Abstract
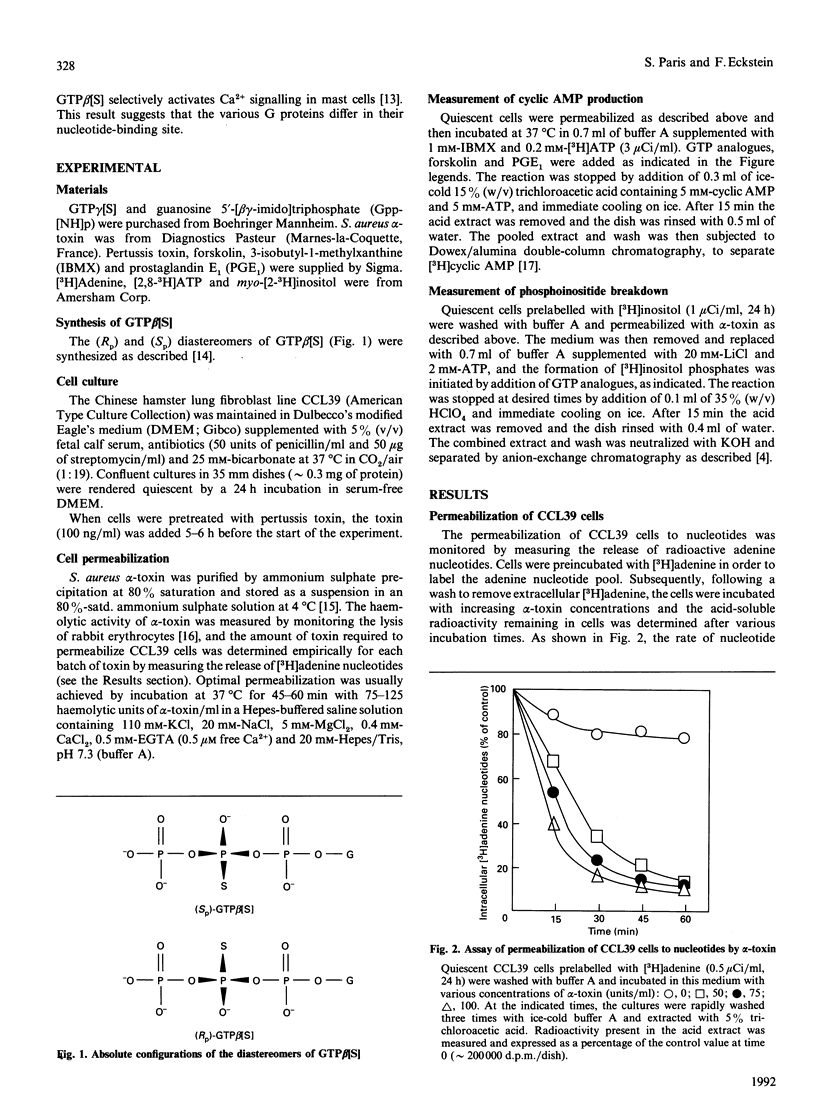
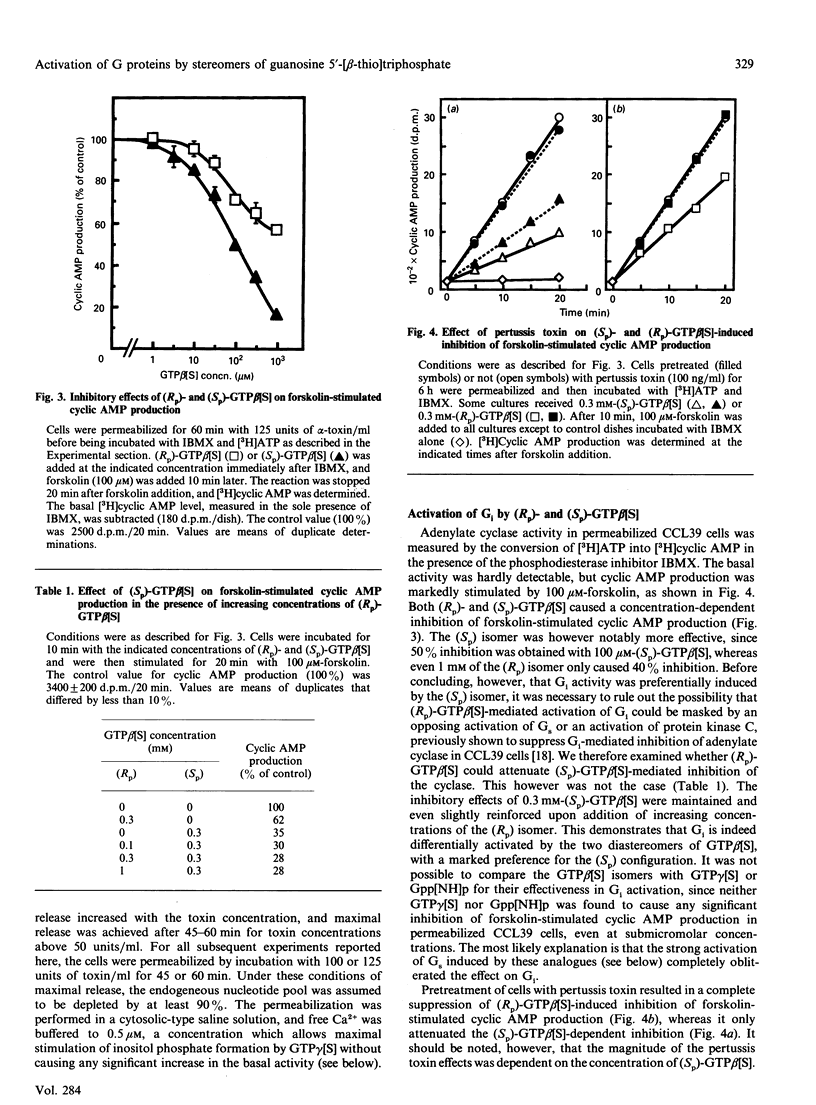
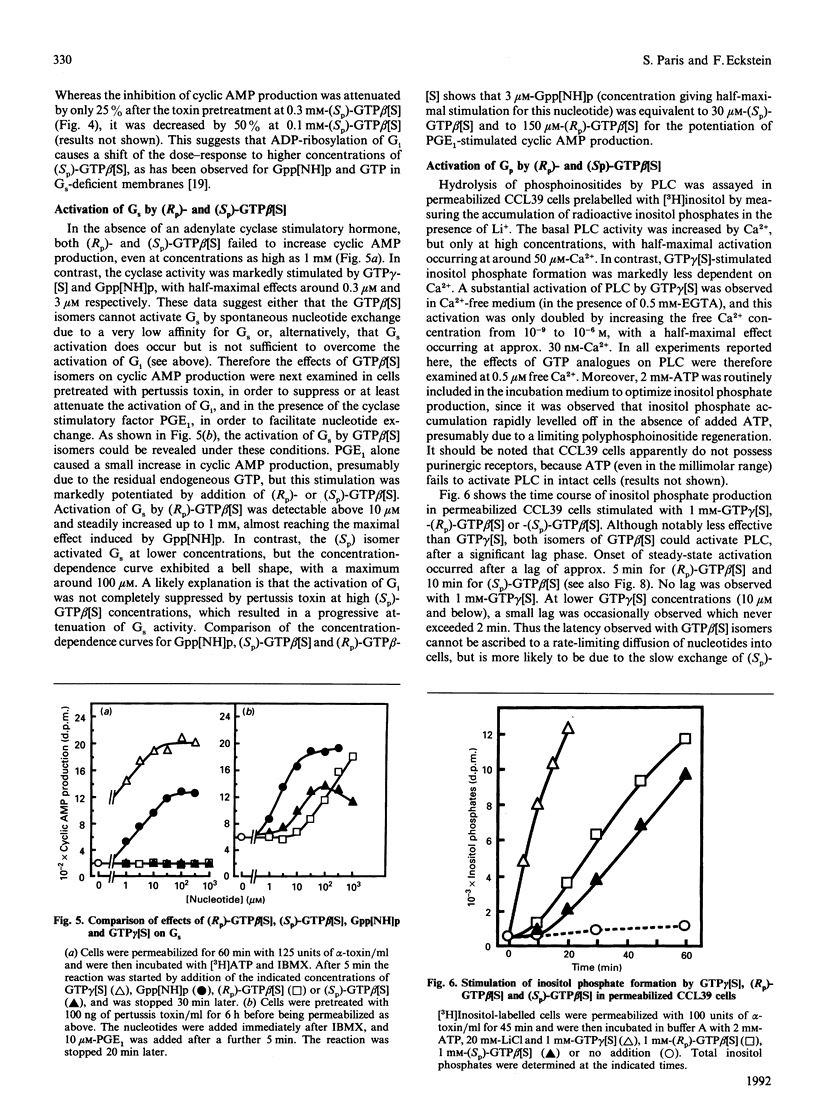
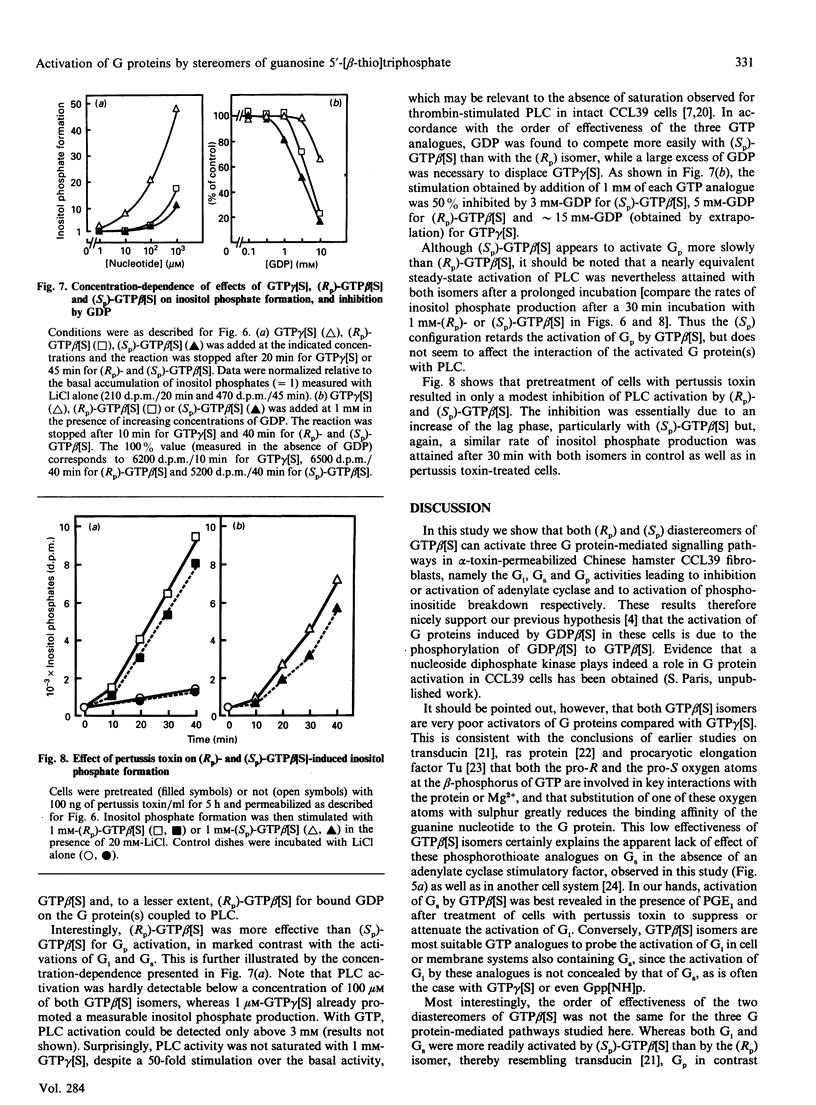
The effects of guanosine 5'-[beta-thio]triphosphate (GTP beta[S]) on G proteins have been examined in Chinese hamster lung fibroblasts (CCL39 line) permeabilized with alpha-toxin from Staphylococcus aureus. Although much less effective than guanosine 5'-[gamma-thio]triphosphate (GTP gamma[S]), both (Rp) and (Sp) diastereomers of GTP beta[S] were found to activate three G protein-mediated pathways: inhibition of forskolin-stimulated adenylate cyclase (mediated by Gi), potentiation of receptor-mediated activation of adenylate cyclase (mediated by Gs), and activation of phosphoinositide breakdown (mediated by Gp). Activation of Gi and Gs occurred above 3 microM-GTP beta[S], but activation of Gp only occurred above 100 microM-GTP beta[S]. Moreover, the order of effectiveness of the two diastereomers was not the same for the three G protein-mediated processes. Whereas both Gi and Gs were more effectively activated (about 5-fold) by (Sp)-GTP beta[S] than by (Rp)-GTP beta[S], Gp showed a marked preference for the (Rp) isomer. Indeed, (Rp)-GTP beta[S] induced the formation of inositol phosphates with a shorter latency and was a better competitor of GDP for binding to Gp than the (Sp) isomer. These results point to different guanine nucleotide-binding properties for Gi and Gs on the one hand and Gp on the other. At least two distinct Gp proteins, differing by their sensitivity to pertussis toxin, are present in CCL39 cells. Since pretreatment of cells with pertussis toxin completely suppressed the effects of (Rp)-GTP beta[S] on Gi, while only slightly attenuating its effects on Gp, we believe that it is the pertussis toxin-insensitive Gp which prefers the (Rp) isomer. Therefore (Rp)-GTP beta[S] may be a valuable tool for the selective activation and the biochemical characterization of this pertussis toxin-insensitive Gp.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader M. F., Sontag J. M., Thiersé D., Aunis D. A reassessment of guanine nucleotide effects on catecholamine secretion from permeabilized adrenal chromaffin cells. J Biol Chem. 1989 Oct 5;264(28):16426–16434. [PubMed] [Google Scholar]
- Bader M. F., Thiersé D., Aunis D., Ahnert-Hilger G., Gratzl M. Characterization of hormone and protein release from alpha-toxin-permeabilized chromaffin cells in primary culture. J Biol Chem. 1986 May 5;261(13):5777–5783. [PubMed] [Google Scholar]
- Bernheimer A. W. Assay of hemolytic toxins. Methods Enzymol. 1988;165:213–217. doi: 10.1016/s0076-6879(88)65033-6. [DOI] [PubMed] [Google Scholar]
- Cassel D., Eckstein F., Lowe M., Selinger Z. Determination of the turn-off reaction for the hormone-activated adenylate cyclase. J Biol Chem. 1979 Oct 10;254(19):9835–9838. [PubMed] [Google Scholar]
- Connolly B. A., Romaniuk P. J., Eckstein F. Synthesis and characterization of diastereomers of guanosine 5'-O-(1-thiotriphosphate) and guanosine 5'-O-(2-thiotriphosphate). Biochemistry. 1982 Apr 27;21(9):1983–1989. doi: 10.1021/bi00538a002. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman R. J. Aggregation of IgE receptors induces degranulation in rat basophilic leukemia cells permeabilized with alpha-toxin from Staphylococcus aureus. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1624–1628. doi: 10.1073/pnas.85.5.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Salomon Y. Assay of adenylyl cyclase catalytic activity. Methods Enzymol. 1991;195:3–21. doi: 10.1016/0076-6879(91)95150-i. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Masters S. B., Bourne H. R., Reed R. R. Biochemical characterization of three stimulatory GTP-binding proteins. The large and small forms of Gs and the olfactory-specific G-protein, Golf. J Biol Chem. 1990 Feb 15;265(5):2671–2676. [PubMed] [Google Scholar]
- Kobayashi I., Shibasaki H., Takahashi K., Tohyama K., Kurachi Y., Ito H., Ui M., Katada T. Purification and characterization of five different alpha subunits of guanine-nucleotide-binding proteins in bovine brain membranes. Their physiological properties concerning the activities of adenylate cyclase and atrial muscarinic K+ channels. Eur J Biochem. 1990 Jul 31;191(2):499–506. doi: 10.1111/j.1432-1033.1990.tb19149.x. [DOI] [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Magnaldo I., Pouysségur J. Alpha-thrombin-induced inositol phosphate formation in G0-arrested and cycling hamster lung fibroblasts: evidence for a protein kinase C-mediated desensitization response. J Cell Physiol. 1986 Nov;129(2):167–174. doi: 10.1002/jcp.1041290207. [DOI] [PubMed] [Google Scholar]
- Leupold C. M., Goody R. S., Wittinghofer A. Stereochemistry of the elongation factor Tu X GTP complex. Eur J Biochem. 1983 Sep 15;135(2):237–241. doi: 10.1111/j.1432-1033.1983.tb07643.x. [DOI] [PubMed] [Google Scholar]
- Magnaldo I., Pouysségur J., Paris S. Thrombin exerts a dual effect on stimulated adenylate cyclase in hamster fibroblasts, an inhibition via a GTP-binding protein and a potentiation via activation of protein kinase C. Biochem J. 1988 Aug 1;253(3):711–719. doi: 10.1042/bj2530711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty T. M., Padrell E., Carty D. J., Omri G., Landau E. M., Iyengar R. Go protein as signal transducer in the pertussis toxin-sensitive phosphatidylinositol pathway. Nature. 1990 Jan 4;343(6253):79–82. doi: 10.1038/343079a0. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Pang I. H., Sternweis P. C. Purification of unique alpha subunits of GTP-binding regulatory proteins (G proteins) by affinity chromatography with immobilized beta gamma subunits. J Biol Chem. 1990 Oct 25;265(30):18707–18712. [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Further evidence for a phospholipase C-coupled G protein in hamster fibroblasts. Induction of inositol phosphate formation by fluoroaluminate and vanadate and inhibition by pertussis toxin. J Biol Chem. 1987 Feb 15;262(5):1970–1976. [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Guanosine 5'-O-(3-thiotriphosphate) and guanosine 5'-O-(2-thiodiphosphate) activate G proteins and potentiate fibroblast growth factor-induced DNA synthesis in hamster fibroblasts. J Biol Chem. 1990 Jul 15;265(20):11567–11575. [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Pertussis toxin inhibits thrombin-induced activation of phosphoinositide hydrolysis and Na+/H+ exchange in hamster fibroblasts. EMBO J. 1986 Jan;5(1):55–60. doi: 10.1002/j.1460-2075.1986.tb04177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seuwen K., Kahan C., Hartmann T., Pouyssegur J. Strong and persistent activation of inositol lipid breakdown induces early mitogenic events but not Go to S phase progression in hamster fibroblasts. Comparison of thrombin and carbachol action in cells expressing M1 muscarinic acetylcholine receptors. J Biol Chem. 1990 Dec 25;265(36):22292–22299. [PubMed] [Google Scholar]
- Smrcka A. V., Hepler J. R., Brown K. O., Sternweis P. C. Regulation of polyphosphoinositide-specific phospholipase C activity by purified Gq. Science. 1991 Feb 15;251(4995):804–807. doi: 10.1126/science.1846707. [DOI] [PubMed] [Google Scholar]
- Strathmann M., Simon M. I. G protein diversity: a distinct class of alpha subunits is present in vertebrates and invertebrates. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9113–9117. doi: 10.1073/pnas.87.23.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer T., Monastirsky B., Codina J., Birnbaumer L. Studies on nucleotide and receptor regulation of Gi proteins: effects of pertussis toxin. Mol Endocrinol. 1989 Jul;3(7):1115–1124. doi: 10.1210/mend-3-7-1115. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Chae H. Z., Rhee S. G., Exton J. H. Activation of the beta 1 isozyme of phospholipase C by alpha subunits of the Gq class of G proteins. Nature. 1991 Apr 11;350(6318):516–518. doi: 10.1038/350516a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. J., Smith J. A., Exton J. H. Purification from bovine liver membranes of a guanine nucleotide-dependent activator of phosphoinositide-specific phospholipase C. Immunologic identification as a novel G-protein alpha subunit. J Biol Chem. 1990 Oct 5;265(28):17150–17156. [PubMed] [Google Scholar]
- Tucker J., Sczakiel G., Feuerstein J., John J., Goody R. S., Wittinghofer A. Expression of p21 proteins in Escherichia coli and stereochemistry of the nucleotide-binding site. EMBO J. 1986 Jun;5(6):1351–1358. doi: 10.1002/j.1460-2075.1986.tb04366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka G., Eckstein F., Stryer L. Stereochemistry of the guanyl nucleotide binding site of transducin probed by phosphorothioate analogues of GTP and GDP. Biochemistry. 1985 Dec 31;24(27):8094–8101. doi: 10.1021/bi00348a039. [DOI] [PubMed] [Google Scholar]
- von zur Mühlen F., Eckstein F., Penner R. Guanosine 5'-[beta-thio]triphosphate selectively activates calcium signaling in mast cells. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):926–930. doi: 10.1073/pnas.88.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]


