Figure 3: Iron toxicity modulates mitochondrial redox environment.
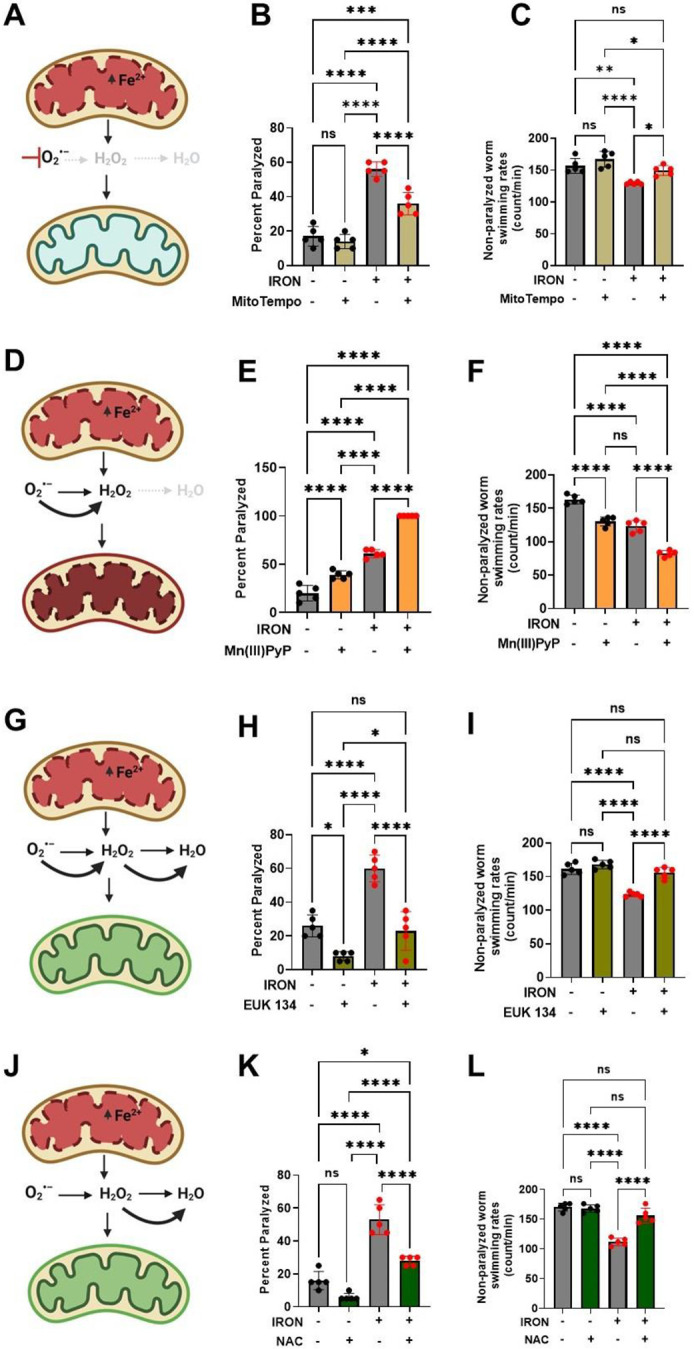
A). Schematic diagram showing specific target of mitoTempo in redox environment. B). MitoTempo ameliorates iron toxicity induced increase in worm paralysis. Staged L4 worms were transferred to plates containing 0 μM iron, 0 μM iron + 10 μM MitoTempo, 35 μM iron and 35 μM iron + 10 μM MitoTempo. Paralysis was scored every 24 h for 5 days. Data are mean ±SEM, N=5 independent biological replicates (where one biological replicate contains 20 worms per plate). ***p=0.0001, ****p<0.0001, one-way ANOVA, Tukey post hoc test. C). MitoTempo restored non-paralyzed worm swimming rates in iron toxicity environment. Staged L4 worms were transferred to plates containing 0 μM iron, 0 μM iron + 10 μM MitoTempo, 35 μM iron and 35 μM iron + 10 μM MitoTempo. Non-paralyzed worms were individually transferred to plate containing 100 μl of buffer. After 30 seconds of equilibration, swimming rates were collected for 15 seconds. Data are mean ±SEM N=5 independent replicates (where 4 independent worm count constitute an N). ns not significant, **p<0.001, ****p<0.0001, one-way ANOVA, Tukey post hoc test. D). Schematic diagram showing specific target of SOD mimetic manganese porphyrin [Mn(III)PyP] in redox environment. E). Mn(III)PyP exacerbates iron toxicity induced increase in worm paralysis. Staged L4 worms were transferred to plates containing 0 μM iron, 0 μM iron + 100 μM Mn(III)PyP, 35 μM iron and 35 μM iron + 100 μM Mn(III)PyP. Paralysis was scored every 24 h for 5 days. Data are mean ±SEM, N=5 independent biological replicates (where one biological replicate contains 20 worms per plate). ****p<0.0001, one-way ANOVA, Tukey post hoc test. F). Mn(III)PyP worsen non-paralyzed worm swimming rates in iron toxicity environment. Staged L4 worms were transferred to plates containing 0 μM iron, 0 μM iron + 100 μM Mn(III)PyP, 35 μM iron and 35 μM iron + 100 μM Mn(III)PyP. Non-paralyzed worms were individually transferred to plate containing 100 μl of buffer. After 30 seconds of equilibration, swimming rates were collected for 15 seconds. Data are mean ±SEM N=5 independent replicates (where 4 independent worm count constitute an N). ****p<0.0001, one-way ANOVA, Tukey post hoc test. G). Schematic diagram showing specific target of EUK 134 (SOD and Catalase mimetic) in mitochondrial redox environment. H). EUK 134 protects against iron-induced toxicity induced in worm paralysis. Staged L4 worms were transferred to plates containing 0 μM iron, 0 μM iron + 100 μM EUK 134, 35 μM iron and 35 μM iron + 100 μM EUK 134. Paralysis was scored every 24 h for 5 days. Data are mean ±SEM, N=5 independent biological replicates (where one biological replicate contains 20 worms per plate). ns not significant ***p=0.0001, ****p<0.0001, one-way ANOVA, Tukey post hoc test. I). EUK 134 restored non-paralyzed worm swimming rates in iron toxicity environment. Staged L4 worms were transferred to plates containing 0 μM iron, 0 μM iron + 100 μM EUK 134, 35 μM iron and 35 μM iron + 100 μM EUK 134. Non-paralyzed worms were individually transferred to plate containing 100 μl of buffer. After 30 seconds of equilibration, swimming rates were collected for 15 seconds. Data are mean ±SEM N=5 independent replicates (where 4 independent worm count constitute an N). ns not significant, **p<0.001, ****p<0.0001, one-way ANOVA, Tukey post hoc test. J). Schematic diagram showing specific target of N-Acetyl Cysteine (NAC) in mitochondrial redox environment. K). NAC protects against iron toxicity induced increase in worm paralysis. Staged L4 worms were transferred to plates containing 0 μM iron, 0 μM iron + 2.5 mM NAC, 35 μM iron and 35 μM iron + 2.5 mM NAC. Paralysis was scored every 24 h for 5 days. Data are mean ±SEM, N=5 independent biological replicates (where one biological replicate contains 20 worms per plate). *p<0.05, ***p=0.0001, ****p<0.0001, one-way ANOVA, Tukey post hoc test. L). NAC restored non-paralyzed worm swimming rates in iron toxicity environment. Staged L4 worms were transferred to plates containing 0 μM iron, 0 μM iron + 2.5 mM NAC, 35 μM iron and 35 μM iron + 2.5 mM NAC. Non-paralyzed worms were individually transferred to plate containing 100 μl of buffer. After 30 seconds of equilibration, swimming rates were collected for 15 seconds. Data are mean ±SEM N=5 independent replicates (where 4 independent worm count constitute an N). ns not significant, ****p<0.0001, one-way ANOVA, Tukey post hoc test
