Abstract
Centrosomes rely upon proteins within the pericentriolar material to nucleate and organize microtubules. Several mRNAs also reside at centrosomes, although less is known about how and why they accumulate there. We previously showed that local Centrocortin (Cen) mRNA supports centrosome separation, microtubule organization, and viability in Drosophila embryos. Here, using Cen mRNA as a model, we examine mechanisms of centrosomal mRNA localization. We find that while the Cen N’-terminus is sufficient for protein enrichment at centrosomes, multiple domains cooperate to concentrate Cen mRNA at this location. We further identify an N’-terminal motif within Cen that is conserved among dynein cargo adaptor proteins and test its contribution to RNA localization. Our results support a model whereby Cen protein enables the accumulation of its own mRNA to centrosomes through a mechanism requiring active translation, microtubules, and the dynein motor complex. Taken together, our data uncover the basis of translation-dependent localization of a centrosomal RNA required for mitotic integrity.
Keywords: RNA localization, centrosome, dynein, transport, cargo adaptor
Summary
Enrichment of Centrocortin (Cen) mRNA at centrosomes is required for mitotic fidelity. This study describes a mechanism underlying co-translational Cen mRNA targeting involving microtubules, the dynein motor, and a highly conserved dynein binding motif within the Cen coding sequence.
Introduction
RNA localization is a highly conserved paradigm used to restrict gene expression to subcellular compartments (Chin and Lecuyer, 2017; Ryder and Lerit, 2018; Das et al., 2021). Several mechanisms enable RNA localization, including active transport, selective protection from degradation, and diffusion coupled to local entrapment (Palacios, 2007; Holt and Bullock, 2009; Das et al., 2021). Based on a small number of well-characterized examples, such as β-actin mRNA, it is widely believed that active transport involves recognition of RNA elements by RNA-binding proteins, which then recruit motor proteins to traffic the mRNA cargo to its destination (Kislauskis et al., 1993; Oleynikov and Singer, 2003; Bullock, 2007; Martin and Ephrussi, 2009; Mofatteh and Bullock, 2017). Often, these RNAs are translated once they reach their destination (Besse and Ephrussi, 2008; Jung et al., 2014). However, in cases of co-translational transport, the nascent peptide plays a critical role in RNA localization, as classically shown for transcripts localizing to the endoplasmic reticulum (recently reviewed in (Gasparski et al., 2022)).
Recent work highlights the centrosome as a subcellular hub for mRNA localization and translational control (Marshall and Rosenbaum, 2000; Lecuyer et al., 2007; Ryder and Lerit, 2018; Zein-Sabatto and Lerit, 2021). Centrosomes undergo cell cycle-dependent oscillations in microtubule-organizing activity dependent upon the recruitment and shedding of the pericentriolar material (PCM) (Gould and Borisy, 1977; Khodjakov and Rieder, 1999; Palazzo et al., 2000). Whether local RNAs contribute to centrosome dynamics or function is a longstanding question subject to renewed interest (Zein-Sabatto and Lerit, 2021; Lerit, 2022).
Localization-based screens in cultured cells, Xenopus, Drosophila, and other systems identified several conserved mRNAs residing at centrosomes, including cyclin B (cyc B), Pericentrin (pcnt)/ Pcnt-like protein (Plp), and Centrocortin (Cen) mRNAs (Raff et al., 1990; Groisman et al., 2000; Lecuyer et al., 2007; Sepulveda et al., 2018; Bergalet et al., 2020; Chouaib et al., 2020; Ryder et al., 2020; Safieddine et al., 2021; Fang and Lerit, 2022). Intriguingly, most RNAs enrich at centrosomes just prior to mitotic onset, with lower levels detected during M-phase (Sepulveda et al., 2018; Ryder et al., 2020). These findings suggest the concentration of RNA at the centrosome is dynamically regulated, perhaps through conserved mechanisms, and further hint at biological relevance.
Within syncytial Drosophila embryos, RNA localization to centrosomes is also regulated developmentally. Drosophila embryos proceed through 14 abridged and synchronous nuclear divisions prior to cellularization (Foe and Alberts, 1983). Most localized RNAs progressively enrich at interphase centrosomes as the nuclear cycles (NCs) proceed (Ryder et al., 2020; Fang and Lerit, 2022). For example, during NC 10, Cen mRNA localizes to centrosomes primarily as single molecules. However, by NC 13, significantly more Cen mRNA enriches at centrosomes within distinct, micron-scale ribonucleoprotein (RNP) granules containing Cen mRNA and protein and the multifunctional RNA-binding protein, fragile-X mental retardation protein (FMRP), a negative regulator of Cen mRNA translation (Ryder et al., 2020).
Cen was originally identified based on its direct binding to Centrosomin (Cnn), an essential PCM scaffolding factor (Kao and Megraw, 2009). That study further showed that Cen mutants display mitotic errors and embryonic lethality. Critically, proper localization of Cen mRNA to the centrosome is also important for mitotic fidelity. The 3’-UTR of the anterior morphogen bicoid (bcd) contains localization elements sufficient to direct heterologous RNAs to the anterior pole (Macdonald and Struhl, 1988). By fusing the Cen coding sequence (CDS) to the bcd 3’-UTR, we demonstrated that Cen mRNA mislocalization results in centrosome separation errors, disorganized microtubules, DNA damage, and embryonic lethality (Ryder et al., 2020). What directs Cen mRNA to the centrosome remains little understood, however.
Because the early Drosophila embryo is largely transcriptionally quiescent, and its development relies upon maternally endowed stores of RNAs and proteins until the maternal-to-zygotic transition (Tadros and Lipshitz, 2009), the rapid accumulation of RNA at interphase centrosomes is suggestive of an active transport mechanism. However, this remains to be tested. We and others showed the Cen CDS is necessary and sufficient for RNA localization (Bergalet et al., 2020; Ryder et al., 2020). Consistent with a targeting mechanism requiring the nascent peptide, the accumulation of Cen mRNA at centrosomes is sensitive to the protein synthesis inhibitor harringtonine (Bergalet et al., 2020). This finding also aligns with the discovery that mRNAs localize to centrosomes in mammalian cells while they are translated (Safieddine et al., 2021). While co-translational transport has emerged as the prevailing model for all centrosome-localized mRNAs studied to date, the underlying mechanisms directing these RNAs to the centrosome remains largely unknown.
Here, we investigate how Cen RNPs localize to the centrosome. We identify cis- and trans-elements needed for proper localization of Cen mRNA. We show that an N-terminal Cen fragment is sufficient for protein localization to the centrosome but insufficient to form and localize Cen mRNA granules. Nevertheless, the N’-terminus of Cen is necessary for the accumulation of Cen mRNA at centrosomes. Our data indicate that multiple domains within the Cen CDS work together to coordinate effective RNA localization. Supporting this notion, we identified a conserved dynein light intermediate chain (DLIC) binding site within the Cen N-terminus that, together with other components of the dynein motor complex, promotes Cen mRNA granule organization and localization. We propose a model whereby Cen protein serves as a dynein cargo adaptor to potentiate the co-translational localization of its cognate mRNA.
Results
Engaged polysomes are necessary for Cen mRNA granule formation and localization to centrosomes
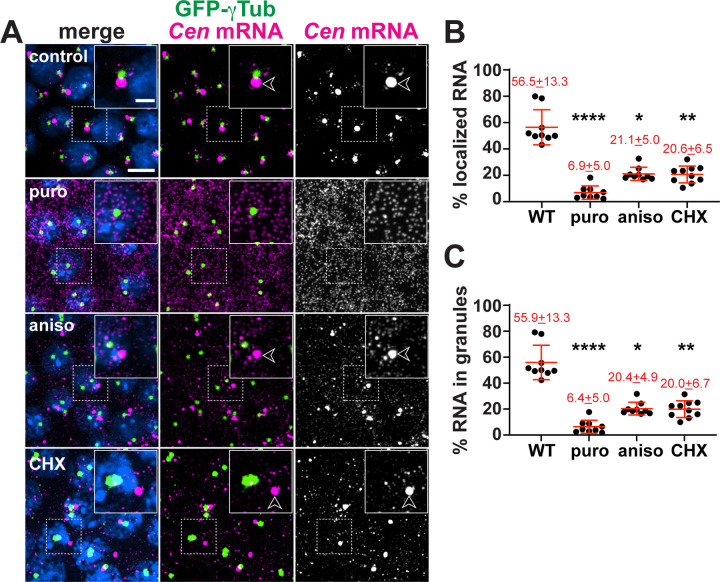
Cen mRNA localization displays differential sensitivity to various classes of translational inhibitors (Bergalet et al., 2020). Using single molecule fluorescence in situ hybridization (smFISH) and computational analysis of the resulting images (Ryder et al., 2020; Ryder and Lerit, 2020), we quantified Cen mRNA localization relative to GFP-γTubulin (GFP-γTub) labelled centrosomes in embryos treated with puromycin (puro), a tRNA analog that terminates translation elongation and promotes ribosome dissociation, versus anisomycin (aniso) and cycloheximide (CHX), drugs that block elongation without releasing the nascent peptide (Figure 1A) (Nathans, 1964; Grollman, 1967; Schneider-Poetsch et al., 2010). Each of the translational inhibitors we examined impaired Cen mRNA accumulation at centrosomes, also resulting in a corresponding reduction in the percent of RNA localizing within higher order RNP granules (defined as four or more overlapping RNA objects (Ryder et al., 2020)) (Figure 1B,C). These responses were particularly evident upon treatment with puro, where RNA localization and granule formation were largely abolished. Thus, Cen mRNA localization is dependent upon intact polysomes. Our findings further suggest that sequences within the nascent peptide direct Cen mRNA localization.
Figure 1. Co-translational transport of Cen mRNA to centrosomes.
(A) Maximum-intensity projections of NC 13 embryos expressing GFP-gTub (green) stained with Cen smFISH probes (magenta) and DAPI (blue) to label nuclei following incubation with DMSO (control) or the translation inhibitors puromycin (puro), anisomycin (aniso), or cycloheximide (CHX). Arrowheads mark Cen RNPs. Quantification shows the percentage of Cen mRNA (B) localizing to the centrosome and (C) organized within granules, defined as ≥4 overlapping RNA objects (Ryder et al., 2020). Mean ± SD is displayed (red). Significance by ANOVA with Dunnett’s multiple comparison test with *, P<0.05; **, P<0.01; and ****, P<0.0001. Scale bars: 5 μm; 2 μm (insets).
The Cen N-terminus is necessary, but not sufficient, to localize Cen RNA to centrosomes
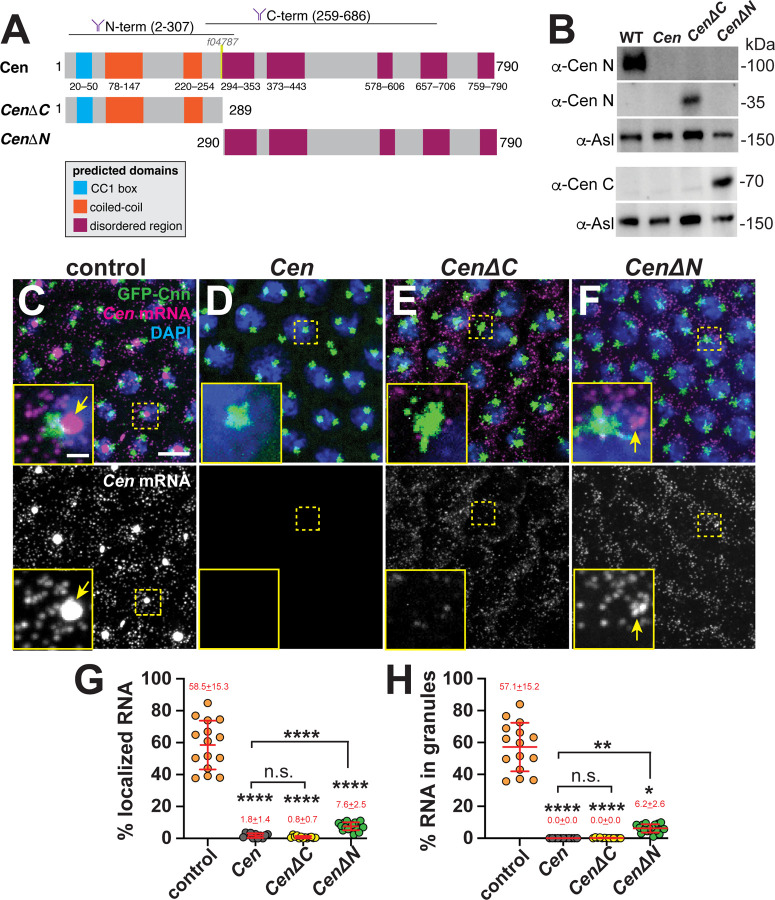
To identify domains important for Cen mRNA localization, we first truncated the Cen protein into N- (CenΔC, comprising amino acids (AAs) 1–289) or C-terminal (CenΔN, comprising AAs 290–790) pieces and expressed these in the Cen null genetic background (Figure 2A). Immunoblotting confirmed the truncated products were expressed at comparable levels in early embryo extracts and migrated at the expected molecular sizes, as detected by antibodies with epitopes in the N’- or C’-regions of Cen (Figure 2B; (Kao and Megraw, 2009)). By interphase of NC 13, most Cen mRNA normally localizes to the centrosome within granules (arrows, Figure 2C). Demonstrating specificity, the smFISH signals were absent in Cen mutants, which harbor a P-element insertion (f04787) in the CDS and are RNA and protein nulls (Figure 2A,D; (Bergalet et al., 2020; Ryder et al., 2020)). Because our probes tile the CDS, Cen smFISH signals were detected in both CenΔC and CenΔN backgrounds (Figure 2E and F). However, the percentages of Cen mRNA overlapping the centrosome surface (0 μm distance from GFP-Centrosomin (GFP-Cnn)) and within granules were dramatically reduced in the truncation lines relative to controls (Figure 2G and H). While small RNA granules were occasionally detected in CenΔN embryos (arrows, Figure 2F, H), neither fragment was sufficient to restore endogenous levels of RNA localization. We conclude that neither the N’- nor C’-termini of Cen are sufficient for robust RNA localization; rather, both regions likely function cooperatively.
Figure 2. Multiple Cen domains support mRNA localization to the centrosome.
(A) Schematic of the full-length and truncated Cen protein products with positions of predicted domains (Paysan-Lafosse et al., 2023), antibody epitopes (Kao and Megraw, 2009), and the transposon f04787 within null mutants indicated. (B) Immunoblots from 0.5–2.5 hr embryo extracts from the indicated genotypes showing truncated Cen protein products in the CenΔC (~35 kDa) and CenΔN (~70 kDa) samples relative to the Asl loading control. The N-terminal anti-Cen antibody was used for the top two blots (α-Cen N), while the C-terminal anti-Cen antibody was used below (α-Cen C; see also (Kao and Megraw, 2009)). (C–F) Maximum-intensity projections of Cen smFISH (magenta) in NC 13 interphase embryos expressing GFP-Cnn (green) with DAPI-stained nuclei (blue). (C) Control embryo with Cen mRNA localized at centrosomes (arrow). In contrast, (D) Cen mutants and (E) CenΔC embryos fail to localize Cen mRNA to centrosomes. (F) Although CenΔN is partially sufficient to form small RNA granules (arrow) near centrosomes, neither fragment recapitulates WT localization. In all experiments, CenΔC and CenΔN are expressed in the Cen null background. Percentage of Cen mRNA (G) overlapping with centrosomes or (H) in granules 0 μm from the Cnn surface. Each dot represents a measurement from N= 15 control, 11 Cen, 13 CenΔC, and 17 CenΔN embryos. Mean ± SD is displayed (red). Significance was determined by (G) one-way ANOVA followed by Dunnett’s T3 multiple comparison test or (H) Kruskal-Wallis test followed by Dunn’s multiple comparison test with n.s., not significant; *, P<0.05; **, P<0.01; and ****, P<0.0001. Scale bars: 5μm; 1μm (insets).
Cen protein also localizes to the centrosome within Cen RNPs (Bergalet et al., 2020; Ryder et al., 2020). Therefore, we next tested whether the truncated protein products localized to centrosomes. We confirmed that anti-Cen N’ and anti-Cen C’ antibodies detect Cen at centrosomes in control embryos, and these signals are absent in Cen null mutants (Figures 2A and 3A–C, F; (Kao and Megraw, 2009)). By comparison, while CenΔC localized to centrosomes, CenΔN did not, as detected by the N’- versus C’-terminal antibodies, respectively (Figure 3D–F). Contrary to full-length Cen, we observed that the CenΔC protein appeared to localize near the center of the centrosome rather than the outer PCM flares (cf. Figure 3A, B vs. D); however, what directs Cen to distinct PCM zones remains unclear. We conclude that the N-terminus is necessary and sufficient for Cen protein localization (Figure 3D–F). Moreover, our results show that Cen mRNA and protein distributions may be uncoupled (cf. Figures 2E vs. 3D).
Figure 3. he N-terminal fragment is necessary and sufficient for Cen protein localization to the centrosome.
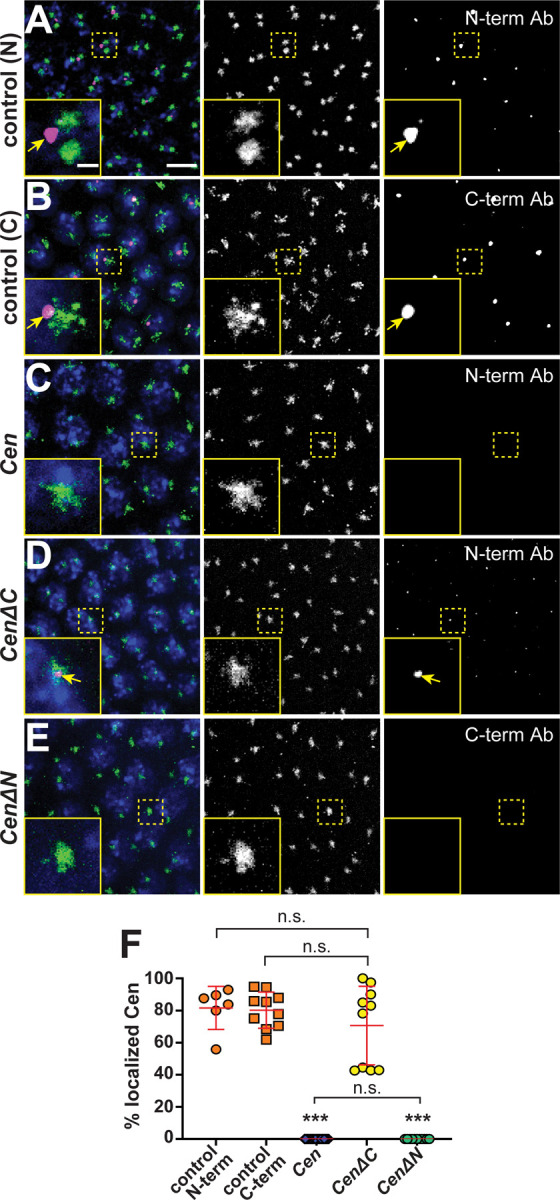
T Maximum-intensity projections of NC 13 interphase embryos expressing GFP-Cnn (green) labeled with anti-Cen antibodies (magenta) and DAPI (blue nuclei). Control embryos labeled with (A) anti-Cen N-terminal or (B) C-terminal antibodies (Ab) show Cen localized at centrosomes (arrows). (C) Cen protein is not detected in null mutants. (D) The N-terminal fragment (CenΔC) is sufficient to direct Cen to the centrosome (arrows), while the C-terminal fragment (CenΔN; (E)) is not. Both transgenes are expressed in the Cen null background. (F) The percentage of Cen protein signals overlapping with centrosomes (0 μm from Cnn surface). Each dot represents a measurement from N= 6 control (N-terminal Cen Ab), 10 control (C-terminal Cen Ab), 23 Cen null (N-terminal Cen Ab), 10 CenΔC (N-terminal Cen Ab), and 11 CenΔN embryos (C-terminal Cen Ab). Significance was determined by Kruskal-Wallis test followed by Dunn’s multiple comparison test with n.s., not significant and ***, P<0.001. Scale bars: 5μm; 1μm (insets).
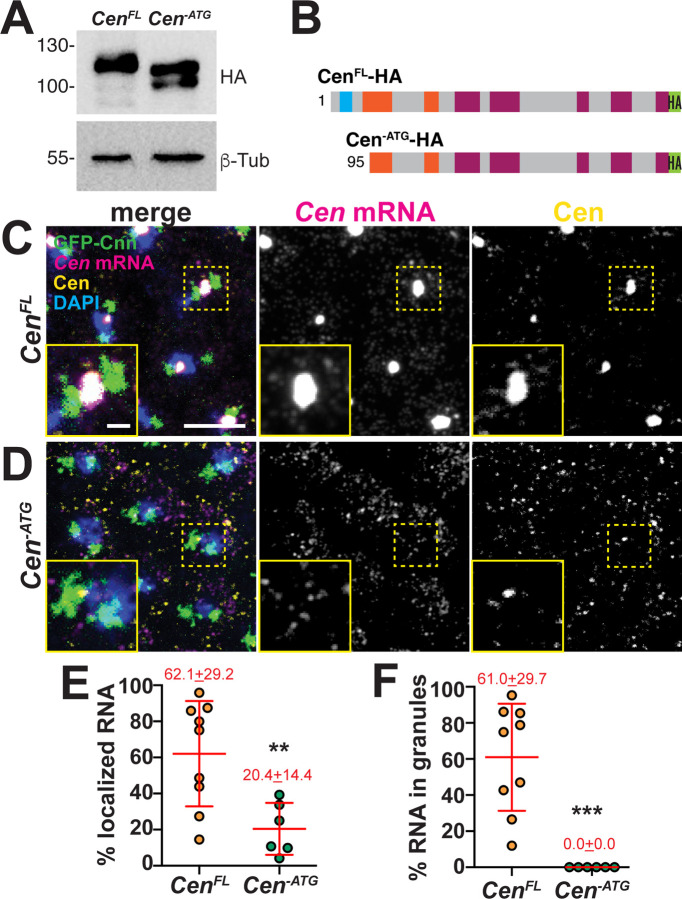
As an independent approach to experimentally uncouple Cen mRNA and protein, we deleted the translation initiation codon (Cen-ATG) and expressed this or a full-length control (CenFL) HA-tagged transgene in the Cen null background. Unexpectedly, Cen-ATG was translated in ovaries and embryos, yielding a protein ~30 kDa smaller than CenFL, as detected by western blotting (Figure 4A). These data indicate Cen has one or more cryptic translation start sites. Consistent with this finding, the first 90–100 N’-terminal AAs of Cen-ATG were undetectable by mass spectrometry (Figures S1 and 4B). Although Cen-ATG did not block Cen translation as intended, it did permit further analysis of the role of the N’-terminus for Cen mRNA localization. The amount of Cen mRNA localizing to centrosomes was reduced by two-thirds and granules failed to form in Cen-ATG embryos relative to CenFL controls (Figure 4C–F). In addition, despite comparable expression levels, less Cen-ATG protein localized to centrosomes compared to CenFL (insets Figure 4C, D). Taken together, our analysis indicates that the first ~100 AA are important for Cen mRNA and protein localization to centrosomes.
Figure 4. The first 100 AA of Cen direct RNA localization.
(A) Immunoblots from ovarian extracts from the indicated genotypes showing Cen protein products, as detected with anti-HA antibodies, relative to the β-Tub loading control. Truncated products are detected in the Cen-ATG lysate. (B) Schematic of the CenFL and Cen-ATG HA-tagged protein products showing predicted translation start sites, based on mass spectrometry analysis (see Figure S1). Maximum intensity projections of NC 13 (C) CenFL and (D) Cen-ATG embryos expressing GFP-Cnn and stained with Cen smFISH probes (magenta), C-term anti-Cen antibodies (yellow), and DAPI (blue) to label nuclei. Quantifications show (E) the percentage of RNA overlapping with centrosomes or (F) organized within granules 0 μm from the Cnn surface. Each dot represents a measurement from N= 9 CenFL and 6 Cen-ATG embryos. In all experiments, both transgenes were expressed in the Cen null background. Mean ± SD is displayed (red). Significance was determined by two-tailed Mann-Whitney test with **, p=0.0076 and ***, p=0.0004. Scale bars: 5μm; 1μm (insets).
A conserved predicted DLIC binding motif facilitates Cen mRNA localization to centrosomes
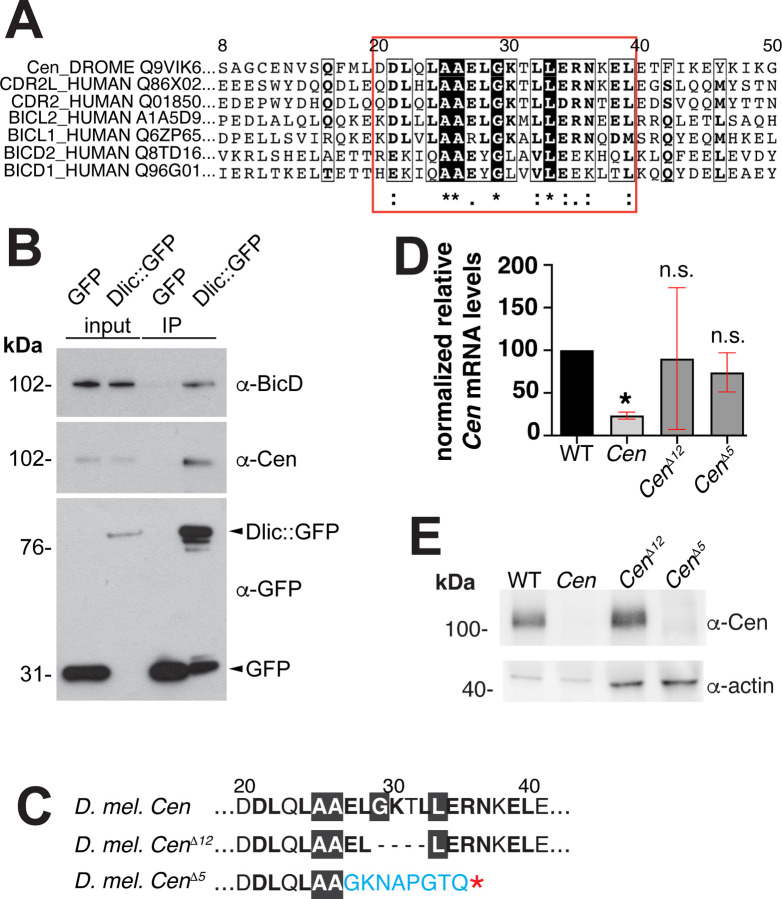
Analysis of the Cen protein secondary structure revealed two predicted N’-terminal coiled-coil domains and several disordered regions clustered at the C-terminus (Cen; Figure 2A; (Apweiler et al., 2000)). Through primary sequence alignments, we identified a region within the first 50 AA of Cen that is very similar to the previously identified CC1 box motif of several dynein cargo adaptors, which include BicD family members, Spindly, and Hook proteins (red box, Figure 5A; (Gama et al., 2017; Lee et al., 2018)). This motif is also conserved in the human Cen paralogs, CDR2L and CDR2 (Figure 5A). The CC1 box mediates the interaction of dynein cargo adaptors with a short helix of the DLIC subunit of the dynein motor complex. Together with adjacent coiled-coil sequences that interact with Dynein heavy chain (Dhc) and the dynein activating complex, dynactin, this interaction tethers cargo to the motor and releases dynein from its autoinhibited state (Gama et al., 2017; Lee et al., 2018; Lee et al., 2020; Chaaban and Carter, 2022). Through immunoprecipitation, we tested whether Cen also associates with DLIC in embryonic extracts. Similar to the positive control BicD, Cen co-precipitated with GFP-DLIC, but not GFP alone (Figure 5B). Taken together, these data are consistent with Cen representing a novel dynein cargo adaptor.
Figure 5. Identification of the conserved Cen DLIC binding site.
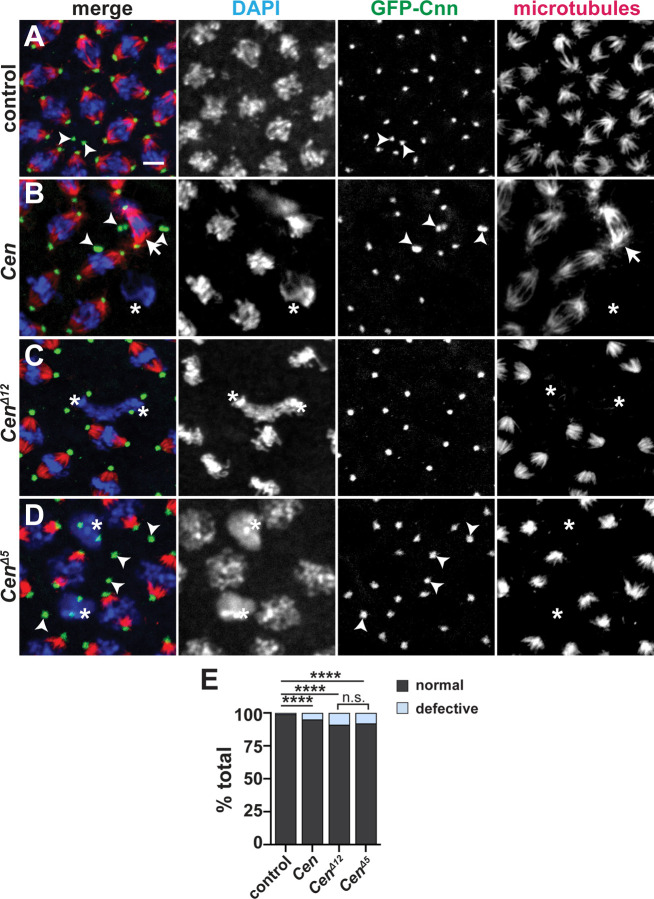
(A) Clustal Omega sequence alignment of Drosophila Cen with the human paralogs CDR2 and CDR2L and several dynein activating cargo adaptors. Red box marks the conserved DLIC-binding motif (CC1 box). (B) Dlic-GFP associates with BicD (Dienstbier et al., 2009) and Cen in 0–5-hour embryonic extracts. Input and immunoprecipitated samples (IP) for GFP control and Dlic-GFP are indicated. (C) The Cen CC1 box was mutated, yielding an in-frame deletion of the 12 nucleotides that comprise amino acids (AA) 29–32 (GKTL; CenΔ12), while the CenΔ5 mutant is defined by a frameshift after AA 26 and a premature stop (asterisk). (D) Relative levels of Cen mRNA normalized to RP49 and the WT control in 0–2-hour embryos (up to NC 14) by qPCR. Bars show mean ± SD from three independent experiments. *, P<0.05 by Kruskal-Wallis multiple comparison test relative to WT; n.s., not significant. (E) Blot shows Cen protein detected in 0–2-hour embryos with a C’-terminal anti-Cen antibody relative to the actin loading control. No Cen protein was detected in null or CenΔ5 extracts.
To test if the conserved CC1 box supports Cen mRNA localization to centrosomes, we disrupted it by CRISPR/Cas-9 genome editing. We successfully generated several mutants, of which, CenΔ12 represents the largest in-frame deletion recovered and removes AAs 29–33. We also examined CenΔ5, which causes a frameshift mutation after AA 26, resulting in a predicted truncated product (Figure 5C). Both mutations disrupt highly conserved residues with the CC1 box motif, including an invariant glycine that creates a cavity in the coiled coil for binding the DLIC helix (Lee et al., 2020). We confirmed by qPCR that both CenΔ12 and CenΔ5 mutant lines express Cen mRNA at levels comparable to wild-type (WT; Figure 5D). In contrast, while Cen protein is produced in CenΔ12 embryos, none was detectable in CenΔ5 extracts by western blot (Figure 5E), presumably due to protein destabilization.
To assay whether the CC1 box contributes to Cen mRNA localization, we compared RNA distributions in CenΔ12 and CenΔ5 embryos relative to controls. In younger, interphase NC 11 embryos, enrichments of Cen mRNA at centrosomes were modestly reduced in CenΔ12 embryos relative to controls, but this did not reach statistical significance. Conversely, while numerous Cen transcripts were still detected in CenΔ5 embryos, Cen mRNA localization to centrosomes was eliminated (Figure S2). These findings corroborate earlier evidence that sequences within the Cen protein are essential for Cen mRNA localization.
We next examined Cen mRNA distributions relative to the non-localizing gapdh mRNA during interphase NC 13, when the majority of Cen RNA localizes to the centrosome within granules (0 μm distance from Cnn surface; Figure 6A, D,E). Relative to controls, less Cen mRNA enriched at centrosomes in CenΔ12 (~15% reduction; mean±S.D.= 60.2±12.1% in CenΔ12 versus 70.5±9.1% in controls; p=0.0343 by Kruskal-Wallis test; Figures 6A, B, and D). These data indicate that impairing the DLIC binding site compromises Cen mRNA localization. In contrast, the CenΔ5 mutation abolished both Cen mRNA localization and RNA granule assembly (Figure 6C–E). Taken as a whole, these data indicate the CC1 box motif within Cen is functionally important and further imply DLIC contributes to Cen mRNA localization to centrosomes.
Figure 6. The CC1 box supports Cen mRNA localization.
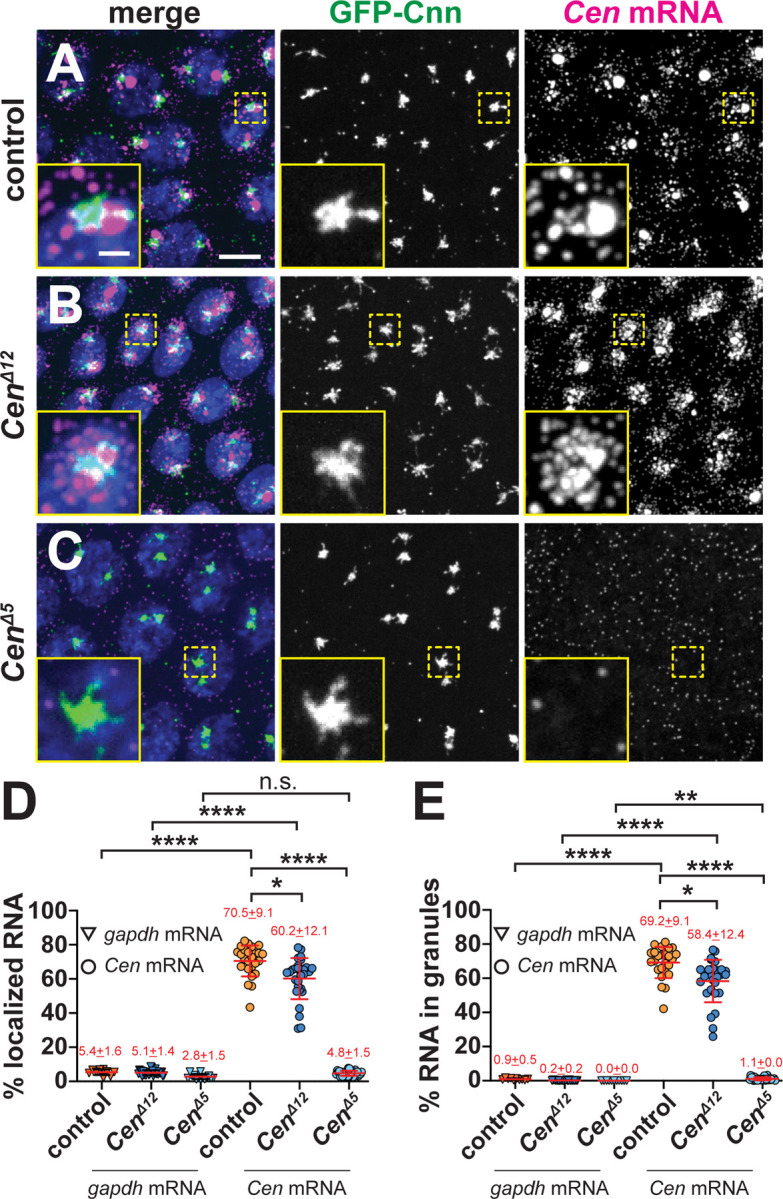
Maximum-intensity projections of NC 13 interphase embryos expressing GFP-Cnn (green) stained with Cen smFISH probes (magenta) and DAPI (blue nuclei). (A) Control embryos show Cen mRNA enriched at centrosomes in RNP granules, which are reduced in (B) CenΔ12 samples. (C) Cen mRNA localization and granule formation are abolished in CenΔ5 embryos. Quantification of the percentage of Cen or gapdh mRNA (D) overlapping with the centrosome surface and (E) residing in granules (0 μm distance from Cnn). Each dot represents a single measurement from control (N= 10 gapdh and 25 Cen mRNA), CenΔ12 (N= 30 gapdh and 30 Cen mRNA), and CenΔ5 (N= 14 gapdh and 27 Cen mRNA) labelled embryos. Mean ± SD displayed (red). Significance was determined by Kruskal-Wallis test followed by Dunn’s multiple comparison test relative to controls with n.s., not significant; *, P<0.05; **, P<0.01; and ****, P<0.0001. Scale bar: 5μm; 1μm (insets).
Mitotic spindle morphogenesis is sensitive to local Cen mRNA dosage
To further examine the functional significance of the conserved Cen CC1 box, we examined mitotic spindle morphogenesis. Proper dosage of Cen mRNA at the centrosome is needed for mitotic fidelity (Ryder et al., 2020). Similar to Cen null mutants, CenΔ12 and CenΔ5 embryos displayed elevated frequencies of aberrant spindles and defective centrosome separation relative to controls (Figure 7A–E). These findings show that Cen activity supports spindle formation.
Figure 7. Disruption of the Cen CC1 box impairs spindle morphology.
Maximum-intensity projections of metaphase NC 12 embryos from embryos expressing GFP-Cnn (green, centrosomes) and stained for α-Tub to label microtubules (red) and DAPI (blue nuclei). (A) Control embryo showing bipolar spindles. Various spindle defects are noted in (B) Cen null, (C) CenΔ12, and (D) CenΔ5 embryos, including spindle inactivation (asterisks), detached centrosomes (arrowheads), and bent spindles (arrows). (E) Frequency of spindle defects from N=1622 spindles from n=7 control, N=1473 spindles from n=7 Cen null, n=2138 spindles from n=15 CenΔ12, and N=1842 spindles from n=12 CenΔ5 embryos. ****, P<0.00001 by Chi-square test. Scale bar: 5 μm.
Microtubules enrich Cen mRNA at centrosomes
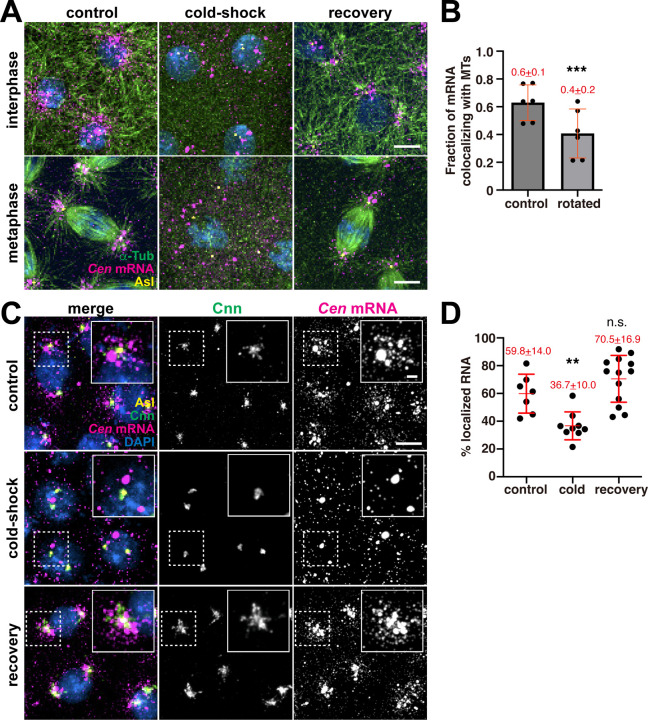
A role for the CC1 box raised the possibility that Cen mRNA is transported by dynein along microtubules to the centrosome. Indeed, microtubules are nucleated from centrosomes with their minus ends embedded within the PCM (Mitchison and Kirschner, 1984; Soltys and Borisy, 1985; Vertii et al., 2016). While microtubules serve as tracks for the localization of many RNAs, including PCNT and ASPM mRNAs in mammalian cells, their requirement for the localization of other centrosomal mRNAs has not been tested (Sepulveda et al., 2018; Safieddine et al., 2021). To assay the relationship between microtubules and Cen mRNA, we first confirmed the coincidence of endogenous Cen mRNA and microtubules labeled with α-Tub antibodies by calculating a Mander’s coefficient of colocalization (Figure 8A, B; control). These co-occurring Cen mRNA and α-Tub signals were not due to spurious overlap, as rotating the RNA channel by 90° significantly reduced the extent of colocalization (Figure 8B). Therefore, a proportion of Cen mRNA overlaps with microtubules.
Figure 8. Microtubules enrich Cen mRNA at centrosomes.
(A) Microtubule regrowth assay. Representative images of NC 11 embryos labeled with Cen smFISH probes (magenta) and antibodies for α-Tub (green) and Asl (yellow). Nuclei are labeled with DAPI (blue) in control, cold-shock, and recovery conditions. (B) Graph shows the Mander’s coefficient of colocalization for Cen mRNA overlapping with microtubules. Each dot is a measurement from N=6 interphase NC 10–11 control embryos. The RNA channel was rotated 90° to test for specificity of colocalization. (C) Maximum intensity projections of NC 12 interphase embryos from the indicated conditions labeled with Cen smFISH probes (magenta), Cnn (green) and Asl (yellow) antibodies, and DAPI (blue). Insets show Cnn structure and Cen mRNA distribution are affected by microtubule destabilization. (D) Quantification of the percentage of Cen mRNA localizing to centrosomes (<1 μm distance from Asl surface) from N=7 control, 9 cold-shocked, and 13 recovered NC 12 interphase embryos. Mean ± SD is displayed (red). Significance was determined by (B) two-tailed t-test and (D) one-way ANOVA followed by Dunnett’s multiple comparisons test relative to the control with n.s., not significant; **, P<0.01; and ***, P<0.001. Scale bars: 5 μm; 2 μm (insets).
Next, we conducted a microtubule regrowth assay to determine whether microtubules are required for Cen mRNA localization. Cold-shock induced microtubule depolymerization was sufficient to disperse Cen mRNA at all stages examined (Figure 8A; cold-shock). Examination of RNA distributions in age-matched embryos relative to centrosomes labeled with Cnn and Asterless (Asl) antibodies revealed that microtubule disruption was sufficient to decrease Cen mRNA localization to centrosomes by nearly 40%, as compared to controls (Figure 8C, D). The condensation of Cnn into a more compact structure following cold-shock (Figure 8C) serves as an internal control, as identical responses were previously noted following acute microtubule depolymerization via colchicine (Megraw et al., 2002; Lerit et al., 2015). Moreover, when the embryos were allowed to briefly recover at room temperature to permit microtubule regrowth, Cen mRNA re-decorated microtubules and re-localized to centrosomes to untreated levels (Figure 8A; recovery; and C,D). These data demonstrate that microtubules allow robust localization of Cen mRNA to centrosomes.
Cen mRNA localization is dynein-dependent
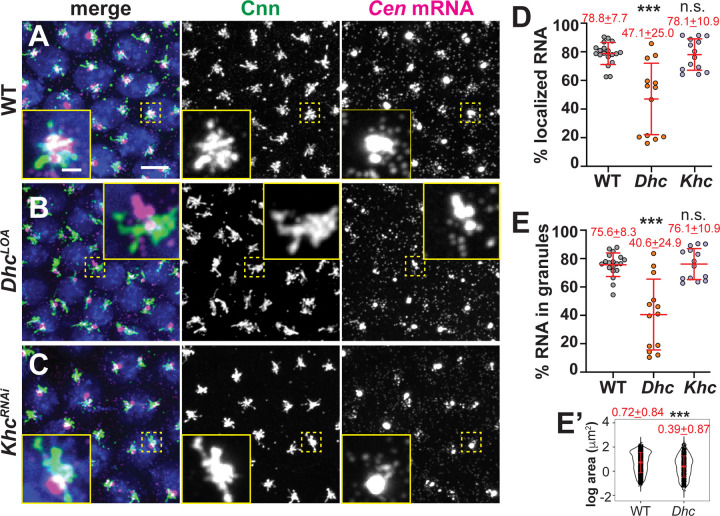
To further test whether dynein traffics Cen mRNA to centrosomes, we compared Cen distributions in embryos with impaired Dhc activity relative to controls. Because dynein is essential for viability (Gepner et al., 1996), we collected embryos from mothers homozygous for a hypomorphic mutation in the Dhc gene (Dhc64C) (Salvador-Garcia et al., 2023) that is equivalent to the legs at odd angles (LOA; hereafter, DhcLOA) allele first described in mouse (Nolan et al., 2000; Hafezparast et al., 2003). Supporting a requirement of the dynein motor complex for Cen mRNA localization, the DhcLOA mutation led to significantly less Cen mRNA at centrosomes (~40% reduction), as well as ~50% less Cen mRNA within granules, as compared to controls (Figure 9A–E). Moreover, the Cen RNPs that did form in the Dhc mutants were also smaller (Figure 9B, E’). These data confirm that dynein supports Cen RNA granule assembly and localization. In contrast, depletion of the plus-end-directed microtubule motor, kinesin, using a shRNA (KhcRNAi) sufficient to reduce Khc protein levels (Veeranan-Karmegam et al., 2016) did not significantly alter Cen RNA localization at the centrosome (Figure 9C–E). Our collective data implicate the dynein transport complex in promoting Cen mRNA accumulation at centrosomes.
Figure 9. Dynein targets Cen mRNA to centrosomes.
Maximum-intensity projections of NC 13 interphase embryos labeled with Cen smFISH (magenta), anti-Cnn antibodies (green; centrosomes), and DAPI (blue nuclei) in (A) WT, (B) DhcLOA hypomorphic, or (C) KhcRNAi embryos. Quantification shows the percentage of total mRNA that (D) overlaps with centrosomes and (E) resides in granules at centrosomes (0 μm distance from Cnn). Each dot represents a measurement from N= 19 WT, 13 DhcLOA and 14 KhcRNAi embryos. (E’) Log transformed RNA granule area from N=4127 granules from n=23 WT embryos and N=1412 granules from n=13 DhcLOA embryos; each dot represents a single granule. Mean ± SD displayed (red). Significance by (D and E) Kruskal-Wallis test followed by Dunn’s multiple comparison test relative to WT and (E’) unpaired t-test with n.s., not significant and ***, P<0.001. Scale bar: 5μm; 1 μm (inset).
The RNA-binding protein Egl enhances Cen mRNA localization
To direct RNA trafficking along microtubules in Drosophila oocytes and blastoderm embryos, the RNA-binding protein Egl loads various transcripts onto dynein (Dienstbier et al., 2009). Dynein light chain binds Egl and promotes its dimerization, which optimizes Egl binding to mRNA and subsequently the dynein cargo adaptor BicD (McClintock et al., 2018; Sladewski et al., 2018; Goldman et al., 2019). Egl is required for oocyte specification and polarization, precluding analysis of egl null embryos (Mach and Lehmann, 1997; Navarro et al., 2004). Therefore, to test whether Egl contributes to Cen mRNA localization, we examined egl-deficient embryos expressing an egl mutant transgene (EglRBD3) that contains alanine substitutions of 8 positively charged residues within the Egl RNA-binding domain (RBD) and, consequently, disrupts subcellular localization of various mRNA cargoes in Drosophila oocytes (Goldman et al., 2021). As a control, we also examined egl-deficient embryos expressing a full-length (EglWT) transgene. This analysis revealed that interphase NC 13 EglRBD3 embryos had ~15% less Cen mRNA at the centrosome or within RNA granules than EglWT (Figure 10A–D). Additionally, those Cen RNPs that formed in the absence of Egl RNA-binding activity were nearly 20% smaller than controls (Figure 10D’). Thus, Egl contributes to Cen mRNA granule assembly or maintenance to promote the accumulation of Cen mRNA at centrosomes.
Figure 10. The Egl RBD supports Cen mRNA localization.
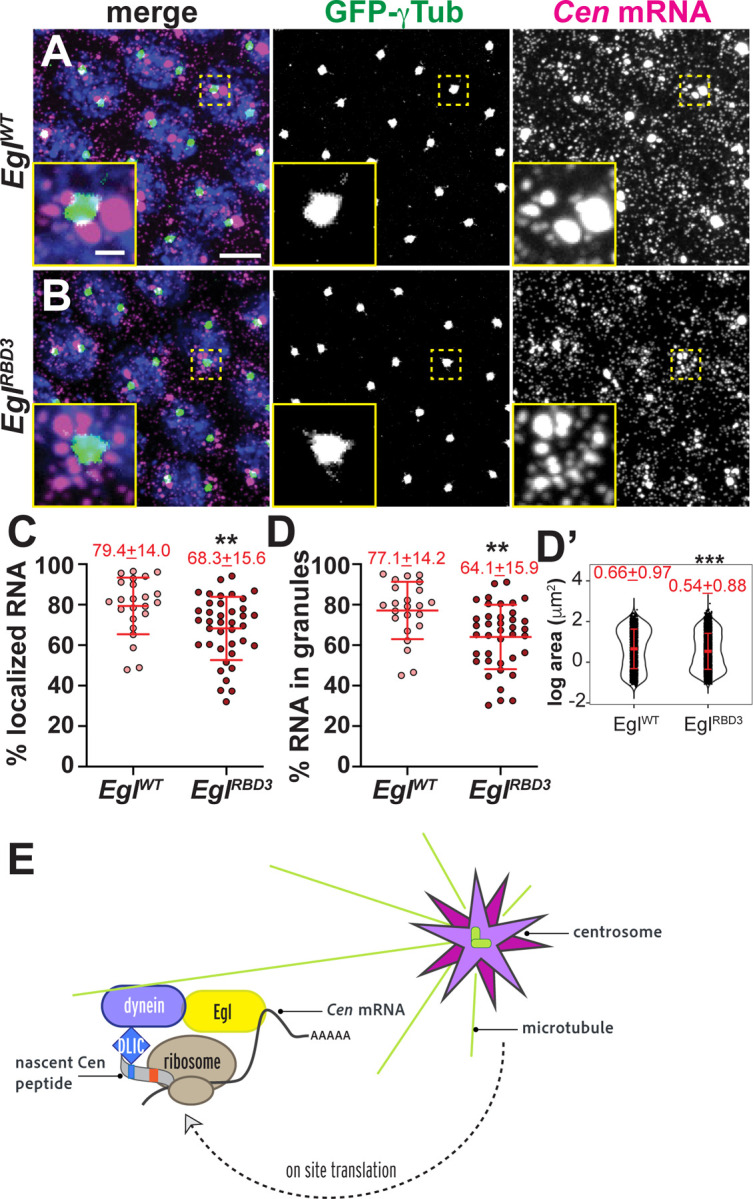
Maximum-intensity projections of NC 13 interphase embryos expressing GFP-γ-Tub (green) labeled with Cen smFISH (magenta) and DAPI (blue) in (A) control EglWT and (B) EglRBD3 embryos. Quantification of the percentage of (C) total Cen mRNA and (D) mRNA in granules within the PCM zone (≤0.5 μm from γ-Tub surface). Each dot represents a measurement from 23 EglWT and 39 EglRBD3 embryos. (D’) Log transformed RNA granule area from N=2304 granules from n=23 EglWT embryos and N=4924 granules from n=39 EglRBD3 embryos; each dot represents a single granule. Mean ± SD displayed (red). Significance by unpaired t-test with **, P<0.01; and *** P<0.001. Scale bar: 5μm; 1μm (insets). (E) Model of the co-translational transport of Cen mRNA to centrosomes. Upon translation, an N’-terminal DLIC-binding CC1 box motif is exposed on the Cen nascent peptide, which associates with the dynein motor complex. We speculate Egl may bind to Cen mRNA. The Cen transport complex transits along microtubules via dynein to the centrosome. Additional on-site translation (Bergalet et al., 2020) may contribute to granule formation and/or stabilization.
Discussion
While RNA localization to centrosomes is a longstanding observation, how local RNAs affect centrosome behavior remains relatively unstudied. That the localization of some centrosomal RNAs is conserved across taxa strongly implies a functional role. Cen mRNA serves as a valuable model to study this paradigm, as mislocalizing Cen mRNA leads to centrosome defects (Ryder et al., 2020). Further, the Cen 3’-UTR is important for targeting the antisense ik2 mRNA, which codes for an actin regulatory factor (Oshima et al., 2006; Bergalet et al., 2020). Here, we examined what directs Cen mRNA to centrosomes.
We found the accumulation of Cen mRNA at centrosomes is puromycin-sensitive, highlighting the relevance of the nascent peptide for RNA localization. We then mapped domains within the Cen protein structure that enable RNA localization. Unexpectedly, we found Cen mRNA and protein localization can be separated. While the Cen N’-terminus is necessary but not sufficient for RNA localization, it is sufficient for Cen protein accumulation at centrosomes. These results argue for the presence of multiple domains that function cooperatively to target Cen mRNA to centrosomes.
We further defined the first 100 AA as important for Cen mRNA and protein localization. Within this region, we uncovered a conserved CC1 box that contributes to RNA localization. Nevertheless, it is feasible that neighboring sequences also contribute to dynein or dynactin association, as shown for BicDR1 (Chaaban and Carter, 2022). CC1 boxes are found within dynein activating cargo adaptors, which directly bind DLIC and tether cargoes to the dynein motor complex (Gama et al., 2017; Lee et al., 2018; Lee et al., 2020; Chaaban and Carter, 2022). Dynein cargo adaptors also recruit the multi-subunit dynactin complex to the homodimeric Dhc, which enables dynein to translocate along the microtubule over long distances in association with cargo (Reck-Peterson et al., 2018). Known CC1 box-containing dynein activating cargo adaptors include the BicD, BicDR, and Spindly proteins. Our study positions Cen within this protein family. To support this, we show Cen biochemically associates with DLIC and that mutation of the CC1 box or various components of the dynein complex compromises Cen mRNA localization. The conservation of the CC1 box within CDR2 and CDR2L further suggests these mammalian proteins similarly function as dynein adaptors.
We therefore propose a model wherein the dynein complex directly binds the Cen CC1 box and, potentially, neighboring protein sequences as they emerge from the ribosome, activating the dynein motor complex, and directing Cen protein and mRNA to the centrosome (Figure 10E). While it is presently unknown if Egl directly binds Cen mRNA, it is tempting to speculate that such an interaction within the 3’-portion of Cen mRNA may be why this region of the Cen CDS is necessary for its localization. Thus, our model proposes that Cen mRNA localization requires both protein recognition via DLIC and RNA recognition by Egl. Such interactions are predicted to add valency and, therefore, robustness to the Cen transport complex. Of course, future work is needed to fully validate such a model.
Intriguingly, a recent study identified the RNAs for most Drosophila orthologs of the dynein cargo adaptors are enriched at the apical domain of follicle cell epithelia, where microtubules are nucleated. Further, the localization of those mRNAs was also translation-dependent (Cassella and Ephrussi, 2022). Similar observations were also noted for the centrosomal localization of NIN and BICD2 mRNAs in mammalian cells (Safieddine et al., 2021) and parallel our findings of Cen mRNA in the syncytial embryo. Taken together, these findings strongly suggest that the translation- and dynein-dependent localization of dynein cargo adaptor RNAs to microtubule-organizing centers is a conserved feature.
For many RNAs, the assembly into higher order granules generally represents a translationally repressed state (Das et al., 2021). Paradoxically, the size of the Cen RNP seems to scale with Cen protein levels, as loss of the translational repressor FMRP (or Cen over-expression) enlarges the granule (Ryder et al., 2020). Moreover, we found that treatment with the translational inhibitor puromycin led to the rapid dissolution of the Cen RNP and depletion of its RNA at centrosomes. Thus, active translation not only directs Cen mRNA localization – it is required to maintain it. These findings are consistent with a requirement for continuous active transport or anchoring of Cen mRNA to the centrosome. Live imaging is required to distinguish these models and to directly visualize Cen translation en route to the centrosome, or within the granule itself.
Materials and methods
Fly stocks
The following Drosophila strains and transgenic lines were used: y1w1118 (Bloomington Drosophila Stock Center, BDSC #1495) was the WT control; PBAC-GFP-Cnn, expressing Cnn tagged at the N-terminus with EGFP under endogenous regulatory elements (Lerit et al., 2015); Ubi-GFP-γ-Tub23C, expressing GFP-γ-Tub under the Ubiquitin promotor (Lerit and Rusan, 2013); Dynein heavy chain (Dhc64C gene) DhcLOA is a hypomorphic allele that codes for F597Y mutant Dhc (modeled after the murine Dync1h1 F580Y mutation (Salvador-Garcia et al., 2023); Ubi-GFP-Dlic (Pandey et al., 2007); Cenf04787 is defined by a PiggyBac insertion in the Cen coding sequence and is a null mutation (BDSC #18805) (Bellen et al., 2004; Kao and Megraw, 2009). The maternal α-Tub promoter was used to drive GAL4 expression (matGAL4; BDSC #7063) of pUASp-Egl WT-FLAG- Egl shRNA and pUASp-Egl RBD3-FLAG-Egl shRNA (Goldman et al., 2021), which were generous gifts from G. Gonsalvez (Augusta University), KhcRNAi (BDSC #35409; TRiP GL00330). Reduction of Khc by this TRiP line was previously demonstrated by western blot (Veeranan-Karmegam et al., 2016). UASp-Cen∆C, UASp-Cen∆N, UASp-CenFL-HA, and UASp-Cen-ATG-HA were generated for this study (see below), expressed in the Cen null background, and driven with a single copy of matGAL4. To examine maternal effects, syncytial stage embryos were derived from mutant and/or transgenic mothers.
Flies were raised on cornmeal-based Drosophila medium (Bloomington formulation; Lab-Express, Inc.), and crosses were maintained at 25°C in a light and temperature-controlled chamber.
Construction of transgenic and mutant animals
Cen CRISPR mutants
Strains with deletions within the region of the genome encoding the CC1 box of Cen were generated with the CRISPR/Cas9 reagents generated by the CRISPR Fly Design project (Port et al., 2014). A pCFD3 plasmid expressing a gRNA targeting the Cen sequence 5’-TACAATTGGCAGCAGAGCT-3’ (pCFD3-Cen) was generated by annealing overlapping oligos and ligating them with a Bbs1-cut backbone, as described previously (Port et al., 2014). The CenΔ5 allele was generated in an experiment in which a 100 ng/μl solution of pCFD3-cen was injected into nos-cas9 embryos (CFD2 strain; (Port et al., 2014)). The CenΔ12 allele was generated in an experiment in which embryos were generated by crossing nos-cas9 CFD2 females with males that had a stable integration of pCFD3-Cen at the attP40 docking site. In both these experiments, embryos were injected with a 150 ng/μl of a donor oligonucleotide (Ultramer; IDT) that contained codon changes that would, after precise homology-directed repair (HDR) of the Cas9 cleavage site, change A25 and A26 residues within the CC1 box to V residues. Previous work has shown that the equivalent mutation strongly reduces binding of cargo adaptors to dynein and dynactin (Schlager et al., 2014). The target site was amplified by PCR from genomic DNA extracted from the offspring of flies that developed from the embryos and analyzed by Sanger sequencing (Port and Bullock, 2016). Whilst the mutations carried by the donor oligonucleotide were not recovered, indicating that HDR was not successful, the Δ12 allele was found.
Cen truncation lines
To generate the Cen truncations, the Cen coding sequence was divided into two pieces after the 289th amino acid and PCR amplified using Phusion High-Fidelity DNA Polymerase from the cDNA clone pOT-LD41224 (Drosophila Genomics Resource Center (DGRC)). This site was chosen because it does not disrupt predicted secondary structure motifs. The following primers were used to amplify the respective pieces:
Cen N-terminal piece:
Forward: 5’- GGAAGTGGTGGTAGTGGAGGAAGTGAGGAATCCAATCACGGTTCGG-3’
Reverse 3’- TCGGCGCGCCCACCCTTTTAATCCCTCAGGCAGCGACT-5’
Cen C-terminal piece:
Forward: 5’- GAAGTGGTGGTAGTGGAGGAAGTATTAACGAAAGCAACACCAATATGGA-3’
Reverse: 5’- GGCGCGCCCACCCTTTTACTTTTGACGAAACTGATGATGATGAC-3’
Each Cen truncation was ligated into the pENTR-D Gateway vector (Invitrogen) using Gibson Assembly. The following primers and Phusion PCR were used to linearize the pENTR-D vector and add overlapping ends for ligation:
Vector for Cen N-terminal piece:
Forward: 5’- GTAGTCGCTGCCTGAGGGATTAAAAGGGTGGGCGCGC-3’
Reverse: 5’- CGCATAGTCAGGAACATCGTATGGGTACATGGTGAAGGGGGCGGC-3’
Vector for Cen C-terminal piece:
Forward: 5’- CAAGAGTCATCATCATCAGTTTCGTCAAAAGTAAAAGGGTGGGCGCGC-3’
Reverse: 5’- CGCATAGTCAGGAACATCGTATGGGTACATGGTGAAGGGGGCGGC-3’
A 3x HA tag plus linker was also incorporated by Gibson Assembly as a premade oligo with the following sequence. The 3x HA tag is underlined:
N-terminal 3x HA tag plus linker:
5’- ATGTACCCATACGATGTTCCTGACTATGCGGGCTATCCCTATGACGTCCCGGACTATGCAGGATCCTATCCATATGACGTTCCAGATTACGCTGGCGGCAGCGGTGGAAGTGGTGGTAGTGGAGGAAGT-3’
Cen FL and -ATG HA-tagged lines
To generate the full-length Cen HA-tagged construct, the full Cen CDS (including the ATG codon) was amplified using Phusion from pOT-LD41224 (DGRC) using the following primers: Full length Cen plus ATG:
Forward: 5’- GGCCGCCCCCTTCACCATGGAGGAATCCAATCACGGTTC-3’
Reverse: 5’- TCCACCGCTGCCGCCCTTTTGACGAAACTGATGATGATGAC-3’
The pENTR-D vector was linearized by Phusion PCR using the following primers:
Forward: 5’- CCTATCCATATGACGTTCCAGATTACGCTTAAAAGGGTGGGCGCGCC-3’
Reverse: 5’- CGAACCGTGATTGGATTCCTCCATGGTGAAGGGGGCGGC-3’
The full-length Cen was inserted into the pENTR-D vector with a C-terminal 3x HA tag plus linker by Gibson assembly. The C-terminal 3x HA tag plus linker was added as a premade oligo with the following sequence. The 3x HA tag is underlined:
C-terminal 3x HA tag plus linker:
5’- GGCGGCAGCGGTGGAAGTGGTGGTAGTGGAGGAAGTTACCCATACGATGTTCCTGACTATGCGGGCTATCCCTATGACGTCCCGGACTATGCAGGATCCTATCCATATGACGTTCCAGATTACGCTTAA-3’
To generate the Cen -ATG HA-tagged construct, the Cen coding sequence was amplified from pOT-LD41224 using the following primers to remove the initiating ATG codon then ligated by Gibson Assembly, as described for the full-length construct:
Forward: 5’- CCGCGGCCGCCCCCTTCACCGAGGAATCCAATCACGGTTC-3’
Reverse: 5’- CCGTGATTGGATTCCTCGGTGAAGGGGGCGGC-3’
For all lines, sequence-verified single colony clones were shuttled into the destination vector pPWattB (UASp-Gateway with attB sites for Phi31C transformation) using the Gateway cloning system (Invitrogen). Constructs were inserted at the attP2 (Chromosome III) locus and transgenic animals were generated by BestGene, Inc.
Sequence alignment
Protein sequences were obtained from UniProt (UniProt, 2023), aligned using the Clustal Omega multiple sequence alignment tool (Madeira et al., 2022), then displayed using ESPript 3.0 (Robert and Gouet, 2014) using the percent equivalent similarity and the black-and-white color schemes.
Immunofluorescence
Embryos (0.5–2.5 hr) were collected then dechorionated, fixed in 4% paraformaldehyde, and blocked in BBT (PBS supplemented with 0.1% Tween-20 and 0.1% BSA) as described in (Lerit et al., 2015). Primary antibodies were diluted in BBT in incubated done overnight at 4 °C with nutation. On the following day, samples were washed three times with BBT then blocked again with BBT supplemented with 2% normal goat serum (NGS) prior to incubation with secondary antibodies and DAPI for 2 hours at room temperature. Samples were mounted in AquaPoly/Mount (VWR, 87001–902).
To visualize microtubules, embryos were fixed in a solution of 1:1 of heptane:37% formaldehyde for 3 min with intermittent mixing, then manually devitellinized using 30G PrecisionGlide needles (BD) (Theurkauf, 1994). Embryos were then blocked in BBT, rinsed in PBS, blocked again with Image-iT FX signal enhancer (ThermoScientific), then incubated with antibodies overnight at 4 °C with primary antibodies diluted in BBT. On the following day, samples were processed and mounted as described above.
The following antibodies were used: rabbit anti-Cen (UT393, 1:500; gift from T. Megraw, Florida State University) (Kao and Megraw, 2009) recognizes the C-terminus of Cen, rabbit anti-Cen (1:500; gift from T. Megraw) (Kao and Megraw, 2009) recognizes the N-terminus of Cen, mouse anti-α-Tub DM1a (1:500; Sigma, T6199), and rabbit anti-Cnn (1:4000; gift from T. Megraw). Secondary antibodies and stains: Alexa Fluor 488, 568, or 647 (1:500, Invitrogen). DAPI was used at 10 ng/mL (Thermo Fisher).
Single molecule fluorescence in situ hybridization (smFISH)
smFISH experiments were conducted as previously described in (Ryder et al., 2020). All steps were done using RNase-free solutions. In short, 0–2-hour embryos were aged 30 minutes then fixed in 4% paraformaldehyde and rehydrated stepwise into 0.1% PBST. Rehydrated embryos were then washed with wash buffer (WB; 10% formamide and 2× SSC supplemented fresh each experiment with 0.1% Tween-20 and 2 μg/mL nuclease-free BSA) at room temperature and incubated in a freshly made hybridization buffer (HB; 100 mg/mL dextran sulfate and 10% formamide in 2× SSC supplemented fresh each experiment with 0.1% Tween-20, 2 μg/mL nuclease-free BSA and 10 mM ribonucleoside vanadyl complex (RVC; S1402S; New England Biolabs)) in a 37 °C water bath. Embryos were incubated overnight in a 37 °C water bath in HB with a final concentration of 0.4 μM Stellaris smFISH probes (Cen or GAPDH) conjugated to Quasar 570 dye (LGC Biosearch Technologies). The following day, the hybridized embryos were washed with WB three times, then with 0.1% PBST, and stained with DAPI (1:1000). Vectashield mounting medium (Vector Laboratories, H-1000) was used to mount the slides. Complete probe sequences are reported in Ryder et al., 2020.
Dual smFISH and immunofluorescence
Dual smFISH and IF experiments were as previously described (Ryder et al., 2020; Fang and Lerit, 2022). All steps were done using RNase-free solutions. Embryos were rehydrated and washed first in 0.1% PBST (PBS plus 0.1% Tween-20) and then in WB, as above. Embryos were then incubated with 100 μL of HB for 10–20 minutes in a 37 °C water bath, followed by an overnight incubation in 25 μL of HB containing 0.5 μM smFISH probes and primary antibody in a 37 °C water bath. On the next day, embryos were washed four times for 30 minutes in prewarmed WB, stained with secondary antibody and DAPI (1:1000) for 2 hours at room temperature, washed with 0.1% PBST, and mounted with Vectashield mounting medium (H-1000; Vector Laboratories). Slides were stored at 4 °C and imaged within 1 week.
Pharmacological inhibition of translation
0.5–2.5 hr embryos were collected and incubated in a 1:1 solution (450 μL: 450 μL) of heptane: Robb’s medium (1 mM calcium chloride, 10 mM glucose, 100 mM HEPES (pH 7.2), 1.2 mM MgCl2, 55 mM KOAc, 40 mM NaOAc, and 100 mM sucrose) containing the appropriate drug or an equivalent volume of DMSO. The concentrations and incubation times for each drug were: 3 mM puromycin (Sigma-Aldrich P8833) for 10 min; 0.1 mM anisomycin (Sigma-Aldrich A9789) for 15 min; and 0.71 mM cycloheximide (VWR, 97064–724) for 15 min. After drug incubation, Robb’s medium was removed, and 450 μl of 4% paraformaldehyde in PBS was added, and embryos were fixed for 20 min, then devitellinized. Samples were processed for smFISH or dual smFISH + IF, as above.
Microtubule regrowth assay
For cold-shock, embryos were transferred to a 1.5 mL tube and incubated on ice for 5 minutes to disrupt the microtubules, then immediately fixed. For microtubule regrowth (recovery), cold-shocked embryos were incubated in room-temperature PBS for 5 minutes, then immediately fixed. Control, cold-shocked, and recovery embryos were then processed for sequential smFISH and immunofluorescence, as follows. Embryos were fixed in 37% formaldehyde and manually devitellinized, as described above, rinsed in 0.1% PBST, incubated in Image-IT FX for 30 minutes, washed again in 0.1% PBST, and then washed in WB buffer for 10 minutes. Embryos were incubated in HB buffer for 10–20 minutes in a 37 °C water bath prior to an overnight incubation in 25 μL of HB containing 0.5 μM smFISH probes. On the next day, embryos were washed in WB buffer, 2 X SSC with 0.1% Tween-20, and then 0.1% PBST sequentially. Next, embryos were blocked in 0.1% BBT buffer (PBS supplemented with 0.1% BSA and 0.1% Tween-20). Embryos were then incubated overnight at 4 °C with primary antibody in 0.1% BBT, further blocked in 0.1% BBT supplemented with 2% NGS, and incubated for 2 hours at room temperature with secondary antibodies and DAPI. Embryos were mounted in Vectashield mounting medium prior to imaging. Slides were stored at 4 °C and imaged within 1 week.
Microscopy
Images were acquired on a Nikon Ti-E system fitted with a Yokogawa CSU-X1 spinning disk head (Yokogawa Corporation of America), Orca Flash 4.0 v2 digital complementary metal-oxide-semiconductor camera (Hamamatsu Corporation), Perfect Focus system (Nikon), and a Nikon LU-N4 solid state laser launch (15 mW 405, 488, 561, and 647 nm) using the following objectives: 100× 1.49 NA Apo TIRF oil immersion or 40× 1.3 NA Plan Fluor oil immersion. Images were acquired at ambient temperature (∼25°C) using either Vectashield or Aqua-Poly/Mount imaging medium, as described, using Nikon Elements AR software.
Image Analysis
Images for figures were assembled using Fiji (NIH; (Schindelin et al., 2012)) and Adobe Illustrator. The software was used to separate or merge channels, crop regions of interest, generate maximum intensity projections, and adjust brightness and contrast.
RNA detection and measurements
Raw, single channel .tif files of centrosomes and RNA were segmented in three dimensions using a code adapted from the Allen Institute for Cell Science Cell Segmenter then run through the open-source, Python-based SubcellularDistribution pipeline (Ryder and Lerit, 2020) to calculate the percentage of RNA overlapping with centrosomes, percent of RNA in granules, and granular intensities. Granules at a distance 0μm or 0.5μm with a normalized intensity greater than 4 were log transformed and plotted using R. Unless otherwise noted, all RNA measurements were calculated based on the percentage of Cen mRNA residing within 0 μm (i.e., overlapping) from the Cnn surface.
We examined Cen mRNA distributions in EglWT versus EglRBD3 embryos by smFISH relative to the PCM marker γ-Tub-GFP rather than GFP-Cnn because the GFP-Cnn and Egl transgenes both reside on Chromosome III. Because γ-Tub occupies a significantly smaller radius of the PCM than Cnn (about 600 vs 1400 nm, by structured illumination microscopy; (Lerit et al., 2015)), for these experiments, we took a conservative measurement of the percentage of Cen mRNA residing within 0.5 μm from the γ-Tub surface.
Because cold-shock compresses the volume of Cnn, for the microtubule regrowth experiments, the percentage of Cen mRNA residing within 1 μm from the surface of the core centriolar protein, Asl, was measured from all samples (control, cold-shock, and recovery).
Spindle morphology defects
To quantify spindle morphology, mitotic embryos imaged at 40x were examined for the following morphologies: bent spindles, multipolar or fused spindles, acentrosomal spindle poles, and defective centrosome separation. If any spindles within an embryo contained one of these phenotypes, the embryo was considered positive for a spindle morphology defect. Three independent biological replicates were performed for each genotype.
Colocalization analysis
Single optical slices were analyzed for co-occurrence of Cen RNA with microtubules. Mander’s M1 coefficient was calculated from a 66.56 μm2 area using the JacoP plugin for ImageJ in which a manual threshold was applied to remove background signal (Bolte and Cordelieres, 2006).
Immunoprecipitation
To examine the interaction between DLIC and Cen, the immunoprecipitation was performed as in (Dix et al., 2013) using 0–5 hour embryos lysed in a buffer containing 25 mM HEPES pH 7.0, 50 mm KCl, 1 mM MgCl2, 2 mM DTT and 250 mM sucrose, supplemented with 1x Complete protease inhibitors (Roche). Transgenic strains expressing GFP-Dlic (Pandey et al., 2007) were used for immunoprecipitation using agarose GFP-Trap beads (Chromotek).
To determine the amino acid residues corresponding to the truncated Cen-ATG protein product, we immunoprecipitated Cen from ovaries harvested from well-fed 1–2-day old Cen null females expressing CenFL or Cen-ATG transgenes lysed in RCB buffer containing 50 mM HEPES, pH 7.4, 150 mM NaCl, 2.5 mM MgCl2, 0.01% Triton x-100, and 250 mM sucrose supplemented with 1x Complete protease inhibitors, 1 mM DTT, and 1 μg/mL Pepstatin A. Protein concentration was normalized across samples using a Pierce BCA assay (Thermo Scientific, cat. 23225). We used ~50-pairs of ovaries, yielding about 10 mg of protein per reaction. For each reaction, 50 μL of Pierce Protein A/G magnetic beads (Thermo Scientific, cat. 88802) were prewashed in RCB, and half of the bead slurry was used to preclear the lysate for 30 min to minimize nonspecific binding. The precleared lysate was reserved and incubated with 10 μL of a 1:50 dilution of rabbit anti-HA antibody (C29F4, Cell Signaling Technology) for 2-hr at RT. The remaining 25 μL of prewashed beads was then added and incubated for 1-hr at RT. Beads were washed well in RCB, then processed for immunoblotting (10 μg protein per lane) or shipped to MS Bioworks for mass spectrometry (see below).
Mass Spectrometry
Mass spectrometry and analysis was performed by MS Bioworks, LLC (Ann Arbor, MI). 3 × 20 μL per immunoprecipitated Cen-HA sample were separated on a 4–12% Bis-Tris NuPAGE Novex mini-gel (Invitrogen) using the MOPS buffer system. The gel was stained with Coomassie, and target bands excised. Gel segments were digested with three enzymes using a robot (DigestPro, CEM) with the following protocol. First, they were washed with 25 mM ammonium bicarbonate followed by acetonitrile. Next, samples were reduced with 10 mM DTT at 60°C followed by alkylation with 50mM iodoacetamide at RT. Next, samples were digested with trypsin/ chymotrypsin/ elastase (Promega) at 37°C for 4h. The reaction was quenched with formic acid and the supernatant was analyzed directly without further processing. The gel digests were analyzed by nano LC/MS/MS with a Waters M-class HPLC system interfaced to a ThermoFisher Fusion Lumos. Peptides were loaded on a trapping column and eluted over a 75 μm analytical column at 350nL/min; both columns were packed with XSelect CSH C18 resin (Waters). A 30 min gradient was employed. The mass spectrometer was operated in data-dependent mode, with MS and MS/MS performed in the Orbitrap at 60,000 FWHM resolution and 15,000 FWHM resolution, respectively. APD was turned on. The instrument was run with a 3s cycle for MS and MS/MS. From the FL immunoprecipitation, 152 proteins were identified, and 4703 spectra were matched. Cen was the second-most abundant protein. From the -ATG immunoprecipitation, 302 proteins were identified, and 9706 spectra were matched. Cen was the fourth-most abundant protein.
Immunoblotting
Western blotting was performed as in (Dix et al., 2013). Alternatively, appropriately aged embryos were lysed in 0.1% PBST using an electric homogenizer on ice then immediately boiled in 5x SDS loading dye for 5 minutes and returned to ice. Samples were resolved by premade gradient SDS-PAGE gel (Bio-Rad) and transferred to nitrocellulose membrane by wet or semi-dry transfer. Membranes were blocked in a 5% dry milk solution diluted in TBST (Tris-based saline with 0.05% Tween-20) and incubated overnight at 4°C with primary antibodies in 1% dry milk in TBST solution. Primary antibodies used: rabbit anti-C terminus of Cen (UT393) or rabbit anti-N terminus of Cen (both 1:5000; gift from T. Megraw, Florida State University, (Kao and Megraw, 2009)), guinea pig anti-Asterless (1:5000; gift from G. Rogers, University of Arizona), mouse anti-actin JLA20-S (1:1000; DSHB), mouse anti-β-Tub E7 (1:15,000; DSHB), rabbit anti-HA (1: 5000; C29F4, Cell Signaling Technology), mouse anti-BicD 1B11 (1:1000; (Suter and Steward, 1991)), and mouse anti-GFP clones 7.1 and 13.1 (1:1000; Roche).
The following day, membranes were washed with TBST and incubated with secondary antibodies for 1 hour at room temperature. Secondary antibodies were diluted 1:2500 in TBST and included goat anti-mouse HRP (31430; Thermo Fisher Scientific), goat anti-rabbit HRP (31460, ThermoFisher Scientific), and goat anti-guinea pig HRP (A18769, ThermoFisher Scientific). Bands were visualized with Clarity ECL substrate (1705061; Bio-Rad) on a Bio-Rad ChemiDoc imaging system.
qPCR
Embryos aged 0.5–2.5 hours were dechorionated with bleach, flash frozen in liquid nitrogen, and stored at −80 °C. A volume of 100 μL of embryos per biological replicate was homogenized in TRIzol (Invitrogen) and RNA was extracted using phenol: chloroform extraction. The extracted RNA was then treated with Ambion TURBO DNase (Thermo Fisher Scientific, AM2238). cDNA was then synthesized using an iScript cDNA synthesis kit (Bio-Rad, 170–8891).
Three technical replicates per biological replicate were run concurrently in a 96-well plate (Bio-Rad, HSP9601) using iQ SYBR Green Supermix (Bio-Rad, 170–8882). Data was collected on a Bio-Rad CFX96 Real-time machine. Levels of Cen expression were normalized to Ribosomal protein L32 (RP49). The following primers were used:
RP49 (amplicon 75 base pairs)
Forward: 5’- CATACAGGCCCAAGATCGTG-3’
Reverse: 5’- ACAGCTTAGCATATCGATCCG-3’
Centrocortin (amplicon 78 base pairs)
Forward: 5’- AAAGTACCCCCGGTAACACC-3’
Reverse: 5’-TGAGGATACGACGCTCTGTG-3’
Statistical Analysis
All statistical analyses were conducted using GraphPad Prism software, except granule area was calculated using R-software. Data were first tested for any outliers using a ROUT test with a Q= 1% and normality using the D’Agostino and Pearson normality test. This was followed by Student’s two-tailed t test, ANOVA, Fisher’s exact test, or the appropriate nonparametric tests. Data were plotted with mean ± SD displayed.
Supplementary Material
Acknowledgments
We thank Drs. Nasser Rusan, Greg Rogers, Timothy Megraw, Jordan Raff, and Graydon Gonsalvez for gifts of reagents and Drs. Gary Bassell and Sulagna Das for critical reading of this manuscript. We acknowledge Dr. Richard Jones from MS Bioworks, LLC for technical expertise and assistance with mass spectrometry and analysis. We are also grateful to Dr. Pearl Ryder for early contributions to this work. Stocks obtained from the Bloomington Drosophila Stock Center (NIH grant P40OD018537); antibodies from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at the University of Iowa Department of Biology; and reagents from the Drosophila Genomics Resource Center (DGRC), supported by NIH grant 2P40OD010949, were used in this study.
This work was supported by NIH grants 5K12GM000680 (HZ-S), 1F31NS134380 (JB), 5K99GM143517 (JF), and R01GM138544 (DAL); the UK Medical Research Council (as part of United Kingdom Research and Innovation (file reference number MC_U105178790) (SLB); and the NIH administrative supplements 3R01GM138544-01S1 (for JL) and 3R01GM138544-03S1 (for CAH). DAL is also supported by a Research Scholar Grant (RSG-22-874157-01-CCB) from the American Cancer Society.
Acknowledgments
- αTub
α-Tubulin
- Aniso
Anisomycin
- Asl
Asterless
- BicD
Bicaudal
- CDR2
Cerebellar degeneration-related protein 2
- CDR2L
Cerebellar degeneration-related protein 2-like
- Cen
Centrocortin
- CHX
Cycloheximide
- Cnn
Centrosomin
- Dhc
dynein heavy chain
- DLIC
dynein light intermediate chain
- Egl
Egalitarian
- γTub
γ-Tubulin
- Khc
kinesin heavy chain
- NC
nuclear cycle
- PCM
pericentriolar material
- Puro
puromycin
- RBD
RNA-binding domain
Footnotes
The authors declare no competing financial interests.
Data Availability Statement
All data are available in the published article and its online supplemental material. Source files of uncropped versions of the immunoblots presented in the figures are included.
References
- Apweiler R., Attwood T.K., Bairoch A., Bateman A., Birney E., Biswas M., Bucher P., Cerutti L., Corpet F., Croning M.D., Durbin R., Falquet L., Fleischmann W., Gouzy J., Hermjakob H., Hulo N., Jonassen I., Kahn D., Kanapin A., Karavidopoulou Y., Lopez R., Marx B., Mulder N.J., Oinn T.M., Pagni M., Servant F., Sigrist C.J., Zdobnov E.M., and InterPro C.. 2000. InterPro--an integrated documentation resource for protein families, domains and functional sites. Bioinformatics. 16:1145–1150. [DOI] [PubMed] [Google Scholar]
- Bellen H.J., Levis R.W., Liao G., He Y., Carlson J.W., Tsang G., Evans-Holm M., Hiesinger P.R., Schulze K.L., Rubin G.M., Hoskins R.A., and Spradling A.C.. 2004. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 167:761–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergalet J., Patel D., Legendre F., Lapointe C., Benoit Bouvrette L.P., Chin A., Blanchette M., Kwon E., and Lecuyer E.. 2020. Inter-dependent Centrosomal Co-localization of the cen and ik2 cis-Natural Antisense mRNAs in Drosophila. Cell Rep. 30:3339–3352 e3336. [DOI] [PubMed] [Google Scholar]
- Besse F., and Ephrussi A.. 2008. Translational control of localized mRNAs: restricting protein synthesis in space and time. Nat Rev Mol Cell Biol. 9:971–980. [DOI] [PubMed] [Google Scholar]
- Bolte S., and Cordelieres F.P.. 2006. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 224:213–232. [DOI] [PubMed] [Google Scholar]
- Bullock S.L. 2007. Translocation of mRNAs by molecular motors: think complex? Semin Cell Dev Biol. 18:194–201. [DOI] [PubMed] [Google Scholar]
- Cassella L., and Ephrussi A.. 2022. Subcellular spatial transcriptomics identifies three mechanistically different classes of localizing RNAs. Nat Commun. 13:6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaaban S., and Carter A.P.. 2022. Structure of dynein-dynactin on microtubules shows tandem adaptor binding. Nature. 610:212–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A., and Lecuyer E.. 2017. RNA localization: Making its way to the center stage. Biochim Biophys Acta Gen Subj. 1861:2956–2970. [DOI] [PubMed] [Google Scholar]
- Chouaib R., Safieddine A., Pichon X., Imbert A., Kwon O.S., Samacoits A., Traboulsi A.M., Robert M.C., Tsanov N., Coleno E., Poser I., Zimmer C., Hyman A., Le Hir H., Zibara K., Peter M., Mueller F., Walter T., and Bertrand E.. 2020. A Dual Protein-mRNA Localization Screen Reveals Compartmentalized Translation and Widespread Co-translational RNA Targeting. Dev Cell. 54:773–791 e775. [DOI] [PubMed] [Google Scholar]
- Das S., Vera M., Gandin V., Singer R.H., and Tutucci E.. 2021. Intracellular mRNA transport and localized translation. Nat Rev Mol Cell Biol. 22:483–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienstbier M., Boehl F., Li X., and Bullock S.L.. 2009. Egalitarian is a selective RNA-binding protein linking mRNA localization signals to the dynein motor. Genes Dev. 23:1546–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix C.I., Soundararajan H.C., Dzhindzhev N.S., Begum F., Suter B., Ohkura H., Stephens E., and Bullock S.L.. 2013. Lissencephaly-1 promotes the recruitment of dynein and dynactin to transported mRNAs. J Cell Biol. 202:479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J., and Lerit D.A.. 2022. Orb-dependent polyadenylation contributes to PLP expression and centrosome scaffold assembly. Development. 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe V.E., and Alberts B.M.. 1983. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci. 61:31–70. [DOI] [PubMed] [Google Scholar]
- Gama J.B., Pereira C., Simoes P.A., Celestino R., Reis R.M., Barbosa D.J., Pires H.R., Carvalho C., Amorim J., Carvalho A.X., Cheerambathur D.K., and Gassmann R.. 2017. Molecular mechanism of dynein recruitment to kinetochores by the Rod-Zw10-Zwilch complex and Spindly. J Cell Biol. 216:943–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparski A.N., Mason D.E., Moissoglu K., and Mili S.. 2022. Regulation and outcomes of localized RNA translation. Wiley Interdiscip Rev RNA. 13:e1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepner J., Li M., Ludmann S., Kortas C., Boylan K., Iyadurai S.J., McGrail M., and Hays T.S.. 1996. Cytoplasmic dynein function is essential in Drosophila melanogaster. Genetics. 142:865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman C.H., Neiswender H., Baker F., Veeranan-Karmegam R., Misra S., and Gonsalvez G.B.. 2021. Optimal RNA binding by Egalitarian, a Dynein cargo adaptor, is critical for maintaining oocyte fate in Drosophila. RNA Biol. 18:2376–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman C.H., Neiswender H., Veeranan-Karmegam R., and Gonsalvez G.B.. 2019. The Egalitarian binding partners Dynein light chain and Bicaudal-D act sequentially to link mRNA to the Dynein motor. Development. 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R.R., and Borisy G.G.. 1977. The pericentriolar material in Chinese hamster ovary cells nucleates microtubule formation. J Cell Biol. 73:601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman I., Huang Y.S., Mendez R., Cao Q., Theurkauf W., and Richter J.D.. 2000. CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell. 103:435–447. [DOI] [PubMed] [Google Scholar]
- Grollman A.P. 1967. Inhibitors of protein biosynthesis. II. Mode of action of anisomycin. J Biol Chem. 242:3226–3233. [PubMed] [Google Scholar]
- Hafezparast M., Klocke R., Ruhrberg C., Marquardt A., Ahmad-Annuar A., Bowen S., Lalli G., Witherden A.S., Hummerich H., Nicholson S., Morgan P.J., Oozageer R., Priestley J.V., Averill S., King V.R., Ball S., Peters J., Toda T., Yamamoto A., Hiraoka Y., Augustin M., Korthaus D., Wattler S., Wabnitz P., Dickneite C., Lampel S., Boehme F., Peraus G., Popp A., Rudelius M., Schlegel J., Fuchs H., Hrabe de Angelis M., Schiavo G., Shima D.T., Russ A.P., Stumm G., Martin J.E., and Fisher E.M.. 2003. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 300:808–812. [DOI] [PubMed] [Google Scholar]
- Holt C.E., and Bullock S.L.. 2009. Subcellular mRNA localization in animal cells and why it matters. Science. 326:1212–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H., Gkogkas C.G., Sonenberg N., and Holt C.E.. 2014. Remote control of gene function by local translation. Cell. 157:26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao L.R., and Megraw T.L.. 2009. Centrocortin cooperates with centrosomin to organize Drosophila embryonic cleavage furrows. Curr Biol. 19:937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A., and Rieder C.L.. 1999. The sudden recruitment of gamma-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J Cell Biol. 146:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislauskis E.H., Li Z., Singer R.H., and Taneja K.L.. 1993. Isoform-specific 3’-untranslated sequences sort alpha-cardiac and beta-cytoplasmic actin messenger RNAs to different cytoplasmic compartments. J Cell Biol. 123:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuyer E., Yoshida H., Parthasarathy N., Alm C., Babak T., Cerovina T., Hughes T.R., Tomancak P., and Krause H.M.. 2007. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell. 131:174–187. [DOI] [PubMed] [Google Scholar]
- Lee I.G., Cason S.E., Alqassim S.S., Holzbaur E.L.F., and Dominguez R.. 2020. A tunable LIC1-adaptor interaction modulates dynein activity in a cargo-specific manner. Nat Commun. 11:5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.G., Olenick M.A., Boczkowska M., Franzini-Armstrong C., Holzbaur E.L.F., and Dominguez R.. 2018. A conserved interaction of the dynein light intermediate chain with dynein-dynactin effectors necessary for processivity. Nat Commun. 9:986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerit D.A. 2022. Signed, sealed, and delivered: RNA localization and translation at centrosomes. Mol Biol Cell. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerit D.A., Jordan H.A., Poulton J.S., Fagerstrom C.J., Galletta B.J., Peifer M., and Rusan N.M.. 2015. Interphase centrosome organization by the PLP-Cnn scaffold is required for centrosome function. J Cell Biol. 210:79–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerit D.A., and Rusan N.M.. 2013. PLP inhibits the activity of interphase centrosomes to ensure their proper segregation in stem cells. J Cell Biol. 202:1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald P.M., and Struhl G.. 1988. cis-acting sequences responsible for anterior localization of bicoid mRNA in Drosophila embryos. Nature. 336:595–598. [DOI] [PubMed] [Google Scholar]
- Mach J.M., and Lehmann R.. 1997. An Egalitarian-BicaudalD complex is essential for oocyte specification and axis determination in Drosophila. Genes Dev. 11:423–435. [DOI] [PubMed] [Google Scholar]
- Madeira F., Pearce M., Tivey A.R.N., Basutkar P., Lee J., Edbali O., Madhusoodanan N., Kolesnikov A., and Lopez R.. 2022. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 50:W276–W279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W.F., and Rosenbaum J.L.. 2000. Are there nucleic acids in the centrosome? Curr Top Dev Biol. 49:187–205. [DOI] [PubMed] [Google Scholar]
- Martin K.C., and Ephrussi A.. 2009. mRNA localization: gene expression in the spatial dimension. Cell. 136:719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock M.A., Dix C.I., Johnson C.M., McLaughlin S.H., Maizels R.J., Hoang H.T., and Bullock S.L.. 2018. RNA-directed activation of cytoplasmic dynein-1 in reconstituted transport RNPs. Elife. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraw T.L., Kilaru S., Turner F.R., and Kaufman T.C.. 2002. The centrosome is a dynamic structure that ejects PCM flares. J Cell Sci. 115:4707–4718. [DOI] [PubMed] [Google Scholar]
- Mitchison T., and Kirschner M.. 1984. Microtubule assembly nucleated by isolated centrosomes. Nature. 312:232–237. [DOI] [PubMed] [Google Scholar]
- Mofatteh M., and Bullock S.L.. 2017. SnapShot: Subcellular mRNA Localization. Cell. 169:178–178 e171. [DOI] [PubMed] [Google Scholar]
- Nathans D. 1964. Puromycin Inhibition of Protein Synthesis: Incorporation of Puromycin into Peptide Chains. Proc Natl Acad Sci U S A. 51:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro C., Puthalakath H., Adams J.M., Strasser A., and Lehmann R.. 2004. Egalitarian binds dynein light chain to establish oocyte polarity and maintain oocyte fate. Nat Cell Biol. 6:427–435. [DOI] [PubMed] [Google Scholar]
- Nolan P.M., Peters J., Strivens M., Rogers D., Hagan J., Spurr N., Gray I.C., Vizor L., Brooker D., Whitehill E., Washbourne R., Hough T., Greenaway S., Hewitt M., Liu X., McCormack S., Pickford K., Selley R., Wells C., Tymowska-Lalanne Z., Roby P., Glenister P., Thornton C., Thaung C., Stevenson J.A., Arkell R., Mburu P., Hardisty R., Kiernan A., Erven A., Steel K.P., Voegeling S., Guenet J.L., Nickols C., Sadri R., Nasse M., Isaacs A., Davies K., Browne M., Fisher E.M., Martin J., Rastan S., Brown S.D., and Hunter J.. 2000. A systematic, genome-wide, phenotype-driven mutagenesis programme for gene function studies in the mouse. Nat Genet. 25:440–443. [DOI] [PubMed] [Google Scholar]
- Oleynikov Y., and Singer R.H.. 2003. Real-time visualization of ZBP1 association with beta-actin mRNA during transcription and localization. Curr Biol. 13:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K., Takeda M., Kuranaga E., Ueda R., Aigaki T., Miura M., and Hayashi S.. 2006. IKK epsilon regulates F actin assembly and interacts with Drosophila IAP1 in cellular morphogenesis. Curr Biol. 16:1531–1537. [DOI] [PubMed] [Google Scholar]
- Palacios I.M. 2007. How does an mRNA find its way? Intracellular localisation of transcripts. Semin Cell Dev Biol. 18:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo R.E., Vogel J.M., Schnackenberg B.J., Hull D.R., and Wu X.. 2000. Centrosome maturation. Curr Top Dev Biol. 49:449–470. [DOI] [PubMed] [Google Scholar]
- Pandey R., Heeger S., and Lehner C.F.. 2007. Rapid effects of acute anoxia on spindle kinetochore interactions activate the mitotic spindle checkpoint. J Cell Sci. 120:2807–2818. [DOI] [PubMed] [Google Scholar]
- Port F., and Bullock S.L.. 2016. Creating Heritable Mutations in Drosophila with CRISPR-Cas9. Methods Mol Biol. 1478:145–160. [DOI] [PubMed] [Google Scholar]
- Port F., Chen H.M., Lee T., and Bullock S.L.. 2014. Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc Natl Acad Sci U S A. 111:E2967–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff J.W., Whitfield W.G., and Glover D.M.. 1990. Two distinct mechanisms localise cyclin B transcripts in syncytial Drosophila embryos. Development. 110:1249–1261. [DOI] [PubMed] [Google Scholar]
- Reck-Peterson S.L., Redwine W.B., Vale R.D., and Carter A.P.. 2018. The cytoplasmic dynein transport machinery and its many cargoes. Nat Rev Mol Cell Biol. 19:382–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert X., and Gouet P.. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42:W320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder P.V., Fang J., and Lerit D.A.. 2020. centrocortin RNA localization to centrosomes is regulated by FMRP and facilitates error-free mitosis. J Cell Biol. 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder P.V., and Lerit D.A.. 2018. RNA localization regulates diverse and dynamic cellular processes. Traffic. 19:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder P.V., and Lerit D.A.. 2020. Quantitative analysis of subcellular distributions with an open-source, object-based tool. Biol Open. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieddine A., Coleno E., Salloum S., Imbert A., Traboulsi A.M., Kwon O.S., Lionneton F., Georget V., Robert M.C., Gostan T., Lecellier C.H., Chouaib R., Pichon X., Le Hir H., Zibara K., Mueller F., Walter T., Peter M., and Bertrand E.. 2021. A choreography of centrosomal mRNAs reveals a conserved localization mechanism involving active polysome transport. Nat Commun. 12:1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador-Garcia D., Jin L., Hensley A., Golcuk M., Gallaud E., Chaaban S., Port F., Vagnoni A., Planelles-Herrero V.J., McClintock M.A., Derivery E., Carter A.P., Giet R., Gur M., Yildiz A., and Bullock S.L.. 2023. A force-sensitive mutation reveals a spindle assembly checkpoint-independent role for dynein in anaphase progression. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J.Y., White D.J., Hartenstein V., Eliceiri K., Tomancak P., and Cardona A.. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods. 9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlager M.A., Serra-Marques A., Grigoriev I., Gumy L.F., Esteves da Silva M., Wulf P.S., Akhmanova A., and Hoogenraad C.C.. 2014. Bicaudal d family adaptor proteins control the velocity of Dynein-based movements. Cell Rep. 8:1248–1256. [DOI] [PubMed] [Google Scholar]
- Schneider-Poetsch T., Ju J., Eyler D.E., Dang Y., Bhat S., Merrick W.C., Green R., Shen B., and Liu J.O.. 2010. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat Chem Biol. 6:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda G., Antkowiak M., Brust-Mascher I., Mahe K., Ou T., Castro N.M., Christensen L.N., Cheung L., Jiang X., Yoon D., Huang B., and Jao L.E.. 2018. Co-translational protein targeting facilitates centrosomal recruitment of PCNT during centrosome maturation in vertebrates. Elife. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladewski T.E., Billington N., Ali M.Y., Bookwalter C.S., Lu H., Krementsova E.B., Schroer T.A., and Trybus K.M.. 2018. Recruitment of two dyneins to an mRNA-dependent Bicaudal D transport complex. Elife. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltys B.J., and Borisy G.G.. 1985. Polymerization of tubulin in vivo: direct evidence for assembly onto microtubule ends and from centrosomes. J Cell Biol. 100:1682–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter B., and Steward R.. 1991. Requirement for phosphorylation and localization of the Bicaudal-D protein in Drosophila oocyte differentiation. Cell. 67:917–926. [DOI] [PubMed] [Google Scholar]
- Tadros W., and Lipshitz H.D.. 2009. The maternal-to-zygotic transition: a play in two acts. Development. 136:3033–3042. [DOI] [PubMed] [Google Scholar]
- Theurkauf W.E. 1994. Immunofluorescence analysis of the cytoskeleton during oogenesis and early embryogenesis. Methods Cell Biol. 44:489–505. [DOI] [PubMed] [Google Scholar]
- UniProt C. 2023. UniProt: the Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 51:D523–D531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranan-Karmegam R., Boggupalli D.P., Liu G., and Gonsalvez G.B.. 2016. A new isoform of Drosophila non-muscle Tropomyosin 1 interacts with Kinesin-1 and functions in oskar mRNA localization. J Cell Sci. 129:4252–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertii A., Hehnly H., and Doxsey S.. 2016. The Centrosome, a Multitalented Renaissance Organelle. Cold Spring Harb Perspect Biol. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zein-Sabatto H., and Lerit D.A.. 2021. The Identification and Functional Analysis of mRNA Localizing to Centrosomes. Front Cell Dev Biol. 9:782802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in the published article and its online supplemental material. Source files of uncropped versions of the immunoblots presented in the figures are included.