Abstract
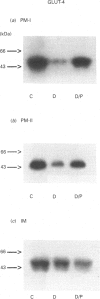
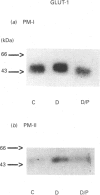
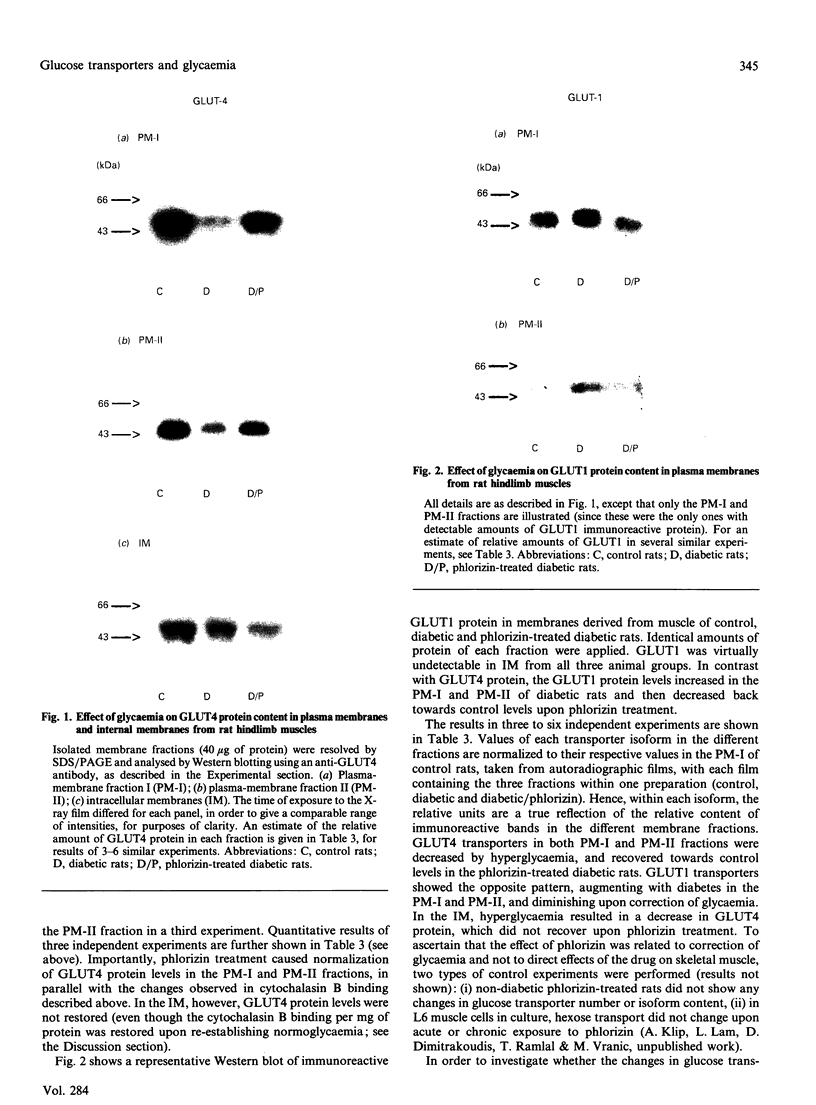
The number of glucose transporters was measured in isolated membranes from diabetic-rat skeletal muscle to determine the role of circulating blood glucose levels in the control of glucose uptake into skeletal muscle. Three experimental groups of animals were investigated in the post-absorptive state: normoglycaemic/normoinsulinaemic, hyperglycaemic/normoinsulinaemic and hyperglycaemic/normoinsulinaemic made normoglycaemic/normoinsulinaemic by phlorizin treatment. Hyperglycaemia caused a reversible decrease in total transporter number, as measured by cytochalasin B binding, in both plasma membranes and internal membranes of skeletal muscle. Changes in GLUT4 glucose transporter protein mirrored changes in cytochalasin B binding in plasma membranes. However, there was no recovery of GLUT4 levels in intracellular membranes with correction of glycaemia. GLUT4 mRNA levels decreased with hyperglycaemia and recovered only partially with correction of glycaemia. Conversely, GLUT1 glucose transporters were only detectable in the plasma membranes; the levels of this protein varied directly with glycaemia, i.e. in the opposite direction to GLUT4 glucose transporters. This study demonstrates that hyperglycaemia, in the absence of hypoinsulinaemia, is capable of down-regulating the glucose transport system in skeletal muscle, the major site of peripheral resistance to insulin-stimulated glucose transport in diabetes. Furthermore, correction of hyperglycaemia causes a complete restoration of the transport system in the basal state (determined by the transporter number in the plasma membrane), but possibly only an incomplete recovery of the transport system's ability to respond to insulin (since there is no recovery of GLUT4 levels in the intracellular membrane insulin-responsive transporter pool). Finally, the effect of hyperglycaemia is specific for glucose transporter isoforms, with GLUT1 and GLUT4 proteins varying respectively in parallel and opposite directions to levels of glycaemia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Charron M. J., Brosius F. C., 3rd, Alper S. L., Lodish H. F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2535–2539. doi: 10.1073/pnas.86.8.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron M. J., Kahn B. B. Divergent molecular mechanisms for insulin-resistant glucose transport in muscle and adipose cells in vivo. J Biol Chem. 1990 May 15;265(14):7994–8000. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cusin I., Terrettaz J., Rohner-Jeanrenaud F., Zarjevski N., Assimacopoulos-Jeannet F., Jeanrenaud B. Hyperinsulinemia increases the amount of GLUT4 mRNA in white adipose tissue and decreases that of muscles: a clue for increased fat depot and insulin resistance. Endocrinology. 1990 Dec;127(6):3246–3248. doi: 10.1210/endo-127-6-3246. [DOI] [PubMed] [Google Scholar]
- Douen A. G., Burdett E., Ramlal T., Rastogi S., Vranic M., Klip A. Characterization of glucose transporter-enriched membranes from rat skeletal muscle: assessment of endothelial cell contamination and presence of sarcoplasmic reticulum and transverse tubules. Endocrinology. 1991 Jan;128(1):611–616. doi: 10.1210/endo-128-1-611. [DOI] [PubMed] [Google Scholar]
- Douen A. G., Ramlal T., Klip A., Young D. A., Cartee G. D., Holloszy J. O. Exercise-induced increase in glucose transporters in plasma membranes of rat skeletal muscle. Endocrinology. 1989 Jan;124(1):449–454. doi: 10.1210/endo-124-1-449. [DOI] [PubMed] [Google Scholar]
- Douen A. G., Ramlal T., Rastogi S., Bilan P. J., Cartee G. D., Vranic M., Holloszy J. O., Klip A. Exercise induces recruitment of the "insulin-responsive glucose transporter". Evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem. 1990 Aug 15;265(23):13427–13430. [PubMed] [Google Scholar]
- Flier J. S., Mueckler M., McCall A. L., Lodish H. F. Distribution of glucose transporter messenger RNA transcripts in tissues of rat and man. J Clin Invest. 1987 Feb;79(2):657–661. doi: 10.1172/JCI112864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Fushiki T., Wells J. A., Tapscott E. B., Dohm G. L. Changes in glucose transporters in muscle in response to exercise. Am J Physiol. 1989 May;256(5 Pt 1):E580–E587. doi: 10.1152/ajpendo.1989.256.5.E580. [DOI] [PubMed] [Google Scholar]
- Gauthier C., Vranic M., Hetenyi G., Jr Importance of glucagon in regulatory rather than emergency responses to hypoglycemia. Am J Physiol. 1980 Feb;238(2):E131–E140. doi: 10.1152/ajpendo.1980.238.2.E131. [DOI] [PubMed] [Google Scholar]
- Harik S. I., Behmand R. A., Arafah B. M. Chronic hyperglycemia increases the density of glucose transporters in human erythrocyte membranes. J Clin Endocrinol Metab. 1991 Apr;72(4):814–818. doi: 10.1210/jcem-72-4-814. [DOI] [PubMed] [Google Scholar]
- Hetenyi G., Jr, Gauthier C., Byers M., Vranic M. Phlorizin-induced normoglycemia partially restores glucoregulation in diabetic dogs. Am J Physiol. 1989 Feb;256(2 Pt 1):E277–E283. doi: 10.1152/ajpendo.1989.256.2.E277. [DOI] [PubMed] [Google Scholar]
- Hirshman M. F., Goodyear L. J., Wardzala L. J., Horton E. D., Horton E. S. Identification of an intracellular pool of glucose transporters from basal and insulin-stimulated rat skeletal muscle. J Biol Chem. 1990 Jan 15;265(2):987–991. [PubMed] [Google Scholar]
- Hirshman M. F., Wallberg-Henriksson H., Wardzala L. J., Horton E. D., Horton E. S. Acute exercise increases the number of plasma membrane glucose transporters in rat skeletal muscle. FEBS Lett. 1988 Oct 10;238(2):235–239. doi: 10.1016/0014-5793(88)80486-1. [DOI] [PubMed] [Google Scholar]
- Ho R. J. Radiochemical assay of long-chain fatty acids using 63Ni as tracer. Anal Biochem. 1970 Jul;36(1):105–113. doi: 10.1016/0003-2697(70)90337-4. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Flier J. S. Regulation of glucose-transporter gene expression in vitro and in vivo. Diabetes Care. 1990 Jun;13(6):548–564. doi: 10.2337/diacare.13.6.548. [DOI] [PubMed] [Google Scholar]
- Karnieli E., Armoni M., Cohen P., Kanter Y., Rafaeloff R. Reversal of insulin resistance in diabetic rat adipocytes by insulin therapy. Restoration of pool of glucose transporters and enhancement of glucose-transport activity. Diabetes. 1987 Aug;36(8):925–931. doi: 10.2337/diab.36.8.925. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Bilan P. J., Cartee G. D., Gulve E. A., Holloszy J. O. Recruitment of GLUT-4 glucose transporters by insulin in diabetic rat skeletal muscle. Biochem Biophys Res Commun. 1990 Oct 30;172(2):728–736. doi: 10.1016/0006-291x(90)90735-6. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T., Young D. A., Holloszy J. O. Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 1987 Nov 16;224(1):224–230. doi: 10.1016/0014-5793(87)80452-0. [DOI] [PubMed] [Google Scholar]
- Klip A., Walker D., Ransome K. J., Schroer D. W., Lienhard G. E. Identification of the glucose transporter in rat skeletal muscle. Arch Biochem Biophys. 1983 Oct 1;226(1):198–205. doi: 10.1016/0003-9861(83)90285-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine R. Cell membrane as a primary site of insulin action. Fed Proc. 1965 Sep-Oct;24(5):1071–1073. [PubMed] [Google Scholar]
- Lussier B., Vranic M., Kovacevic N., Hetenyi G., Jr Glucoregulation in alloxan-diabetic dogs. Metabolism. 1986 Jan;35(1):18–24. doi: 10.1016/0026-0495(86)90090-9. [DOI] [PubMed] [Google Scholar]
- Nesher R., Karl I. E., Kipnis D. M. Dissociation of effects of insulin and contraction on glucose transport in rat epitrochlearis muscle. Am J Physiol. 1985 Sep;249(3 Pt 1):C226–C232. doi: 10.1152/ajpcell.1985.249.3.C226. [DOI] [PubMed] [Google Scholar]
- Ramlal T., Rastogi S., Vranic M., Klip A. Decrease in glucose transporter number in skeletal muscle of mildly diabetic (streptozotocin-treated) rats. Endocrinology. 1989 Aug;125(2):890–897. doi: 10.1210/endo-125-2-890. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Hansen B. F., Hansen S. A. Glucose-induced insulin resistance of skeletal-muscle glucose transport and uptake. Biochem J. 1988 Jun 15;252(3):733–737. doi: 10.1042/bj2520733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G. I., Papachristou D., DeFronzo R. A. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987 May;79(5):1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivitz W. I., DeSautel S. L., Kayano T., Bell G. I., Pessin J. E. Regulation of glucose transporter messenger RNA levels in rat adipose tissue by insulin. Mol Endocrinol. 1990 Apr;4(4):583–588. doi: 10.1210/mend-4-4-583. [DOI] [PubMed] [Google Scholar]
- Song C. S., Bodansky O. Subcellular localization and properties of 5'-nucleotidase in the rat liver. J Biol Chem. 1967 Feb 25;242(4):694–699. [PubMed] [Google Scholar]
- Starke A., Grundy S., McGarry J. D., Unger R. H. Correction of hyperglycemia with phloridzin restores the glucagon response to glucose in insulin-deficient dogs: implications for human diabetes. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1544–1546. doi: 10.1073/pnas.82.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Flier J. S., Lodish H. F., Kahn B. B. Differential regulation of two glucose transporters in rat liver by fasting and refeeding and by diabetes and insulin treatment. Diabetes. 1990 Jun;39(6):712–719. doi: 10.2337/diab.39.6.712. [DOI] [PubMed] [Google Scholar]
- Wallberg-Henriksson H. Glucose transport into skeletal muscle. Influence of contractile activity, insulin, catecholamines and diabetes mellitus. Acta Physiol Scand Suppl. 1987;564:1–80. [PubMed] [Google Scholar]
- Wallberg-Henriksson H., Zetan N., Henriksson J. Reversibility of decreased insulin-stimulated glucose transport capacity in diabetic muscle with in vitro incubation. Insulin is not required. J Biol Chem. 1987 Jun 5;262(16):7665–7671. [PubMed] [Google Scholar]