Abstract
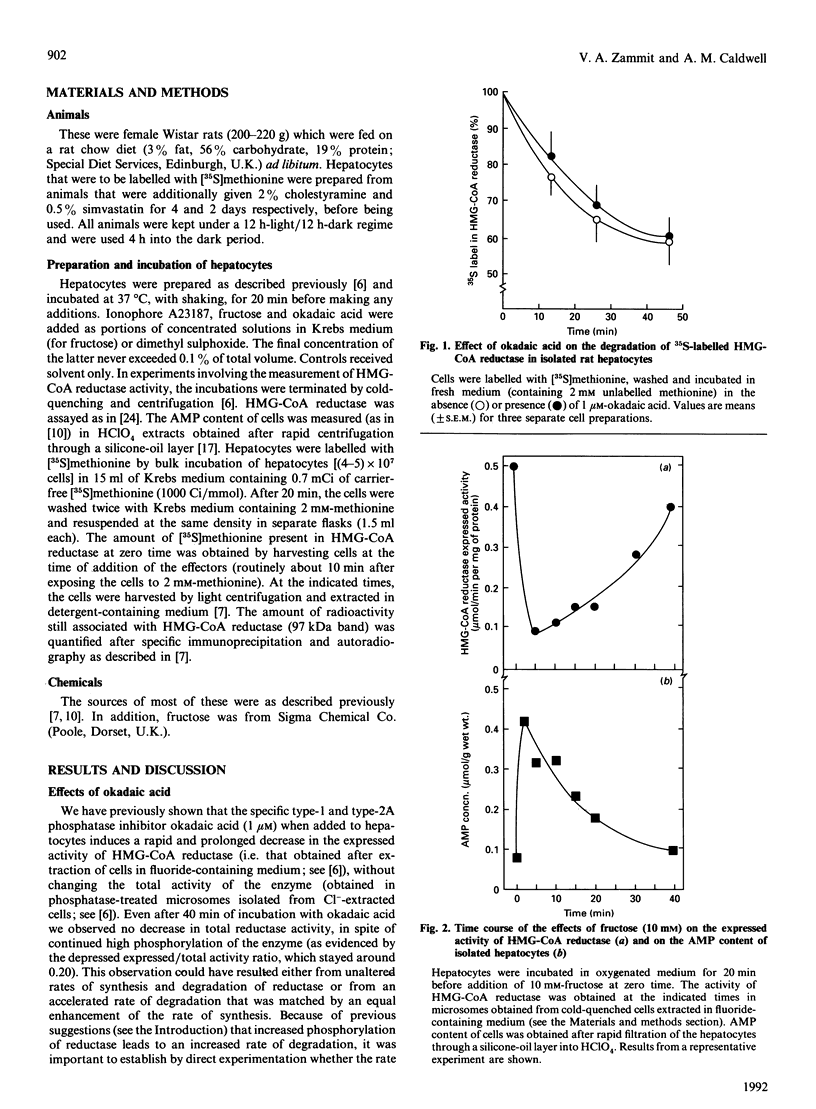
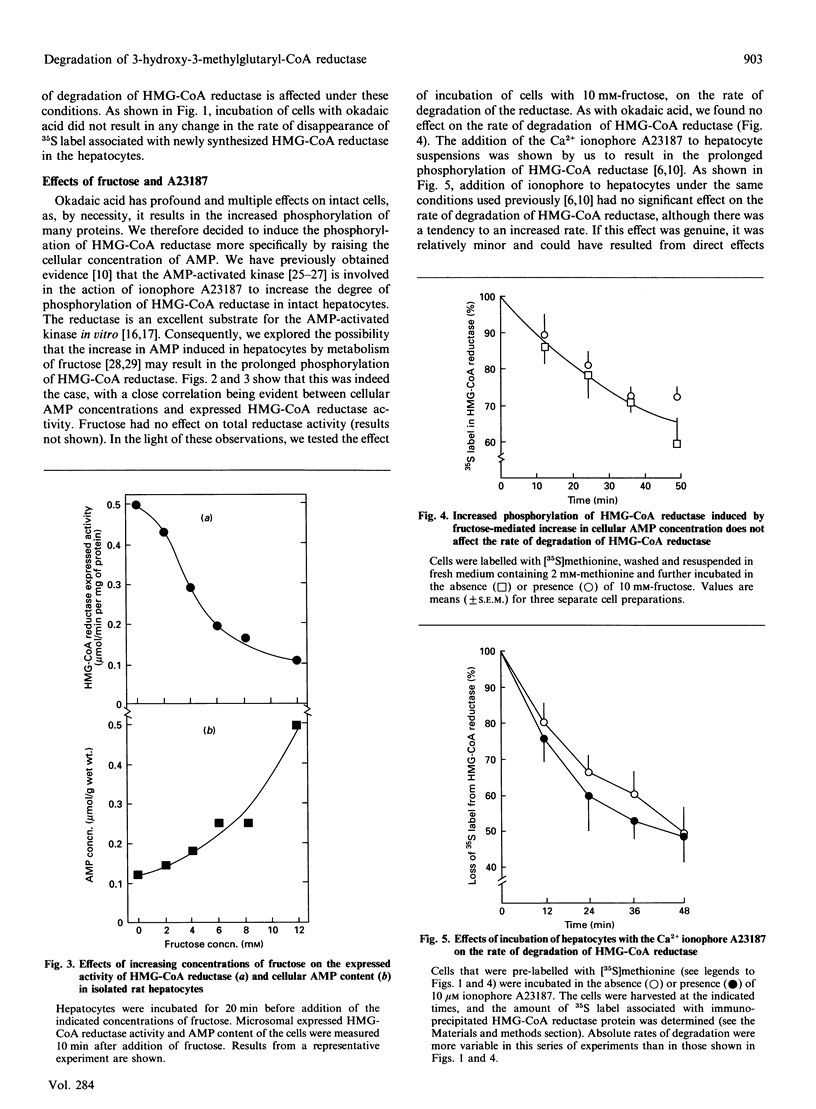
Increased phosphorylation of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase has been suggested to target the protein towards an increased rate of degradation. Our previous observations [Zammit & Caldwell (1990) Biochem. J. 269, 373-379] suggested that, although Ca(2+)-mobilizing hormones and other effectors can alter both the phosphorylation state of the enzyme and its total activity in isolated rat hepatocytes, there appears to be no causal correlation between the two parameters. In the present paper we set out to make direct measurements of the specific rate of degradation of 35S-labelled HMG-CoA reductase in hepatocytes treated with agents that produced very marked and prolonged increases in the degree of phosphorylation of the protein, through different mechanisms. Okadaic acid (which inhibits phosphatases 1 and 2A), fructose (which increases cellular AMP through its metabolism to fructose 1-phosphate) and the Ca2+ ionophore A23187 (which also raises cellular AMP through an unknown mechanism) were all unable to alter the rate of HMG-CoA reductase degradation. We conclude that the basal rate of degradation of HMG-CoA reductase is unaffected by its phosphorylation state and that a transiently increased degree of phosphorylation cannot be the mechanism through which mevalonate increases the rate of degradation of the enzyme in rat hepatocytes and other cell types.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beg Z. H., Stonik J. A., Brewer H. B., Jr Phosphorylation and modulation of the enzymic activity of native and protease-cleaved purified hepatic 3-hydroxy-3-methylglutaryl-coenzyme A reductase by a calcium/calmodulin-dependent protein kinase. J Biol Chem. 1987 Sep 25;262(27):13228–13240. [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A., Brewer H. B., Jr Phosphorylation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and modulation of its enzymic activity by calcium-activated and phospholipid-dependent protein kinase. J Biol Chem. 1985 Feb 10;260(3):1682–1687. [PubMed] [Google Scholar]
- Carling D., Clarke P. R., Zammit V. A., Hardie D. G. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur J Biochem. 1989 Dec 8;186(1-2):129–136. doi: 10.1111/j.1432-1033.1989.tb15186.x. [DOI] [PubMed] [Google Scholar]
- Carling D., Zammit V. A., Hardie D. G. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987 Nov 2;223(2):217–222. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- Ciudad C. J., Carabaza A., Bosch F., Gòmez I Foix A. M., Guinovart J. J. Glycogen synthase activation by sugars in isolated hepatocytes. Arch Biochem Biophys. 1988 Jul;264(1):30–39. doi: 10.1016/0003-9861(88)90566-8. [DOI] [PubMed] [Google Scholar]
- Clarke P. R., Hardie D. G. Calmodulin-dependent multiprotein kinase and protein kinase C phosphorylate the same site on HMG-CoA reductase as the AMP-activated protein kinase. FEBS Lett. 1990 Aug 20;269(1):213–217. doi: 10.1016/0014-5793(90)81157-j. [DOI] [PubMed] [Google Scholar]
- Clarke P. R., Hardie D. G. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 1990 Aug;9(8):2439–2446. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easom R. A., Zammit V. A. A cold-clamping technique for the rapid sampling of rat liver for studies on enzymes in separate cell fractions. Suitability for the study of enzymes regulated by reversible phosphorylation-dephosphorylation. Biochem J. 1984 Jun 15;220(3):733–738. doi: 10.1042/bj2200733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easom R. A., Zammit V. A. Diurnal changes in the fraction of 3-hydroxy-3-methylglutaryl-CoA reductase in the active form in rat liver microsomal fractions. Biochem J. 1984 Jun 15;220(3):739–745. doi: 10.1042/bj2200739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easom R. A., Zammit V. A. Role of reversible phosphorylation in mediating changes in the activity of hepatic 3-hydroxy-3-methylglutaryl-CoA reductase during pregnancy and lactation in the rat. Biochem J. 1987 Dec 15;248(3):993–996. doi: 10.1042/bj2480993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards P. A., Lan S. F., Tanaka R. D., Fogelman A. M. Mevalonolactone inhibits the rate of synthesis and enhances the rate of degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in rat hepatocytes. J Biol Chem. 1983 Jun 25;258(12):7272–7275. [PubMed] [Google Scholar]
- Gil G., Faust J. R., Chin D. J., Goldstein J. L., Brown M. S. Membrane-bound domain of HMG CoA reductase is required for sterol-enhanced degradation of the enzyme. Cell. 1985 May;41(1):249–258. doi: 10.1016/0092-8674(85)90078-9. [DOI] [PubMed] [Google Scholar]
- Inoue S., Bar-Nun S., Roitelman J., Simoni R. D. Inhibition of degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in vivo by cysteine protease inhibitors. J Biol Chem. 1991 Jul 15;266(20):13311–13317. [PubMed] [Google Scholar]
- Kimball S. R., Jefferson L. S. Inhibition of microsomal calcium sequestration causes an impairment of initiation of protein synthesis in perfused rat liver. Biochem Biophys Res Commun. 1991 Jun 28;177(3):1082–1086. doi: 10.1016/0006-291x(91)90649-r. [DOI] [PubMed] [Google Scholar]
- Kimball S. R., Jefferson L. S. Mechanism of the inhibition of protein synthesis by vasopressin in rat liver. J Biol Chem. 1990 Oct 5;265(28):16794–16798. [PubMed] [Google Scholar]
- Luskey K. L., Faust J. R., Chin D. J., Brown M. S., Goldstein J. L. Amplification of the gene for 3-hydroxy-3-methylglutaryl coenzyme A reductase, but not for the 53-kDa protein, in UT-1 cells. J Biol Chem. 1983 Jul 10;258(13):8462–8469. [PubMed] [Google Scholar]
- Marrero P. F., Haro D., Hegardt F. G. Phosphorylation of HMG-CoA reductase induced by mevalonate accelerates its rate of degradation in isolated rat hepatocytes. FEBS Lett. 1986 Mar 3;197(1-2):183–186. doi: 10.1016/0014-5793(86)80323-4. [DOI] [PubMed] [Google Scholar]
- Nakanishi M., Goldstein J. L., Brown M. S. Multivalent control of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mevalonate-derived product inhibits translation of mRNA and accelerates degradation of enzyme. J Biol Chem. 1988 Jun 25;263(18):8929–8937. [PubMed] [Google Scholar]
- Ness G. C., Keller R. K., Pendleton L. C. Feedback regulation of hepatic 3-hydroxy-3-methylglutaryl-CoA reductase activity by dietary cholesterol is not due to altered mRNA levels. J Biol Chem. 1991 Aug 15;266(23):14854–14857. [PubMed] [Google Scholar]
- Parker R. A., Miller S. J., Gibson D. M. Phosphorylation of microsomal HMG CoA reductase increases susceptibility to proteolytic degradation in vitro. Biochem Biophys Res Commun. 1984 Dec 14;125(2):629–635. doi: 10.1016/0006-291x(84)90585-0. [DOI] [PubMed] [Google Scholar]
- Parker R. A., Miller S. J., Gibson D. M. Phosphorylation of native 97-kDa 3-hydroxy-3-methylglutaryl-coenzyme A reductase from rat liver. Impact on activity and degradation of the enzyme. J Biol Chem. 1989 Mar 25;264(9):4877–4887. [PubMed] [Google Scholar]
- Peffley D., Sinensky M. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase synthesis by a non-sterol mevalonate-derived product in Mev-1 cells. Apparent translational control. J Biol Chem. 1985 Aug 25;260(18):9949–9952. [PubMed] [Google Scholar]
- Tanaka R. D., Edwards P. A., Lan S. F., Fogelman A. M. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in avian myeloblasts. Mode of action of 25-hydroxycholesterol. J Biol Chem. 1983 Nov 10;258(21):13331–13339. [PubMed] [Google Scholar]
- Van den Berghe G. Metabolic effects of fructose in the liver. Curr Top Cell Regul. 1978;13:97–135. doi: 10.1016/b978-0-12-152813-3.50008-2. [DOI] [PubMed] [Google Scholar]
- Zammit V. A., Caldwell A. M. Conditions that result in the mobilization and influx of Ca2+ into rat hepatocytes induce the rapid loss of 3-hydroxy-3-methylglutaryl-CoA reductase activity that is not reversed by phosphatase treatment. Biochem J. 1990 Jul 15;269(2):373–379. doi: 10.1042/bj2690373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A., Caldwell A. M., Kolodziej M. P. Rapid decrease in the expression of 3-hydroxy-3-methylglutaryl-CoA reductase protein owing to inhibition of its rate of synthesis after Ca2+ mobilization in rat hepatocytes. Inability of taurolithocholate to mimic the effect. Biochem J. 1991 Oct 15;279(Pt 2):377–383. doi: 10.1042/bj2790377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A., Caldwell A. M. The roles of different protein kinases and of calmodulin in the effects of Ca2+ mobilization on 3-hydroxy-3-methylglutaryl-CoA reductase activity in isolated rat hepatocytes. Biochem J. 1991 Jan 15;273(Pt 2):485–488. doi: 10.1042/bj2730485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A., Easom R. A. Regulation of hepatic HMG-CoA reductase in vivo by reversible phosphorylation. Biochim Biophys Acta. 1987 Feb 18;927(2):223–228. doi: 10.1016/0167-4889(87)90138-8. [DOI] [PubMed] [Google Scholar]


