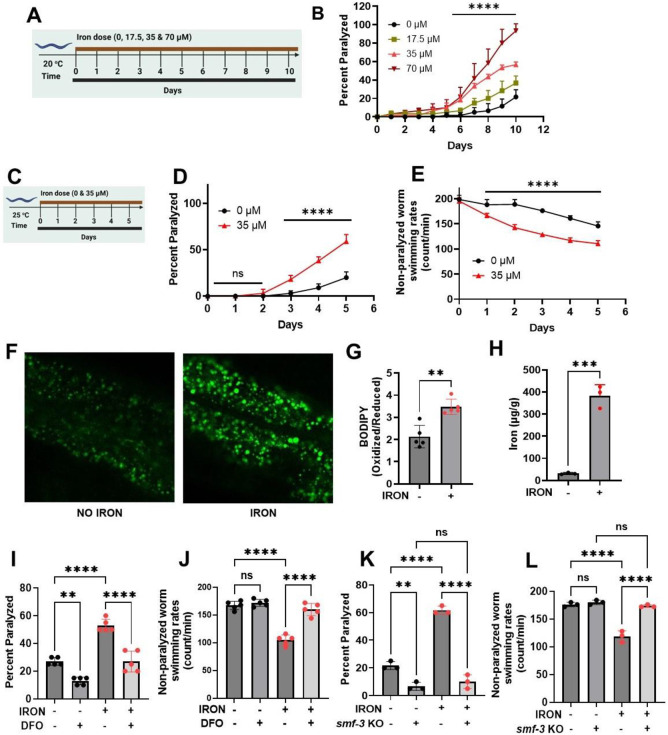
Figure 1: Iron toxicity alters worm physiologic function:
A). Experimental layout showing 10 days of worm exposure to different iron doses (0, 17.5, 35, and 70 μM) at 20 °C. B). Dose- and time-dependent effects of iron exposure on worm paralysis at 20 °C. Staged L4 worms were transferred to plates containing iron (0, 17.5, 35, and 70 μM). Worms were then transferred every 24 h for 10 days. Paralysis (e.g., inability to move upon stimulation) was scored every 24 h for 10 days. Data are mean ±SEM, N=3 independent biological replicates (where one biological replicate contains 20 worms per plate). ****p<0.0001, two-way ANOVA, Tukey post hoc test. C). Experimental layout showing 5 days of worm exposure to iron (0 and 35 μM) at 25 °C. D). Time course of iron exposure on worm paralysis at 25 °C. Staged L4 worms were transferred to plates containing iron (0 or 35 μM). Paralysis was scored every 24 h for 5 days. Data are mean ±SEM, N=5 independent biological replicates (where one biological replicate contains 20 worms per plate). ns not significant, ****p<0.0001, one-way ANOVA, Tukey post hoc test. E). Iron reduced non-paralyzed worm swimming rates at 25 °C. Staged L4 worms were transferred to plate containing iron (0 or 35 μM). Worms were then transferred every 24 h for 5 days. Non-paralyzed worms were individually transferred to plate containing 100 μl of buffer. After 30 seconds of equilibration, swimming rates were collected for 15 seconds. Data are mean ±SEM N=5 independent replicates (where 4 independent worm count constitute an N). ****p<0.0001, one-way ANOVA, Tukey post hoc test. Iron toxicity increases whole worm lipid peroxidation. F) Confocal Image and G) Quantification. Staged L4 worms were transferred to plate containing iron (0 or 35 μM). Worms were then transferred every 24 h for 5 days. Then worms were transferred to plate containing 1.25 μM BODIPY for 60 min and prep for confocal imaging. Image scale 30 μm. Data are mean ±SEM N=5 independent replicates, **p=0.001, one-way ANOVA, Tukey post hoc test. H). Total iron was measured in worms using ICP-MS where dark circle (no iron) and red triangle(iron) bars treated with 35 μM iron. Data are mean ±SEM, N=4 independent biological replicates. ****p<0.0001, Unpaired t test. I). Effects of Deferoxamine (DFO) on iron-induced paralysis. Staged L4 worms were transferred to plates containing 0 μM iron, 0 μM iron + 100 μM DFO 35 μM iron and 35 μM iron + 100 μM DFO. Paralysis was scored every 24 h for 5 days. Data are mean ±SEM, N=5 independent biological replicates (where one biological replicate contains 20 worms per plate). ns not significant, ****p<0.0001, one-way ANOVA, Tukey post hoc test. J). Deferoxamine restored iron-induced impairment of non-paralyzed worm swimming rates. Staged L4 worms were transferred to plates containing 0 μM iron, 0 μM iron + 100 μM DFO 35 μM iron and 35 μM iron + 100 μM DFO . Non-paralyzed worms were individually transferred to plate containing 100 μl of buffer. After 30 seconds of equilibration, swimming rates were collected for 15 seconds on day 5. Data are mean ±SEM N=5 independent replicates. ns not significant, ****p<0.0001, one-way ANOVA, Tukey post hoc test. K). DMT1 (smf-3) knock out abolished iron toxicity mediated worm paralysis. Staged L4 worms were transferred to plates containing iron WT, smf-3 KO, WT + iron (35 μM) and smf-3 KO + iron (35 μM). Paralysis was scored every 24 h for 5 days. Data are mean ±SEM, N=3 independent biological replicates (where one biological replicate contains 20 worms per plate). *p<0.05, ****p<0.0001, one-way ANOVA, Tukey post hoc test. L). DMT1 (smf-3) knock out shielded worms from iron-induced reduction of non-paralyzed worm swimming rates. Staged L4 worms were transferred to plates containing iron WT, smf-3 KO, WT + iron (35 μM) and smf-3 KO + iron (35 μM). Non-paralyzed worms were individually transferred to plate containing 100 μl of buffer. After 30 seconds of equilibration, swimming rates were collected for 15 seconds. Data are mean ±SEM N=3 independent replicates (where 4 independent worm count constitute an N). ns not significant, ***p=0.0001, ****p<0.0001, one-way ANOVA, Tukey post hoc test.