Abstract
1. In bovine adrenal chromaffin cells made permeable either to molecules less than or equal to 3 kDa with alphatoxin or to proteins less than or equal to 150 kDa with streptolysin O, the GTP analogues guanosine 5'-[beta gamma-imido]triphosphate (p[NH]ppG) and guanosine 5'-[gamma-thio]triphosphate (GTP[S]) differently modulated Ca(2+)-stimulated exocytosis. 2. In alphatoxin-permeabilized cells, p[NH]ppG up to 20 microM activated Ca(2+)-stimulated exocytosis. Higher concentrations had little or no effect. At a free Ca2+ concentration of 5 microM, 7 microM-p[NH]ppG stimulated exocytosis 6-fold. Increasing the free Ca2+ concentration reduced the effect of p[NH]ppG. Pretreatment of the cells with pertussis toxin prevented the activation of the Ca(2+)-stimulated exocytosis by p[NH]ppG. 3. In streptolysin O-permeabilized cells, p[NH]ppG did not activate, but rather inhibited Ca(2+)-dependent catecholamine release under all conditions studied. In the soluble cytoplasmic material that escaped during permeabilization with streptolysin O, different G-protein alpha-subunits were detected using an appropriate antibody. Around 15% of the cellular alpha-subunits were detected in the supernatant of permeabilized control cells. p[NH]ppG or GTP[S] stimulated the release of alpha-subunits 2-fold, causing a loss of about 30% of the cellular G-protein alpha-subunits under these conditions. Two of the alpha-subunits in the supernatant belonged to the G(o) type, as revealed by an antibody specific for G(o) alpha. 4. GTP[S], when present alone during stimulation with Ca2+, activated exocytosis in a similar manner to p[NH]ppG. Upon prolonged incubation, GTP[S], in contrast to p[NH]ppG, inhibited Ca(2+)-induced exocytosis from cells permeabilized by either of the pore-forming toxins. This effect was resistant to pertussin toxin. 5. The p[NH]ppG-induced activation of Ca(2+)-stimulated release from alphatoxin-permeabilized chromaffin cells may be attributed to one of the heterotrimeric G-proteins lost during permeabilization with streptolysin O. The inhibitory effect of GTP[S] on exocytosis is apparently not mediated by G-protein alpha-subunits, but by another GTP-dependent process still occurring after permeabilization with streptolysin O.
Full text
PDF
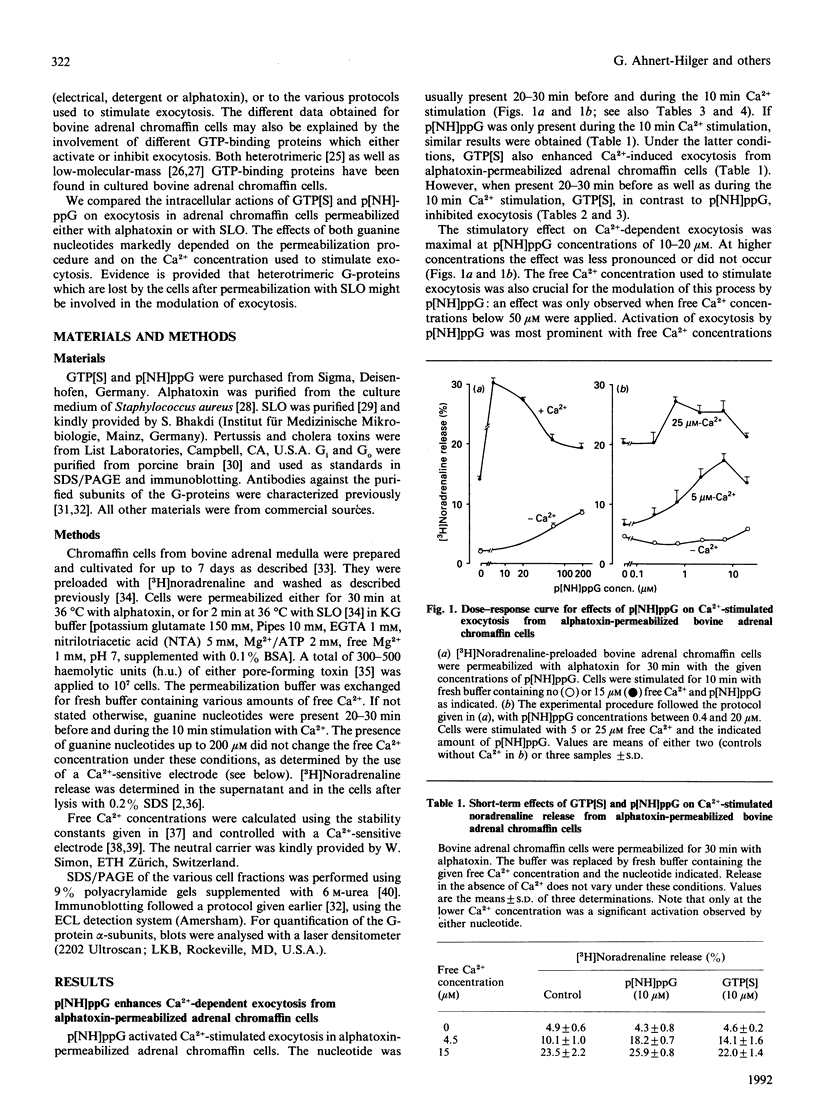
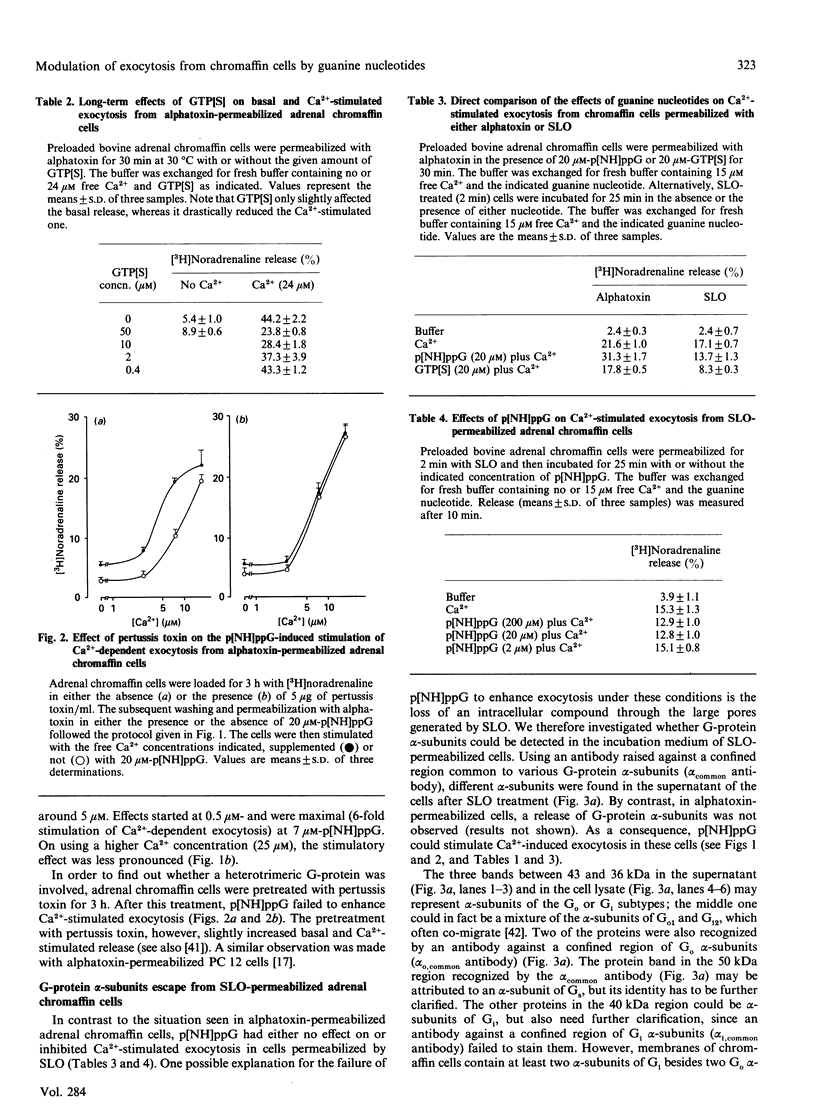
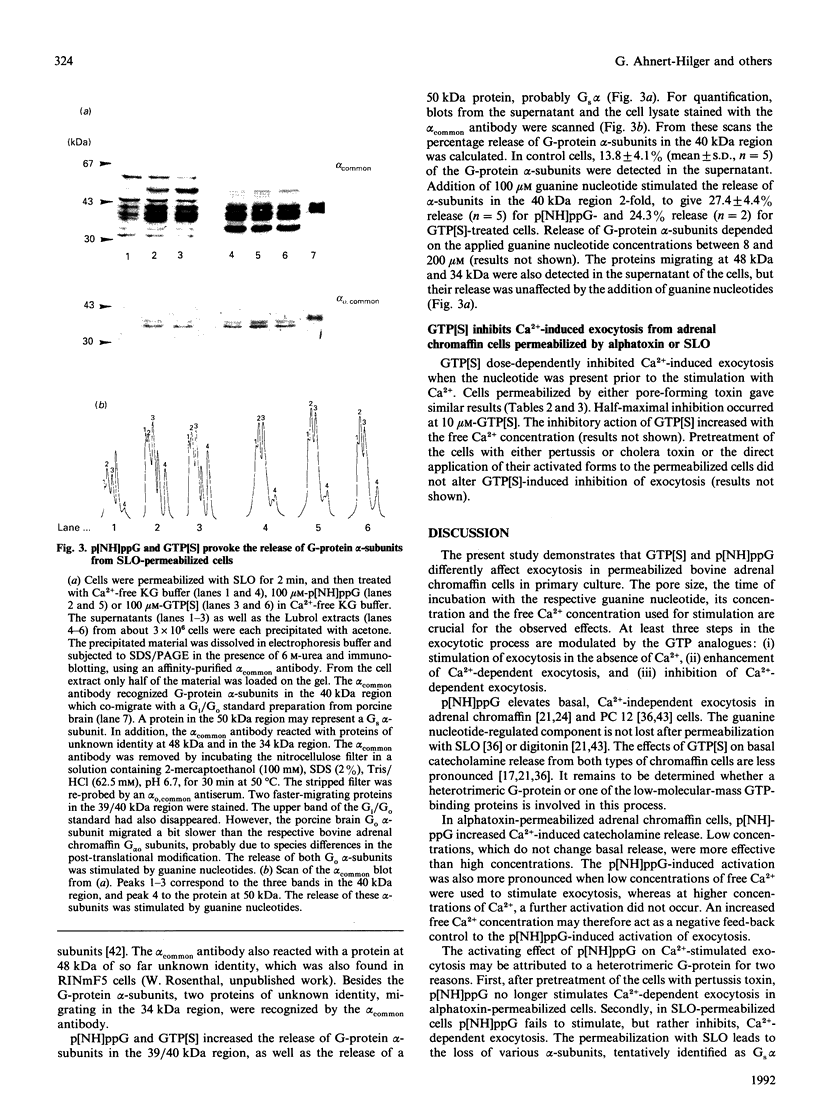


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnert-Hilger G., Bader M. F., Bhakdi S., Gratzl M. Introduction of macromolecules into bovine adrenal medullary chromaffin cells and rat pheochromocytoma cells (PC12) by permeabilization with streptolysin O: inhibitory effect of tetanus toxin on catecholamine secretion. J Neurochem. 1989 Jun;52(6):1751–1758. doi: 10.1111/j.1471-4159.1989.tb07253.x. [DOI] [PubMed] [Google Scholar]
- Ahnert-Hilger G., Bräutigam M., Gratzl M. Ca2+-stimulated catecholamine release from alpha-toxin-permeabilized PC12 cells: biochemical evidence for exocytosis and its modulation by protein kinase C and G proteins. Biochemistry. 1987 Dec 1;26(24):7842–7848. doi: 10.1021/bi00398a046. [DOI] [PubMed] [Google Scholar]
- Ahnert-Hilger G., Mach W., Föhr K. J., Gratzl M. Poration by alpha-toxin and streptolysin O: an approach to analyze intracellular processes. Methods Cell Biol. 1989;31:63–90. doi: 10.1016/s0091-679x(08)61602-7. [DOI] [PubMed] [Google Scholar]
- Ammann D., Bührer T., Schefer U., Müller M., Simon W. Intracellular neutral carrier-based Ca2+ microelectrode with subnanomolar detection limit. Pflugers Arch. 1987 Jul;409(3):223–228. doi: 10.1007/BF00583469. [DOI] [PubMed] [Google Scholar]
- Bader M. F., Sontag J. M., Thiersé D., Aunis D. A reassessment of guanine nucleotide effects on catecholamine secretion from permeabilized adrenal chromaffin cells. J Biol Chem. 1989 Oct 5;264(28):16426–16434. [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Two roles for guanine nucleotides in the stimulus-secretion sequence of neutrophils. Nature. 1986 Feb 6;319(6053):504–507. doi: 10.1038/319504a0. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Roth M., Sziegoleit A., Tranum-Jensen J. Isolation and identification of two hemolytic forms of streptolysin-O. Infect Immun. 1984 Nov;46(2):394–400. doi: 10.1128/iai.46.2.394-400.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–223. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- Bielinski D. F., Morin P. J., Dickey B. F., Fine R. E. Low molecular weight GTP-binding proteins are associated with neuronal organelles involved in rapid axonal transport and exocytosis. J Biol Chem. 1989 Nov 5;264(31):18363–18367. [PubMed] [Google Scholar]
- Bittner M. A., Holz R. W., Neubig R. R. Guanine nucleotide effects on catecholamine secretion from digitonin-permeabilized adrenal chromaffin cells. J Biol Chem. 1986 Aug 5;261(22):10182–10188. [PubMed] [Google Scholar]
- Bourne H. R. Do GTPases direct membrane traffic in secretion? Cell. 1988 Jun 3;53(5):669–671. doi: 10.1016/0092-8674(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Low molecular mass GTP-binding proteins of adrenal chromaffin cells are present on the secretory granule. FEBS Lett. 1989 Mar 13;245(1-2):122–126. doi: 10.1016/0014-5793(89)80204-2. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A., O'Sullivan A. J. A major role for protein kinase C in calcium-activated exocytosis in permeabilised adrenal chromaffin cells. FEBS Lett. 1988 Sep 26;238(1):151–155. doi: 10.1016/0014-5793(88)80246-1. [DOI] [PubMed] [Google Scholar]
- Carroll A. G., Rhoads A. R., Wagner P. D. Hydrolysis-resistant GTP analogs stimulate catecholamine release from digitonin-permeabilized PC12 cells. J Neurochem. 1990 Sep;55(3):930–936. doi: 10.1111/j.1471-4159.1990.tb04580.x. [DOI] [PubMed] [Google Scholar]
- Doucet J. P., Fournier S., Parulekar M., Trifaró J. M. Detection of low molecular mass GTP-binding proteins in chromaffin granules and other subcellular fractions of chromaffin cells. FEBS Lett. 1989 Apr 10;247(1):127–131. doi: 10.1016/0014-5793(89)81254-2. [DOI] [PubMed] [Google Scholar]
- Fernandez J. M., Neher E., Gomperts B. D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. 1984 Nov 29-Dec 5Nature. 312(5993):453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Gomperts B. D., Cockcroft S., Howell T. W., Nüsse O., Tatham P. E. The dual effector system for exocytosis in mast cells: obligatory requirement for both Ca2+ and GTP. Biosci Rep. 1987 May;7(5):369–381. doi: 10.1007/BF01362501. [DOI] [PubMed] [Google Scholar]
- Goud B., Salminen A., Walworth N. C., Novick P. J. A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell. 1988 Jun 3;53(5):753–768. doi: 10.1016/0092-8674(88)90093-1. [DOI] [PubMed] [Google Scholar]
- Hinsch K. D., Rosenthal W., Spicher K., Binder T., Gausepohl H., Frank R., Schultz G., Joost H. G. Adipocyte plasma membranes contain two Gi subtypes but are devoid of Go. FEBS Lett. 1988 Sep 26;238(1):191–196. doi: 10.1016/0014-5793(88)80254-0. [DOI] [PubMed] [Google Scholar]
- Howell T. W., Cockcroft S., Gomperts B. D. Essential synergy between Ca2+ and guanine nucleotides in exocytotic secretion from permeabilized rat mast cells. J Cell Biol. 1987 Jul;105(1):191–197. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Guanine nucleotides and Ca-dependent exocytosis. Studies on two adrenal cell preparations. FEBS Lett. 1985 Sep 23;189(2):345–349. doi: 10.1016/0014-5793(85)81053-x. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. The phorbol ester TPA increases the affinity of exocytosis for calcium in 'leaky' adrenal medullary cells. FEBS Lett. 1983 Aug 22;160(1-2):98–100. doi: 10.1016/0014-5793(83)80944-2. [DOI] [PubMed] [Google Scholar]
- Lind I., Ahnert-Hilger G., Fuchs G., Gratzl M. Purification of alpha-toxin from Staphylococcus aureus and application to cell permeabilization. Anal Biochem. 1987 Jul;164(1):84–89. doi: 10.1016/0003-2697(87)90371-x. [DOI] [PubMed] [Google Scholar]
- Livett B. G. Adrenal medullary chromaffin cells in vitro. Physiol Rev. 1984 Oct;64(4):1103–1161. doi: 10.1152/physrev.1984.64.4.1103. [DOI] [PubMed] [Google Scholar]
- Matsui Y., Kikuchi A., Kondo J., Hishida T., Teranishi Y., Takai Y. Nucleotide and deduced amino acid sequences of a GTP-binding protein family with molecular weights of 25,000 from bovine brain. J Biol Chem. 1988 Aug 15;263(23):11071–11074. [PubMed] [Google Scholar]
- Melançon P., Glick B. S., Malhotra V., Weidman P. J., Serafini T., Orci L., Rothman J. E. A role for GTP-binding proteins in vesicular transport through the Golgi complex. Soc Gen Physiol Ser. 1989;44:175–188. [PubMed] [Google Scholar]
- Morgan A., Burgoyne R. D. Stimulation of Ca2(+)-independent catecholamine secretion from digitonin-permeabilized bovine adrenal chromaffin cells by guanine nucleotide analogues. Relationship to arachidonate release. Biochem J. 1990 Jul 15;269(2):521–526. doi: 10.1042/bj2690521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsse O., Lindau M. The dynamics of exocytosis in human neutrophils. J Cell Biol. 1988 Dec;107(6 Pt 1):2117–2123. doi: 10.1083/jcb.107.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermans S., Schäfer R., Hoffmann B., Bombien E., Spicher K., Hinsch K. D., Schultz G., Rosenthal W. Agonist-sensitive binding of a photoreactive GTP analog to a G-protein alpha-subunit in membranes of HL-60 cells. FEBS Lett. 1990 Jan 15;260(1):14–18. doi: 10.1016/0014-5793(90)80054-m. [DOI] [PubMed] [Google Scholar]
- Orci L., Malhotra V., Amherdt M., Serafini T., Rothman J. E. Dissection of a single round of vesicular transport: sequential intermediates for intercisternal movement in the Golgi stack. Cell. 1989 Feb 10;56(3):357–368. doi: 10.1016/0092-8674(89)90239-0. [DOI] [PubMed] [Google Scholar]
- Peppers S. C., Holz R. W. Catecholamine secretion from digitonin-treated PC12 cells. Effects of Ca2+, ATP, and protein kinase C activators. J Biol Chem. 1986 Nov 5;261(31):14665–14669. [PubMed] [Google Scholar]
- Ribeiro-Neto F. A., Rodbell M. Pertussis toxin induces structural changes in G alpha proteins independently of ADP-ribosylation. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2577–2581. doi: 10.1073/pnas.86.8.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal W., Koesling D., Rudolph U., Kleuss C., Pallast M., Yajima M., Schultz G. Identification and characterization of the 35-kDa beta subunit of guanine-nucleotide-binding proteins by an antiserum raised against transducin. Eur J Biochem. 1986 Jul 15;158(2):255–263. doi: 10.1111/j.1432-1033.1986.tb09745.x. [DOI] [PubMed] [Google Scholar]
- Schrezenmeier H., Ahnert-Hilger G., Fleischer B. A T cell receptor-associated GTP-binding protein triggers T cell receptor-mediated granule exocytosis in cytotoxic T lymphocytes. J Immunol. 1988 Dec 1;141(11):3785–3790. [PubMed] [Google Scholar]
- Schrezenmeier H., Ahnert-Hilger G., Fleischer B. Inactivation of a T cell receptor-associated GTP-binding protein by antibody-induced modulation of the T cell receptor/CD3 complex. J Exp Med. 1988 Aug 1;168(2):817–822. doi: 10.1084/jem.168.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag J. M., Thierse D., Rouot B., Aunis D., Bader M. F. A pertussis-toxin-sensitive protein controls exocytosis in chromaffin cells at a step distal to the generation of second messengers. Biochem J. 1991 Mar 1;274(Pt 2):339–347. doi: 10.1042/bj2740339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B., Gratzl M., Ahnert-Hilger G. Reductive chain separation of botulinum A toxin--a prerequisite to its inhibitory action on exocytosis in chromaffin cells. FEBS Lett. 1989 May 8;248(1-2):23–27. doi: 10.1016/0014-5793(89)80424-7. [DOI] [PubMed] [Google Scholar]
- Toutant M., Aunis D., Bockaert J., Homburger V., Rouot B. Presence of three pertussis toxin substrates and Go alpha immunoreactivity in both plasma and granule membranes of chromaffin cells. FEBS Lett. 1987 May 11;215(2):339–344. doi: 10.1016/0014-5793(87)80174-6. [DOI] [PubMed] [Google Scholar]
- Ullrich S., Wollheim C. B. GTP-dependent inhibition of insulin secretion by epinephrine in permeabilized RINm5F cells. Lack of correlation between insulin secretion and cyclic AMP levels. J Biol Chem. 1988 Jun 25;263(18):8615–8620. [PubMed] [Google Scholar]
- Ullrich S., Wollheim C. B. Galanin inhibits insulin secretion by direct interference with exocytosis. FEBS Lett. 1989 Apr 24;247(2):401–404. doi: 10.1016/0014-5793(89)81379-1. [DOI] [PubMed] [Google Scholar]
- Vallar L., Biden T. J., Wollheim C. B. Guanine nucleotides induce Ca2+-independent insulin secretion from permeabilized RINm5F cells. J Biol Chem. 1987 Apr 15;262(11):5049–5056. [PubMed] [Google Scholar]
- Winslow J. W., Van Amsterdam J. R., Neer E. J. Conformations of the alpha 39, alpha 41, and beta.gamma components of brain guanine nucleotide-binding proteins. Analysis by limited proteolysis. J Biol Chem. 1986 Jun 5;261(16):7571–7579. [PubMed] [Google Scholar]