Abstract
Purpose:
Intravenous fluids are mainstay of management of acute kidney injury (AKI) after sepsis but can cause fluid overload. Recent literature shows that restrictive fluid strategy may be beneficial in some patients with AKI, however, identifying these patients is challenging. We aimed to develop and validate a machine learning algorithm to identify patients who would benefit from a restrictive fluid strategy.
Methods:
We included patients with sepsis who developed AKI within 48 hours of ICU admission and defined restrictive fluid strategy as receiving <500mL fluids within 24 hours after AKI. Our primary outcome was early AKI reversal within 48 hours of AKI onset, and secondary outcomes included sustained AKI reversal and major adverse kidney events (MAKE) at discharge. We used a causal forest, a machine learning algorithm to estimate individual treatment effects and policy tree algorithm to identify patients who would benefit by restrictive fluid strategy. We developed the algorithm in MIMIC-IV and validated it in eICU database.
Results:
Among 2,091 patients in the external validation cohort, policy tree recommended restrictive fluids for 88.2%. Among these, patients who received restrictive fluids demonstrated significantly higher rate of early AKI reversal (48.2% vs 39.6%, p<0.001), sustained AKI reversal (36.7% vs 27.4%, p<0.001) and lower rates of MAKE by discharge (29.3% vs 35.1%, p=0.019). These results were consistent in adjusted analysis.
Conclusion:
Policy tree based on causal machine learning can identify septic patients with AKI who benefit from a restrictive fluid strategy. This approach needs to be validated in prospective trials.
Keywords: Acute Kidney Injury, Restrictive Fluids, Causal Machine Learning, Policy Tree, Individual Treatment Effect
INTRODUCTION:
Acute kidney injury (AKI) is seen in over one-third of critically ill patients with sepsis and is associated with worse outcomes[1]. Oliguria, a frequent clinical indicator of AKI, is common in critically ill patients with sepsis who develop AKI[2]. It is also the second most common reason for the administration of intravenous (IV) fluids in critically ill patients[3]. Since the primary emphasis is on volume optimization, IV fluids are a cornerstone in AKI care. Nonetheless, this approach might not be optimal in all patients. Firstly, administration of fluids does not consistently translate into a corresponding increase in urine output[4]. Secondly, reliance on fluid therapy increases the risk of fluid accumulation, frequently resulting in fluid overload. This phenomenon is itself associated with development and exacerbation of AKI[5–7], and increased mortality rates[7].
Thus, a more personalized approach to IV fluid administration in critically ill patients is warranted. A restrictive approach to administration of IV fluids in patients with acute lung injury has shown to be associated with a shorter duration of mechanical ventilation[8]. Similarly, a restrictive fluid approach to resuscitation for septic shock is safe and associated with less fluid overload[9]. More recently, a restrictive approach to use of IV fluids in critically ill patients with AKI has been shown to be associated with lower cumulative fluid balance and lower rates of initiation of dialysis[10].
However, it remains challenging to identify specific patients with AKI who would benefit from a restrictive fluid strategy. We, therefore, conducted this study to identify septic patients with AKI who would benefit from restrictive fluid therapy.
METHODS:
Study Design and Data Sources
We conducted a retrospective study using data from two critical care databases: the Medical Information Mart for Intensive Care IV (MIMIC-IV)[11] and the eICU Collaborative Research Database (eICU)[12]. MIMIC-IV encompasses de-identified electronic health records of intensive care unit (ICU) patients at Beth Israel Deaconess Medical Center (2008–2019). eICU includes de-identified electronic health records from 208 US ICUs (2014–2015), representing a diverse ICU patient population. We used MIMIC-IV for development and eICU for external validation. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies[13].
Study Population
We included adult, critically ill patients with sepsis who developed AKI. Following Kidney Disease Improving Global Outcomes (KDIGO) guidelines[14], we defined AKI as either a rise in serum creatinine of 0.3 mg/dL or more within 48 hours or an increase of at least 1.5 times the reference serum creatinine within 7 days, or a urine output of less than 0.5 ml/kg/h for at least a 6-hour time-period.
To determine reference creatinine, we first identified the baseline creatinine by calculating the median serum creatinine from measurements within 12 months before hospital admission[15, 16]. If unavailable, we followed the KDIGO guidelines to estimate baseline serum creatinine by back-calculating using the Modification of Diet in Renal Disease equation[17], while assuming an estimated glomerular filtration rate 75 ml/min per 1.73 m2. [14]. We then chose the lower of baseline or admission creatinine as the reference creatinine[18]. Our specific criteria for inclusion were adults aged 18 years or older upon admission, who developed sepsis according to third international consensus definition[19, 20] within 24 hours of admission to intensive care unit (ICU)[21], and developed AKI within 48 hours after admission to ICU. We considered only the first ICU admission for those patients with multiple admissions.
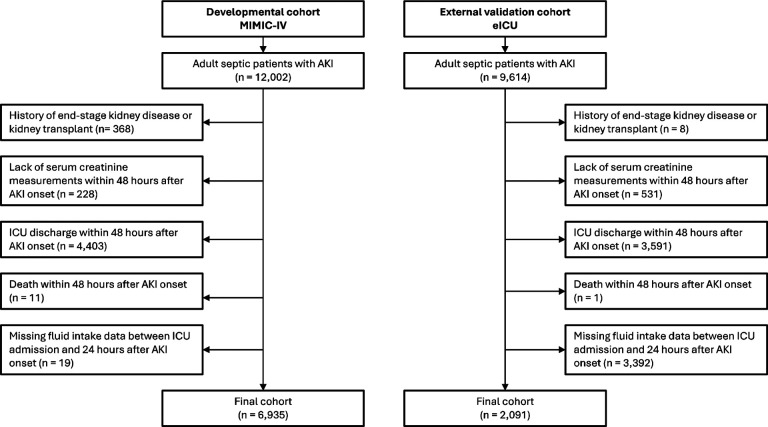
We excluded patients with a history of end stage kidney disease or kidney transplant, lack of serum creatinine measurements within 48 hours after AKI onset, death or transfer out of the ICU within 48 hours of AKI onset, or those with missing fluid administration values from ICU admission to 24 hours after AKI onset. Details regarding the selection process employed in this study are given in Fig. 1.
Fig. 1.
Cohort Inclusion and Exclusion Criteria
Outcomes
The primary outcome of our study was early AKI reversal defined as the patient no longer meeting KDIGO criteria for AKI within 48 hours of AKI onset[14]. The secondary outcomes included sustained AKI reversal, defined as maintaining AKI reversal for 48 hours or longer [22], and MAKE at discharge defined as a composite of in-hospital death, new dialysis or persistent kidney dysfunction[23]. In consistence with previous literature, persistent kidney dysfunction was defined as the final inpatient serum creatinine value as ≥200% of reference creatinine[23].
Treatment
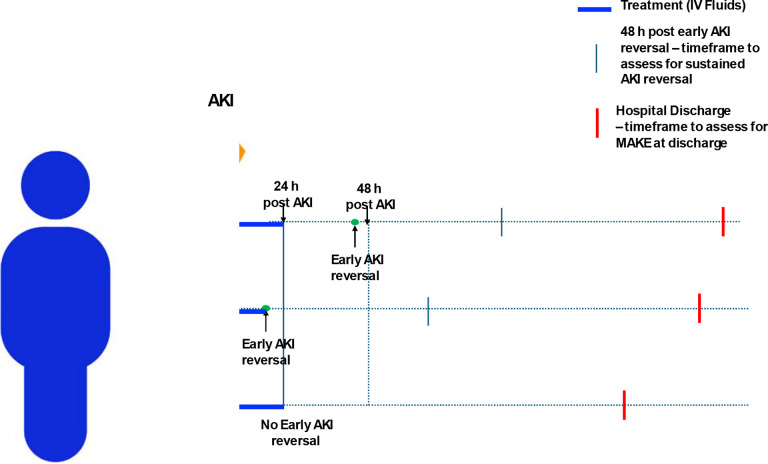
The treatment of interest in this study was the amount of IV fluids administered, including both crystalloids and colloids, from the onset of AKI to either early AKI reversal or up to 24 hours after AKI onset, whichever is sooner. We defined ‘restrictive fluid strategy’ as administrating less than 500mL of IV fluids within this specified time frame (Fig. 2).
Fig. 2.
Overview of Study Design. Patient “a” has early AKI recovery within 48 hours (h) after AKI but after first 24 h, so intravenous (IV) fluids given within first 24h were included, Patient “b” has early AKI recovery within 24 hours (h) after AKI, so IV fluids given till early AKI recovery were included; Patient “c” did not have early AKI recovery, so IV fluids given within first 24h were included; MAKE - Major Adverse Kidney Event
Features
We extracted data on patient demographics, such as age, sex, and race/ethnicity, and for vital signs, SOFA scores, vasopressor administration, use of mechanical ventilation, net fluid balance from ICU admission to onset of AKI. We also extracted data for laboratory values and administration of nephrotoxic medications[24] within the last 48 hours prior to AKI onset. Additionally, we collected data on treatment - the amount of IV fluids administered from the onset of AKI to either early reversal or up to 24 hours after AKI onset, whichever is sooner. Physiologically improbable values were removed based on inputs from content experts. For vital signs, laboratory values and SOFA scores, we collected the highest, lowest and latest values when multiple values were present. We only included features that were present in over 70% of the cohort. Missing data were imputed using the multivariate imputation by chained equations (MICE) method [25].
Development of Policy Tree Approach
We used a novel, machine learning guided strategy (‘Policy Tree’) to identify critically ill septic patients with AKI who would benefit from a restrictive fluid strategy. The development of policy tree approach employed a dual machine learning methodology. In the first step, we estimated individual treatment effects (ITE) to ascertain the impact restrictive fluid strategy in the critical initial phase post-AKI onset at the individual patient level[26]. Subsequently, we applied the policy tree algorithm[27] to construct a decision tree[28] -
Step 1 - Individual Treatment Effects Estimation:
We utilized the causal forest[26] method to estimate ITE within our development cohort. This method is a widely recognized quasi-experimental approach for estimating treatment effects using observational data, as opposed to conducting actual experiments. The causal forest uniquely targets the prediction of unit-level conditional average treatment effects, focusing on maximizing the variance in treatment effects between nodes rather than minimizing prediction error. This process involves two critical steps to ensure accuracy and computational efficiency. Initially, it identifies optimal splits that maximize the expected difference in treatment effects. Following that, during the prediction phase, it estimates treatment effects by aggregating data from similar observations within the forest’s leaves.
Step 2 - Policy Tree Algorithm:
We then used the policy tree algorithm[27] to identify patients who would benefit in terms of early AKI reversal by adhering to restrictive fluid strategy. This algorithm constructs a hierarchical model that categorizes patients based on similar ITE within the same action node and distinct ITE across different action nodes. It considers ITE, relevant features for policy tree construction, the depth of the tree, and the minimum number of observations per node. The algorithm iteratively refines the feature splits to maximize the variance in ITE.
Statistical Analysis
We expressed continuous features as mean and standard deviation, while categorical features as proportions. We examined the differences across groups using bivariate analyses, including analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables, with a P value of < .05 considered statistically significant across all statistical tests.
We then used logistic and linear regression models to assess the impact of adhering to personalized recommendation for restrictive fluid strategy on early AKI reversal, sustained AKI reversal, and major adverse kidney events (MAKE) at discharge. We adjusted these regression models for age, sex, race, reference serum creatinine, SOFA score at the time of sepsis onset, AKI stage at the time of AKI onset, and net fluid balance between ICU admission and AKI onset for each outcome.
All statistical analyses were performed using R software, version 4.3.0[29]. The study received approval from the Institutional Review Board at the Icahn School of Medicine at Mount Sinai (approval no. 19–00951).
RESULTS:
Study Population
Our study included 6,935 patients in MIMIC-IV as the development cohort, and 2,091 patients in eICU as the external validation cohort. The average age in the development cohort was 66.8 ± 15.9 years, with 57.1% males and 66.1% Whites. The average age in the external validation cohort was 66.5 ± 14.8 years with 57.0% males and 80.6% Whites. The baseline characteristics are shown in Table 1 and Supplementary Table S1.
Table 1.
Baseline Characteristics of Patients in Development (MIMIC-IV) and External Validation (eICU) Cohorts.
| Development cohort (MIMIC-IV) | External validation cohort (eICU) | |||||||
|---|---|---|---|---|---|---|---|---|
| Identified to have no benefit from restrictive fluid strategy (N= 712) | Identified to benefit from restrictive fluid strategy (N= 6,223) | Total (N= 6,935) | p value | Identified to have no benefit from restrictive fluid strategy (N= 247) | Identified to benefit from restrictive fluid strategy (N= 1844) | Total (N= 2,091) | p value | |
| Age, year, (mean±SD) | 67.49 ± 15.19 | 66.77 ± 15.98 | 66.84 ± 15.91 | 0.248 | 69.50 ± 14.08 | 66.09 ± 14.88 | 66.49 ± 14.83 | < 0.001 |
| Race, n (%) | < 0.001 | 0.375 | ||||||
| White | 437 (61.4%) | 4150 (66.7%) | 4587 (66.1%) | 193 (78.1%) | 1492 (80.9%) | 1685 (80.6%) | ||
| Black | 100 (14.0%) | 533 (8.6%) | 633 (9.1%) | 25 (10.1%) | 186 (10.1%) | 211 (10.1%) | ||
| Hispanic | 20 (2.8%) | 182 (2.9%) | 202 (2.9%) | 4 (1.6%) | 36 (2.0%) | 40 (1.9%) | ||
| Others | 155 (21.8%) | 1358 (21.8%) | 1513 (21.8%) | 25 (10.1%) | 130 (7.0%) | 155 (7.4%) | ||
| Gender - Male, n (%) | 412 (57.9%) | 3548 (57.0%) | 3960 (57.1%) | 0.664 | 137 (55.5%) | 1054 (57.2%) | 1191 (57.0%) | 0.614 |
| Baseline creatinine, mg/dL, (mean±SD) | 2.16 ± 2.30 | 1.15 ± 0.93 | 1.25 ± 1.19 | < 0.001 | 0.92 ± 0.15 | 0.89 ± 0.17 | 0.90 ± 0.17 | 0.028 |
| AKI stage, n (%) | < 0.001 | < 0.001 | ||||||
| 1 | 473 (66.4%) | 5593 (89.9%) | 6066 (87.5%) | 100 (40.5%) | 1234 (66.9%) | 1334 (63.8%) | ||
| 2 | 35 (4.9%) | 409 (6.6%) | 444 (6.4%) | 51 (20.6%) | 384 (20.8%) | 435 (20.8%) | ||
| 3 | 204 (28.7%) | 221 (3.6%) | 425 (6.1%) | 96 (38.9%) | 226 (12.3%) | 322 (15.4%) | ||
| SOFA at the time of sepsis onset, (mean±SD) | 5.08 ± 2.82 | 4.45 ± 2.36 | 4.52 ± 2.42 | < 0.001 | 4.82 ± 2.44 | 4.71 ± 2.45 | 4.73 ± 2.44 | 0.534 |
| VasopressorsB, n (%) | 250 (35.1%) | 3111 (50.0%) | 3361 (48.5%) | < 0.001 | 35 (14.2%) | 305 (16.5%) | 340 (16.3%) | 0.343 |
| Mechanical ventilationB, n (%) | 2796 (51.7%) | 315 (38.8%) | 3111 (50.0%) | < 0.001 | 197 (15.1%) | 108 (19.9%) | 305 (16.5%) | 0.012 |
| Nephrotoxic drugs administrationA, n (%) | 149 (20.9%) | 1402 (22.5%) | 1551 (22.4%) | 0.331 | 169 (68.4%) | 1282 (69.5%) | 1451 (69.4%) | 0.724 |
Abbreviation: AKI (acute kidney injury), SOFA (sequential organ failure assessment)
Measurements from the last 48 hours prior to AKI onset
Measurements from ICU admission to AKI onset
Policy Tree for Restrictive Fluid Strategy in Critically Ill Septic Patients with AKI
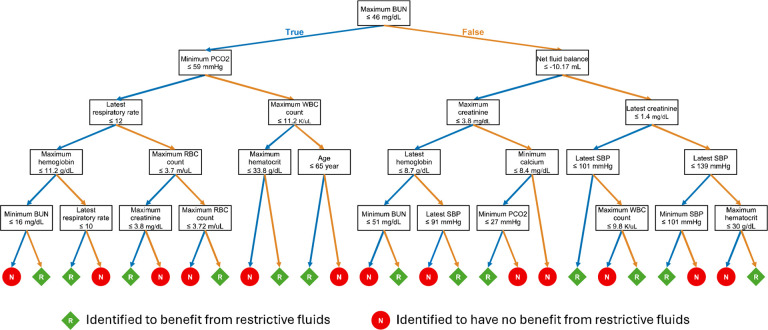
Our policy tree identifies patients with AKI and sepsis who are likely to benefit from a restrictive fluid strategy, defined as administering less than 500mL of IV fluids within 24 hours of AKI onset or until early reversal if it occurs within 24 hours (Fig. 2). The key determinants identified for early AKI reversal were age, systolic blood pressure (SBP), respiratory rate, pCO2, net fluid balance, elements of basic metabolic panel (blood urea nitrogen (BUN), creatinine, calcium) and elements of complete blood count (white blood cells, red blood cells, hemoglobin and hematocrit). The primary decision point in the policy tree was based on the maximum BUN within 48 hours before AKI onset (Fig. 3).
Fig. 3.
Policy Tree for Restrictive Fluid Strategy in Septic Patients with Acute Kidney Injury
The policy tree suggested benefit from restrictive fluid strategy after development of AKI among 6,223 (89.7%) patients in the development cohort and 1,844 (88.2%) patients in the external validation cohort. The baseline characteristics of these patients are shown in Table 1 and Supplementary Table S1. Among the patients identified to benefit from restrictive fluid therapy, only 812 (13%) patients in development cohort and 542 (29.4%) patients in external validation cohort received restrictive fluid therapy. Among patients identified to benefit from restrictive fluid strategy, those that received restrictive fluids were older, with lower net fluid balance and higher urine output from ICU admission to AKI in both development and external validation cohort (Table 2 and Supplementary Table S2).
Table 2.
Baseline Characteristics of Patients Identified to Benefit from Restrictive Fluids: Development (MIMIC-IV) and External Validation (eICU) cohorts.
| Development cohort (MIMIC-IV) | External validation cohort (eICU) | |||||||
|---|---|---|---|---|---|---|---|---|
| Unrestricted fluid intake group (N=5,411) | Restricted fluid intake group (N=812) | Total (N=6,223) | p value | Unrestricted fluid intake group (N=1,302) | Restricted fluid intake group (N=542) | Total (N=1844) | p value | |
| Age, year, (mean±SD) | 66.55 ± 15.91 | 68.19 ± 16.44 | 66.77 ± 15.98 | 0.007 | 65.41 ± 15.02 | 67.72 ± 14.44 | 66.09 ± 14.88 | 0.002 |
| Race, n (%) | 0.872 | 0.03 | ||||||
| White | 3601 (66.5%) | 549 (67.6%) | 4150 (66.7%) | 1034 (79.4%) | 458 (84.5%) | 1492 (80.9%) | ||
| Black | 462 (8.5%) | 71 (8.7%) | 533 (8.6%) | 135 (10.4%) | 51 (9.4%) | 186 (10.1%) | ||
| Hispanic | 158 (2.9%) | 24 (3.0%) | 182 (2.9%) | 28 (2.2%) | 8 (1.5%) | 36 (2.0%) | ||
| Others | 1190 (22.0%) | 168 (20.7%) | 1358 (21.8%) | 105 (8.1%) | 25 (4.6%) | 130 (7.0%) | ||
| Gender - Male, n (%) | 3098 (57.3%) | 450 (55.4%) | 3548 (57.0%) | 0.325 | 755 (58.0%) | 299 (55.2%) | 1054 (57.2%) | 0.265 |
| Baseline creatinine, mg/dL, (mean±SD) | 1.15 ± 0.92 | 1.12 ± 1.03 | 1.15 ± 0.93 | 0.437 | 0.90 ± 0.17 | 0.87 ± 0.18 | 0.89 ± 0.17 | 0.002 |
| AKI stage, n (%) | 0.019 | 0.211 | ||||||
| 1 | 4841 (89.5%) | 752 (92.6%) | 5593 (89.9%) | 858 (65.9%) | 376 (69.4%) | 1234 (66.9%) | ||
| 2 | 368 (6.8%) | 41 (5.0%) | 409 (6.6%) | 274 (21.0%) | 110 (20.3%) | 384 (20.8%) | ||
| 3 | 202 (3.7%) | 19 (2.3%) | 221 (3.6%) | 170 (13.1%) | 56 (10.3%) | 226 (12.3%) | ||
| SOFA at the time of sepsis onset, (mean±SD) | 4.56 ± 2.41 | 3.76 ± 1.85 | 4.45 ± 2.36 | < 0.001 | 4.66 ± 2.41 | 4.85 ± 2.53 | 4.71 ± 2.45 | 0.119 |
| VasopressorsB, n (%) | 2796 (51.7%) | 315 (38.8%) | 3111 (50.0%) | < 0.001 | 197 (15.1%) | 108 (19.9%) | 305 (16.5%) | 0.012 |
| Mechanical ventilationB, n (%) | 2796 (51.7%) | 315 (38.8%) | 3111 (50.0%) | < 0.001 | 197 (15.1%) | 108 (19.9%) | 305 (16.5%) | 0.012 |
| Nephrotoxic drugs administrationA, n (%) | 149 (20.9%) | 1402 (22.5%) | 1551 (22.4%) | 0.331 | 169 (68.4%) | 1282 (69.5%) | 1451 (69.4%) | 0.724 |
Abbreviation: AKI (acute kidney injury), SOFA (sequential organ failure assessment)
Measurements from the last 48 hours prior to AKI onset
Measurements from ICU admission to AKI onset
Outcomes
Primary Outcome:
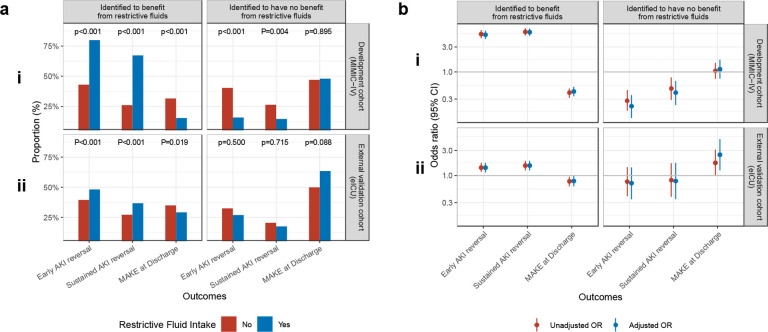
In both development and external validation cohorts, patients suggested by policy tree to benefit from a restrictive fluid strategy who received restrictive fluids had significantly higher rates of early AKI reversal in comparison to those that were suggested to have a benefit from restrictive fluids but did not receive them – 79.9% vs. 43.0%; p<0.001 in development cohort; 48.2% vs. 39.6%; 0.001 in external validation cohort (Fig. 4a). On unadjusted analysis, restrictive fluid strategy among the patients identified to benefit from restrictive fluids, was associated with significantly greater odds of early AKI reversal in both development (OR 5.27; 95% CI: 4.40 – 6.31) and external validation (OR 1.41; 95% CI: 1.16 – 1.73) cohorts (Fig. 4b). This effect was consistent in adjusted analysis where restrictive fluid strategy among patients suggested to benefit from restrictive fluids was associated with greater odds of early AKI reversal in both development (OR 5.12; 95% CI: 4.26 – 6.17) and external validation cohorts (OR 1.41; 95% CI: 1.14 – 1.73) (Fig. 4b). In comparison, in both development and external validation cohorts, patients identified to have no benefit from a restrictive fluid strategy did not show any improvement in outcomes when they received restrictive fluids (Fig. 4a and 4b).
Fig. 4.
Impact of restrictive fluid strategy among patients stratified by predicted benefit from receipt of restrictive fluid strategy: a) Proportion, b) Odds ratio, i) In MIMIC-IV (Development Cohort), ii) in eICU (External Validation Cohort)
Secondary Outcomes:
Among patients identified by the policy tree to benefit from a restrictive fluid strategy, both the development and external validation cohorts showed that those who received restrictive fluids had higher rates of sustained AKI reversal and lower rates of major acute kidney events (MAKE) at discharge compared to those who did not receive restrictive fluids (Fig. 4a). In the external validation cohort, among patients identified to benefit from restrictive fluids, 36.7% of those who received restrictive fluids achieved sustained AKI reversal, compared to only 27.4% of those who did not receive them (p<0.001). Additionally, only 29.3% of patients who received restrictive fluids developed major acute kidney events (MAKE) by discharge, compared to 35.1% of those who did not receive them (p=0.019). In the unadjusted analysis of the external validation cohort, use of restrictive fluids among patients identified to benefit from a restrictive fluid strategy was associated with a significantly higher odds sustained AKI reversal (OR 1.54; 95% CI: 1.24 – 1.90) and lower odds of developing MAKE at discharge (OR 0.77; 95% CI: 0.62 – 0.95). This effect remained consistent in the adjusted analysis, where a restrictive fluid strategy among patients identified to benefit from it was associated with significantly higher odds of sustained AKI reversal (OR 1.54; 95% CI: 1.24 – 1.91) and lower odds of MAKE at discharge (OR 0.78; 95% CI: 0.62 – 0.99). Patients identified as not deriving any benefit from restrictive fluid strategy showed no benefit from receiving restrictive fluids in both adjusted and unadjusted analyses (Fig. 4a and 4b).
DISCUSSION:
In this study we have developed and validated a novel, data-driven, causal machine learning strategy to identify critically ill patients with sepsis who develop AKI and would benefit from a restrictive fluid strategy. We show that among patients predicted to benefit from and administered restrictive fluids, there were increased odds of early AKI reversal and sustained AKI reversal, alongside lower odds of MAKE, even after adjusting for confounders.
Administration of IV fluids to improve cardiac output and consequently kidney blood flow, has been a cornerstone of therapy for AKI. This is based on the assumption that decrease in kidney blood flow is a major driver of AKI [30], However, there are 3 major observations which contradict this hypothesis.
First, even though fluid administration may increase cardiac output and renal blood flow, it does not lead to improvement in renal oxygen delivery or renal microvascular oxygenation[31, 32]. Second, fluid bolus does not always increase urine output. In a prospective, multicenter, observational study including 5 ICUs where patients with oliguria received IV fluids, improvement in urine output was seen in only half of the patients[4]. Third, kidney blood flow is not decreased in sepsis[33]. Instead, the pathogenesis of AKI in critically ill patients includes a complex interplay of inflammatory mediators[34–36], microcirculatory dysfunction[37] and metabolic reprogramming[38, 39]. These result in direct tubular injury, which is not reversible with administration of IV fluids.
IV fluid administration can lead to volume overload, often seen in AKI patients, and is associated with higher morbidity and mortality especially in AKI patients [40, 41]. This excess fluid can cause interstitial edema[42, 43], impaired blood flow and organ dysfunctions. Fluid overload also damages the endothelial glycocalyx layer which is crucial for vascular homeostasis and permeability. Inflammatory mediators in sepsis themselves lead to glycocalyx degradation, a process which is exacerbated by IV fluid resuscitation[44]. This glycocalyx damage increases local inflammation, tissue edema and further end organ injury including acute respiratory distress syndrome (ARDS) and AKI[45]. Fluid overload is also a known risk-factor for development of intra-abdominal hypertension[46], which further contributes to worsening AKI [47].
Thus, there is an increasing focus on minimizing the use of IV fluids in critically ill patients. A randomized study comparing conservative and usual fluid therapy in patients with ARDS found no difference in 60-day mortality[8]. However, the conservative group had improved oxygenation index, lung injury score, lower plateau pressures, increased number of ventilator free days and ICU free days. Studies in patients with sepsis have also found a shorter duration of mechanical ventilation and a trend towards lower mortality for patients managed with conservative fluid strategy [48].
The REVERSE-AKI trial directly evaluated the role of restrictive fluid therapy for management of AKI[10]. This multicenter, randomized, controlled study enrolled adult, critically ill patients with AKI not requiring dialysis. The trial aimed to attain a negative fluid balance in the intervention arm following randomization. By 24 hours, the restrictive fluid group had a cumulative fluid balance of −416mL vs 409mL in the usual care group (p<0.001). At 72 hours, this balance was −1080mL vs 61mL (p=0.03). The trial found that the restrictive fluid group had lower dialysis rates (13% vs 30%; p=0.04) and a trend towards lower duration of AKI (median 2 days vs 3 days; p=0.07). This trial demonstrated that restrictive fluid management in AKI is feasible and may improve outcomes. Building on this, our study identifies critically ill septic patients with AKI who would benefit from restrictive fluid therapy. We have used Policy Tree Algorithm[27], a cutting-edge causal machine learning technique to develop a data driven approach to identify these patients. We show that when identified using this approach, patients who do get restrictive fluids have much higher rates of both early AKI and sustained AKI reversal, and lower rates of MAKE at discharge. Validation of this approach in an external database is a significant strength of this study.
It is important to acknowledge the limitations of our study. First, this is a retrospective study and validation of this approach in prospective, randomized studies is important. Second, we chose the cut-off of 500mL for restrictive fluids based prior literature suggesting 500mL is the typical amount of fluid given during a fluid challenge[3], and was also the amount given in restrictive fluid groups in prior studies[9, 49]. However, future work could focus on different fluid cutoffs. Third, while we adjusted for potential confounders, unmeasured and residual confounding cannot be eliminated.
In conclusion, in this study using a causal learning approach we developed and validated a strategy to identify critically ill patients with AKI who would benefit from a restrictive fluid strategy. This approach should be considered in future prospective, pragmatic trials to improve outcomes of septic patients with AKI.
Supplementary Material
Take-home message.
Intravenous fluids are the mainstay of management of acute kidney injury (AKI) after sepsis but can cause fluid overload. In this study using two large, distinct critical care databases, we developed and validated a causal machine learning based Policy Tree approach to identify septic patients with AKI who benefit from a restrictive fluid strategy, enhancing early and sustained AKI reversal, and reducing major adverse kidney events at discharge.
FUNDING:
This work was supported by the National Institutes of Health (NIH) grants K08DK131286 awarded to A.S., R01DK108803, U01HG007278, U01HG009610, and U01DK116100 awarded to G.N.N. and T32DK007757 and TL1DK136048 awarded to W.O. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH.
Funding Statement
This work was supported by the National Institutes of Health (NIH) grants K08DK131286 awarded to A.S., R01DK108803, U01HG007278, U01HG009610, and U01DK116100 awarded to G.N.N. and T32DK007757 and TL1DK136048 awarded to W.O. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH.
Footnotes
Competing Interests
GNN is a founder of Renalytix, Pensieve, Verici and provides consultancy services to AstraZeneca, Reata, Renalytix, Siemens Healthineer and Variant Bio, serves a scientific advisory board member for Renalytix and Pensieve. He also has equity in Renalytix, Pensieve and Verici. LC is a consultant for Vifor Pharma INC and has received honorarium from Fresenius Medical Care. JAK reports receiving consulting fees from Astute Medical/bioMerieux, Astellas, Alexion, Chugai Pharma, Novartis, Mitsubishi Tenabe and GE Healthcare and is a Full-time employee of Spectral Medical. All remaining authors have declared no conflicts of interest.
REFERENCES:
- 1.Bagshaw SM, Lapinsky S, Dial S, Arabi Y, Dodek P, Wood G, Ellis P, Guzman J, Marshall J, Parrillo JE, Skrobik Y, Kumar A, Cooperative Antimicrobial Therapy of Septic Shock Database Research G, (2009) Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med 35: 871–881 [DOI] [PubMed] [Google Scholar]
- 2.Peerapornratana S, Manrique-Caballero CL, Gomez H, Kellum JA, (2019) Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int 96: 1083–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cecconi M, Hofer C, Teboul JL, Pettila V, Wilkman E, Molnar Z, Della Rocca G, Aldecoa C, Artigas A, Jog S, Sander M, Spies C, Lefrant JY, De Backer D, Investigators F, Group ET, (2015) Fluid challenges in intensive care: the FENICE study: A global inception cohort study. Intensive Care Med 41: 1529–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legrand M, Le Cam B, Perbet S, Roger C, Darmon M, Guerci P, Ferry A, Maurel V, Soussi S, Constantin JM, Gayat E, Lefrant JY, Leone M, support of the An, (2016) Urine sodium concentration to predict fluid responsiveness in oliguric ICU patients: a prospective multicenter observational study. Crit Care 20: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casas-Aparicio GA, Leon-Rodriguez I, Hernandez-Zenteno RJ, Castillejos-Lopez M, Alvarado-de la Barrera C, Ormsby CE, Reyes-Teran G, (2018) Aggressive fluid accumulation is associated with acute kidney injury and mortality in a cohort of patients with severe pneumonia caused by influenza A H1N1 virus. PLoS One 13: e0192592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raimundo M, Crichton S, Martin JR, Syed Y, Varrier M, Wyncoll D, Ostermann M, (2015) Increased Fluid Administration After Early Acute Kidney Injury is Associated with Less Renal Recovery. Shock 44: 431–437 [DOI] [PubMed] [Google Scholar]
- 7.Berthelsen RE, Perner A, Jensen AK, Jensen JU, Bestle MH, (2018) Fluid accumulation during acute kidney injury in the intensive care unit. Acta Anaesthesiol Scand 62: 780–790 [DOI] [PubMed] [Google Scholar]
- 8.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr., Hite RD, Harabin AL, (2006) Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354: 2564–2575 [DOI] [PubMed] [Google Scholar]
- 9.Meyhoff TS, Hjortrup PB, Wetterslev J, Sivapalan P, Laake JH, Cronhjort M, Jakob SM, Cecconi M, Nalos M, Ostermann M, Malbrain M, Pettila V, Moller MH, Kjaer MN, Lange T, Overgaard-Steensen C, Brand BA, Winther-Olesen M, White JO, Quist L, Westergaard B, Jonsson AB, Hjortso CJS, Meier N, Jensen TS, Engstrom J, Nebrich L, Andersen-Ranberg NC, Jensen JV, Joseph NA, Poulsen LM, Herlov LS, Solling CG, Pedersen SK, Knudsen KK, Straarup TS, Vang ML, Bundgaard H, Rasmussen BS, Aagaard SR, Hildebrandt T, Russell L, Bestle MH, Schonemann-Lund M, Brochner AC, Elvander CF, Hoffmann SKL, Rasmussen ML, Martin YK, Friberg FF, Seter H, Aslam TN, Adnoy S, Seidel P, Strand K, Johnstad B, Joelsson-Alm E, Christensen J, Ahlstedt C, Pfortmueller CA, Siegemund M, Greco M, Radej J, Kriz M, Gould DW, Rowan KM, Mouncey PR, Perner A, Group CT, (2022) Restriction of Intravenous Fluid in ICU Patients with Septic Shock. N Engl J Med 386: 2459–2470 [DOI] [PubMed] [Google Scholar]
- 10.Vaara ST, Ostermann M, Bitker L, Schneider A, Poli E, Hoste E, Fierens J, Joannidis M, Zarbock A, van Haren F, Prowle J, Selander T, Backlund M, Pettila V, Bellomo R, team R-As, (2021) Restrictive fluid management versus usual care in acute kidney injury (REVERSE-AKI): a pilot randomized controlled feasibility trial. Intensive Care Med 47: 665–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson AEW, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, Pollard TJ, Hao S, Moody B, Gow B, Lehman LH, Celi LA, Mark RG, (2023) MIMIC-IV, a freely accessible electronic health record dataset. Sci Data 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollard TJ, Johnson AEW, Raffa JD, Celi LA, Mark RG, Badawi O, (2018) The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci Data 5: 180178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S, (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370: 1453–1457 [DOI] [PubMed] [Google Scholar]
- 14.(2012) KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International Supplements 2: 1–138 [Google Scholar]
- 15.Peerapornratana S, Priyanka P, Wang S, Smith A, Singbartl K, Palevsky PM, Chawla LS, Yealy DM, Angus DC, Kellum JA, ProCess, Pro G-AKII, (2020) Sepsis-Associated Acute Kidney Disease. Kidney Int Rep 5: 839–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarbock A, Nadim MK, Pickkers P, Gomez H, Bell S, Joannidis M, Kashani K, Koyner JL, Pannu N, Meersch M, Reis T, Rimmele T, Bagshaw SM, Bellomo R, Cantaluppi V, Deep A, De Rosa S, Perez-Fernandez X, Husain-Syed F, Kane-Gill SL, Kelly Y, Mehta RL, Murray PT, Ostermann M, Prowle J, Ricci Z, See EJ, Schneider A, Soranno DE, Tolwani A, Villa G, Ronco C, Forni LG, (2023) Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol 19: 401–417 [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470 [DOI] [PubMed] [Google Scholar]
- 18.Sakhuja A, Bataineh A, Dealmeida D, Bilderback A, Ambrosino R, Fuhrman DY, Kellum JA, (2021) Creating a High-Specificity Acute Kidney Injury Detection System for Clinical and Research Applications. Am J Kidney Dis 78: 764–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC, (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315: 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moor M, Bennett N, Plecko D, Horn M, Rieck B, Meinshausen N, Buhlmann P, Borgwardt K, (2023) Predicting sepsis using deep learning across international sites: a retrospective development and validation study. EClinicalMedicine 62: 102124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takkavatakarn K, Oh W, Chan L, Hofer I, Shawwa K, Kraft M, Shah N, Kohli-Seth R, Nadkarni GN, Sakhuja A, (2024) Machine learning derived serum creatinine trajectories in acute kidney injury in critically ill patients with sepsis. Crit Care 28: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, Bittleman D, Cruz D, Endre Z, Fitzgerald RL, Forni L, Kane-Gill SL, Hoste E, Koyner J, Liu KD, Macedo E, Mehta R, Murray P, Nadim M, Ostermann M, Palevsky PM, Pannu N, Rosner M, Wald R, Zarbock A, Ronco C, Kellum JA, Acute Disease Quality Initiative W, (2017) Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 13: 241–257 [DOI] [PubMed] [Google Scholar]
- 23.Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, Stollings JL, Kumar AB, Hughes CG, Hernandez A, Guillamondegui OD, May AK, Weavind L, Casey JD, Siew ED, Shaw AD, Bernard GR, Rice TW, Investigators S, the Pragmatic Critical Care Research G, (2018) Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med 378: 829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goswami E, Ogden RK, Bennett WE, Goldstein SL, Hackbarth R, Somers MJG, Yonekawa K, Misurac J, (2019) Evidence-based development of a nephrotoxic medication list to screen for acute kidney injury risk in hospitalized children. Am J Health Syst Pharm 76: 1869–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buuren Sv, Groothuis-Oudshoorn K, (2011) mice: Multivariate Imputation by Chained Equations inR. Journal of Statistical Software 45: 1–67 [Google Scholar]
- 26.Wager S, Athey S, (2018) Estimation and Inference of Heterogeneous Treatment Effects using Random Forests. Journal of the American Statistical Association 113: 1228–1242 [Google Scholar]
- 27.Athey S, Wager S, (2021) Policy Learning With Observational Data. Econometrica 89: 133–161 [Google Scholar]
- 28.von Winterfeldt D, Edwards W (1986) Decision treesDecision Analysis and Behavioral Research. Cambridge University Press, pp. 63–89 [Google Scholar]
- 29.R Core Team R: A Language and Environment for Statistical Computing. In: Editor (ed)^(eds) Book R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, City, pp. [Google Scholar]
- 30.Schrier RW, Wang W, (2004) Acute renal failure and sepsis. N Engl J Med 351: 159–169 [DOI] [PubMed] [Google Scholar]
- 31.Wan L, Bellomo R, May CN, (2007) A comparison of 4% succinylated gelatin solution versus normal saline in stable normovolaemic sheep: global haemodynamic, regional blood flow and oxygen delivery effects. Anaesth Intensive Care 35: 924–931 [DOI] [PubMed] [Google Scholar]
- 32.Legrand M, Mik EG, Balestra GM, Lutter R, Pirracchio R, Payen D, Ince C, (2010) Fluid resuscitation does not improve renal oxygenation during hemorrhagic shock in rats. Anesthesiology 112: 119–127 [DOI] [PubMed] [Google Scholar]
- 33.Di Giantomasso D, May CN, Bellomo R, (2003) Vital organ blood flow during hyperdynamic sepsis. Chest 124: 1053–1059 [DOI] [PubMed] [Google Scholar]
- 34.Maiden MJ, Otto S, Brealey JK, Finnis ME, Chapman MJ, Kuchel TR, Nash CH, Edwards J, Bellomo R, (2016) Structure and Function of the Kidney in Septic Shock. A Prospective Controlled Experimental Study. Am J Respir Crit Care Med 194: 692–700 [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Holthoff JH, Seely KA, Pathak E, Spencer HJ 3rd, Gokden N, Mayeux PR, (2012) Development of oxidative stress in the peritubular capillary microenvironment mediates sepsis-induced renal microcirculatory failure and acute kidney injury. Am J Pathol 180: 505–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seely KA, Holthoff JH, Burns ST, Wang Z, Thakali KM, Gokden N, Rhee SW, Mayeux PR, (2011) Hemodynamic changes in the kidney in a pediatric rat model of sepsis-induced acute kidney injury. Am J Physiol Renal Physiol 301: F209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Backer D, Donadello K, Taccone FS, Ospina-Tascon G, Salgado D, Vincent JL, (2011) Microcirculatory alterations: potential mechanisms and implications for therapy. Ann Intensive Care 1: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez H, Kellum JA, Ronco C, (2017) Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat Rev Nephrol 13: 143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singer M, De Santis V, Vitale D, Jeffcoate W, (2004) Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet 364: 545–548 [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Chen Z, Diao Y, Yang Y, Fu P, (2015) Associations of fluid overload with mortality and kidney recovery in patients with acute kidney injury: A systematic review and meta-analysis. J Crit Care 30: 860 e867–813 [DOI] [PubMed] [Google Scholar]
- 41.Hayashi Y, Shimazui T, Tomita K, Shimada T, Miura RE, Nakada TA, (2023) Associations between fluid overload and outcomes in critically ill patients with acute kidney injury: a retrospective observational study. Sci Rep 13: 17410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patil VP, Salunke BG, (2020) Fluid Overload and Acute Kidney Injury. Indian J Crit Care Med 24: S94–S97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen B, (2021) Fluid Overload. Front Vet Sci 8: 668688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hippensteel JA, Uchimido R, Tyler PD, Burke RC, Han X, Zhang F, McMurtry SA, Colbert JF, Lindsell CJ, Angus DC, Kellum JA, Yealy DM, Linhardt RJ, Shapiro NI, Schmidt EP, (2019) Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care 23: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan RC, Rockstrom MD, Schmidt EP, Hippensteel JA, (2021) Endothelial glycocalyx degradation during sepsis: Causes and consequences. Matrix Biol Plus 12: 100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vatankhah S, Sheikhi RA, Heidari M, Moradimajd P, (2018) The relationship between fluid resuscitation and intra-abdominal hypertension in patients with blunt abdominal trauma. Int J Crit Illn Inj Sci 8: 149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suphatheerawatr N, Jaturapisanukul S, Prommool S, Kurathong S, Pongsittisak W, (2023) Intra-abdominal hypertension among medical septic patients associated with worsening kidney outcomes (IAH-WK study). Medicine (Baltimore) 102: e32807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shahnoor H, Divi R, Addi Palle LR, Sharma A, Contractor B, Krupanagaram S, Batool S, Ali N, (2023) The Effects of Restrictive Fluid Resuscitation on the Clinical Outcomes in Patients with Sepsis or Septic Shock: A Meta-Analysis of Randomized-Controlled Trials. Cureus 15: e45620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jessen MK, Andersen LW, Thomsen MH, Kristensen P, Hayeri W, Hassel RE, Messerschmidt TG, Solling CG, Perner A, Petersen JAK, Kirkegaard H, (2022) Restrictive fluids versus standard care in adults with sepsis in the emergency department (REFACED): A multicenter, randomized feasibility trial. Acad Emerg Med 29: 1172–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.