escherichia coli seryl-tRNA synthetase at 2.5 A. Nature. 1990 Sep 20;347(6290):249–255. doi: 10.1038/347249a0. [DOI] [PubMed] [Google Scholar]
- Diamond A. M., Montero-Puerner Y., Lee B. J., Hatfield D. Selenocysteine inserting tRNAs are likely generated by tRNA editing. Nucleic Acids Res. 1990 Nov 25;18(22):6727–6727. doi: 10.1093/nar/18.22.6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., Dudock B., Hatfield D. Structure and properties of a bovine liver UGA suppressor serine tRNA with a tryptophan anticodon. Cell. 1981 Aug;25(2):497–506. doi: 10.1016/0092-8674(81)90068-4. [DOI] [PubMed] [Google Scholar]
- Forchhammer K., Böck A. Selenocysteine synthase from Escherichia coli. Analysis of the reaction sequence. J Biol Chem. 1991 Apr 5;266(10):6324–6328. [PubMed] [Google Scholar]
- Forchhammer K., Leinfelder W., Böck A. Identification of a novel translation factor necessary for the incorporation of selenocysteine into protein. Nature. 1989 Nov 23;342(6248):453–456. doi: 10.1038/342453a0. [DOI] [PubMed] [Google Scholar]
- Forstrom J. W., Zakowski J. J., Tappel A. L. Identification of the catalytic site of rat liver glutathione peroxidase as selenocysteine. Biochemistry. 1978 Jun 27;17(13):2639–2644. doi: 10.1021/bi00606a028. [DOI] [PubMed] [Google Scholar]
- Ganther H. E. Reduction of the selenotrisulfide derivative of glutathione to a persulfide analog by glutathione reductase. Biochemistry. 1971 Oct 26;10(22):4089–4098. doi: 10.1021/bi00798a013. [DOI] [PubMed] [Google Scholar]
- Garcia G. E., Stadtman T. C. Selenoprotein A component of the glycine reductase complex from Clostridium purinolyticum: nucleotide sequence of the gene shows that selenocysteine is encoded by UGA. J Bacteriol. 1991 Mar;173(6):2093–2098. doi: 10.1128/jb.173.6.2093-2098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. J., Gupta P. L. The determination of very small amounts of selenium in plant samples. Analyst. 1969 Apr;94(117):292–299. doi: 10.1039/an9699400292. [DOI] [PubMed] [Google Scholar]
- Hatfield D. L., Smith D. W., Lee B. J., Worland P. J., Oroszlan S. Structure and function of suppressor tRNAs in higher eukaryotes. Crit Rev Biochem Mol Biol. 1990;25(2):71–96. doi: 10.3109/10409239009090606. [DOI] [PubMed] [Google Scholar]
- Hatfield D., Diamond A., Dudock B. Opal suppressor serine tRNAs from bovine liver form phosphoseryl-tRNA. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6215–6219. doi: 10.1073/pnas.79.20.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D., Lee B. J., Hampton L., Diamond A. M. Selenium induces changes in the selenocysteine tRNA[Ser]Sec population in mammalian cells. Nucleic Acids Res. 1991 Feb 25;19(4):939–943. doi: 10.1093/nar/19.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D., Portugal F. H. Seryl-tRNA in mammalian tissues: chromatographic differences in brain and liver and a specific response to the codon, UGA. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1200–1206. doi: 10.1073/pnas.67.3.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes W. C., Tappel A. L. In vitro synthesis of glutathione peroxidase from selenite. Translational incorporation of selenocysteine. Biochim Biophys Acta. 1983 Mar 10;739(2):225–234. doi: 10.1016/0167-4781(83)90033-7. [DOI] [PubMed] [Google Scholar]
- Hill K. E., Lloyd R. S., Yang J. G., Read R., Burk R. F. The cDNA for rat selenoprotein P contains 10 TGA codons in the open reading frame. J Biol Chem. 1991 Jun 5;266(16):10050–10053. [PubMed] [Google Scholar]
- Hitaka T., Mizutani T., Watanabe K., Totsuka T. The high content of natural suppressor serine tRNA in dystrophic mouse muscle. Biochem J. 1990 Feb 15;266(1):201–206. doi: 10.1042/bj2660201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Hoshino H., Harada F. Minor serine tRNA containing anticodon NCA (C4 RNA) from human and mouse cells. Biochem Int. 1983 Nov;7(5):635–645. [PubMed] [Google Scholar]
- Kumazawa Y., Yokogawa T., Hasegawa E., Miura K., Watanabe K. The aminoacylation of structurally variant phenylalanine tRNAs from mitochondria and various nonmitochondrial sources by bovine mitochondrial phenylalanyl-tRNA synthetase. J Biol Chem. 1989 Aug 5;264(22):13005–13011. [PubMed] [Google Scholar]
- Lee B. J., Worland P. J., Davis J. N., Stadtman T. C., Hatfield D. L. Identification of a selenocysteyl-tRNA(Ser) in mammalian cells that recognizes the nonsense codon, UGA. J Biol Chem. 1989 Jun 15;264(17):9724–9727. [PubMed] [Google Scholar]
- Leinfelder W., Forchhammer K., Veprek B., Zehelein E., Böck A. In vitro synthesis of selenocysteinyl-tRNA(UCA) from seryl-tRNA(UCA): involvement and characterization of the selD gene product. Proc Natl Acad Sci U S A. 1990 Jan;87(2):543–547. doi: 10.1073/pnas.87.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinfelder W., Zehelein E., Mandrand-Berthelot M. A., Böck A. Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine. Nature. 1988 Feb 25;331(6158):723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- McBride O. W., Rajagopalan M., Hatfield D. Opal suppressor phosphoserine tRNA gene and pseudogene are located on human chromosomes 19 and 22, respectively. J Biol Chem. 1987 Aug 15;262(23):11163–11166. [PubMed] [Google Scholar]
- Mizutani T., Hashimoto A. Purification and properties of suppressor seryl-tRNA: ATP phosphotransferase from bovine liver. FEBS Lett. 1984 Apr 24;169(2):319–322. doi: 10.1016/0014-5793(84)80342-7. [DOI] [PubMed] [Google Scholar]
- Mizutani T., Kurata H. Reinvestigation of phosphorylation of tRNA in Escherichia coli. Nucleic Acids Symp Ser. 1990;(22):87–88. [PubMed] [Google Scholar]
- Mizutani T., Kurata H., Yamada K. Study of mammalian selenocysteyl-tRNA synthesis with [75Se]HSe. FEBS Lett. 1991 Sep 2;289(1):59–63. doi: 10.1016/0014-5793(91)80908-l. [DOI] [PubMed] [Google Scholar]
- Mizutani T., Narihara T., Hashimoto A. Purification and properties of bovine liver seryl-tRNA synthetase. Eur J Biochem. 1984 Aug 15;143(1):9–13. doi: 10.1111/j.1432-1033.1984.tb08331.x. [DOI] [PubMed] [Google Scholar]
- Mizutani T. Some evidence of the enzymatic conversion of bovine suppressor phosphoseryl-tRNA to selenocysteyl-tRNA. FEBS Lett. 1989 Jul 3;250(2):142–146. doi: 10.1016/0014-5793(89)80707-0. [DOI] [PubMed] [Google Scholar]
- Mäenpä P. H., Bernfield M. R. A specific hepatic transfer RNA for phosphoserine. Proc Natl Acad Sci U S A. 1970 Oct;67(2):688–695. doi: 10.1073/pnas.67.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P. Aminoacyl tRNA synthetases: general scheme of structure-function relationships in the polypeptides and recognition of transfer RNAs. Annu Rev Biochem. 1987;56:125–158. doi: 10.1146/annurev.bi.56.070187.001013. [DOI] [PubMed] [Google Scholar]
- Schuckelt R., Brigelius-Flohé R., Maiorino M., Roveri A., Reumkens J., Strassburger W., Ursini F., Wolf B., Flohé L. Phospholipid hydroperoxide glutathione peroxidase is a selenoenzyme distinct from the classical glutathione peroxidase as evident from cDNA and amino acid sequencing. Free Radic Res Commun. 1991;14(5-6):343–361. doi: 10.3109/10715769109093424. [DOI] [PubMed] [Google Scholar]
- Sharp S. J., Stewart T. S. The characterization of phosphoseryl tRNA from lactating bovine mammary gland. Nucleic Acids Res. 1977 Jul;4(7):2123–2136. doi: 10.1093/nar/4.7.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C. Biosynthesis and function of selenocysteine-containing enzymes. J Biol Chem. 1991 Sep 5;266(25):16257–16260. [PubMed] [Google Scholar]
- Stadtman T. C. Selenium biochemistry. Annu Rev Biochem. 1990;59:111–127. doi: 10.1146/annurev.bi.59.070190.000551. [DOI] [PubMed] [Google Scholar]
- Sukenaga Y., Ishida K., Takeda T., Takagi K. cDNA sequence coding for human glutathione peroxidase. Nucleic Acids Res. 1987 Sep 11;15(17):7178–7178. doi: 10.1093/nar/15.17.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunde R. A., Evenson J. K. Serine incorporation into the selenocysteine moiety of glutathione peroxidase. J Biol Chem. 1987 Jan 15;262(2):933–937. [PubMed] [Google Scholar]
- Tachibana Y., Mizutani T. Protection of bovine seryl-transfer ribonucleic acid (seryl-tRNA) synthetase from chemical modification by its substrates, and some kinetic parameters. Chem Pharm Bull (Tokyo) 1988 Oct;36(10):4019–4025. doi: 10.1248/cpb.36.4019. [DOI] [PubMed] [Google Scholar]
- Zinoni F., Heider J., Böck A. Features of the formate dehydrogenase mRNA necessary for decoding of the UGA codon as selenocysteine. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4660–4664. doi: 10.1073/pnas.87.12.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]