Summary:
Peripheral perfusion in large anterolateral thigh flaps may be inadequate if perforator zones are not properly planned during flap design and harvest, and variations in vascular anatomy can contribute to operative difficulty and morbidity. Intraflap anastomosis of extrinsic perforators may allow for augmentation of perfusion while avoiding significant intramuscular dissection. Adaptation of the perforator exchange technique, previously described in autologous breast reconstruction, optimizes vascular flow in anterolateral thigh flaps. Here, we present a technique for intraflap perforator anastomosis (the thigh perforator exchange) and illustrate its use in a subset of patients. This technique is relatively simple and rapid to perform with no vascular complications observed in our series.
Takeaways
Question: Is there a technique to augment perfusion in anterolateral thigh (ALT) free flaps when perforator anatomy is suboptimal, predisposing to partial flap loss?
Findings: Thigh perforator exchange (TPEX) can be used in select cases to optimize flap perfusion by performing an intraflap perforator anastomosis. Despite the theoretical risk of thrombosis associated with the additional anastomosis, no patient in our series required a return to the operating room and all flaps survived without partial or total flap loss.
Meaning: TPEX may be an effective technique to minimize flap loss for ALT flaps with suboptimal vascular anatomy.
INTRODUCTION
Large tissue defects require recruitment of comparable tissue volumes for adequate reconstruction. The anterolateral thigh (ALT) flap has become a reconstructive workhorse for its long vascular pedicle, large skin paddle, and low donor site morbidity.1 However, variations in vascular anatomy can contribute to operative difficulty and morbidity.2 In large flap designs, distal perfusion may be inadequate if perforator zones are not planned properly. Although incorporation of additional perforators may alleviate this problem, this may result in significant intramuscular dissection and increased donor site morbidity. In these cases, the authors have adopted the perforator exchange technique, previously described in autologous breast reconstruction, to optimize vascular flow in ALT flaps. Here, we present a technique for intraflap perforator anastomosis [the thigh perforator exchange (TPEX)] in a series of seven patients to illustrate its utility.
OPERATIVE TECHNIQUE
It is the preference of the authors to routinely obtain preoperative bilateral lower extremity computed tomography angiography (CTA) scans to guide selection of the ALT donor site and target perforators. In the event that the preoperative CTA suggests that the dominant perforator is proximal and short, the decision to perform a TPEX can be made preoperatively. Flap dissection proceeds in a standard fashion with a subfascial anterior incision and subsequent elevation to explore for the selected perforators.3 Although the decision to perform a TPEX can be made preoperatively based on CTA findings, the decision is more commonly made intraoperatively based on the size of the final flap design and the course of encountered perforators.
Although dissection of the main pedicle is performed, care is taken to maintain length on larger muscular branches to allow for anastomosis in the event that a TPEX is indicated. Once the decision to perform a TPEX is made, a second perforator is isolated and divided. The selected perforator is then transposed and anastomosed to a branch from the main pedicle to augment perfusion (Figs. 1 and 2).
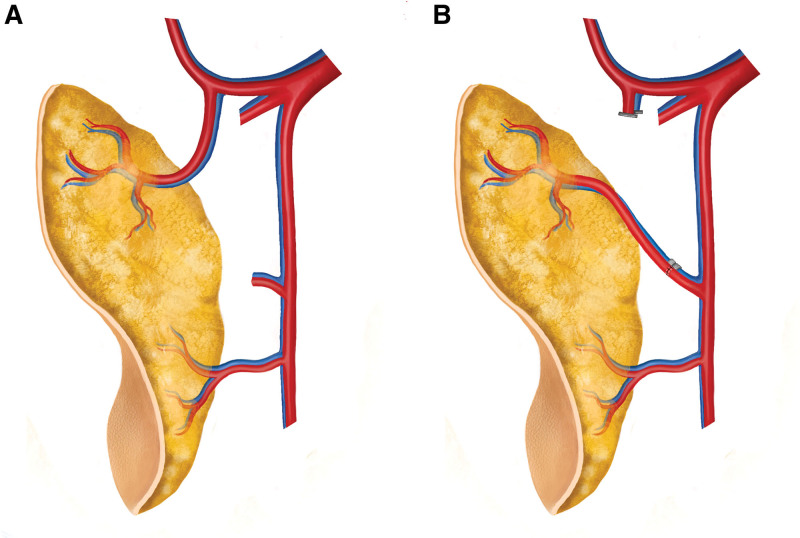
Fig. 1.
Schematic diagram illustrating the TPEX technique in an anterolateral thigh flap. A, Native anatomic configuration for which TPEX may be indicated. A dominant proximal perforator is found arising from the ascending branch of the lateral femoral circumflex artery (typically a TFL perforator). B, Perforator exchange involving rerouting of the ascending branch perforator and its vena comitans (via intraflap anastomosis) to a distal branch of the flap pedicle (the descending branch of the lateral femoral circumflex vessels).
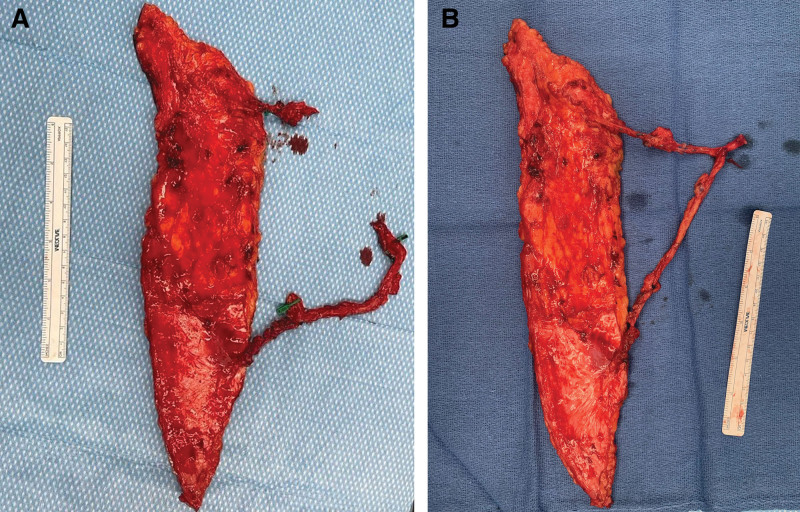
Fig. 2.
Intraoperative photographs exhibiting an anterolateral thigh free flap thigh perforator exchange (TPEX). A, An 8 × 30 cm (240 cm2) anterolateral thigh free flap demonstrating distinct eccentric perforators at the proximal and distal aspects of the flap. B, Following intra-flap anastomosis between the proximal perforator to a branch of the distal main pedicle. The flap is now ready for inset and anastomosis of the engineered pedicle to a vessel at the recipient site.
CASE PRESENTATION
A 35-year-old man presented with right Gustilo 3B tibia and fibula fractures following a motorcycle crash with a resultant large soft tissue deficit. Following bony fixation and temporization with negative pressure wound therapy, a free ALT flap was selected for coverage. CTA revealed adequate cutaneous perforators as well as sufficient recipient vessels. A 35 × 12 cm flap was planned.
Intraoperatively, flap elevation revealed three small perforators originating from the descending branch of the lateral femoral circumflex artery. Additionally, a large perforator supplying the tensor fasciae latae (TFL) was identified proximally, but it did not originate from the same vascular pedicle. Given the large dimensions of the flap and the size of the TFL perforator, the decision was made to preserve this additional perforator and perform a TPEX. The proximal perforator was dissected to length and divided while the other three perforators remained intact to the main vascular pedicle. The TFL perforator and vena comitans were anastomosed to a large muscular branch on the main pedicle with handsewn 9-0 nylon simple interrupted suture and a 2.0 mm venous coupler for arterial and venous anastomoses, respectively. After confirming vessel patency, the flap was transferred to the recipient site for anastomosis to the posterior tibial vessels. The patient recovered appropriately with no postoperative complications throughout the remaining hospital admission.
RESULTS
The results of this technique are summarized in Table 1. Four patients underwent ALT reconstruction secondary to cancer extirpation, while in three patients, it was due to trauma. The rationale for TPEX utilization in all patients was due to the large flap size and eccentric perforators. Among the six flaps with documented dimensions, the average flap area was 252 cm2. Notably, there were no total or partial flap losses. Although an additional anastomosis may theoretically increase the risk of thrombosis, no vascular complications were observed, and no patients required a return to the operating room due to vascular compromise. Three patients experienced minor superficial dehiscence at the recipient site.
Table 1.
Patients Undergoing TPEX in Anterolateral Thigh Free Flaps (n = 7)
| Age (y)/Sex | Defect Etiology | ALT Flap Size (cm, cm2) | Total Perforators | Complications |
|---|---|---|---|---|
| 54/M | H&N SCC | 16 × 9 (144) | 2 | Superficial dehiscence |
| 63/F | H&N SCC | 25 × 8 (200) | 2 | None |
| 55/M | H&N SCC | 32 × 8 (256) | 2 | Superficial dehiscence |
| 57/M | H&N SCC | Not documented | 2 | None |
| 61/F | LE Trauma | 25 × 10 (250) | 2 | None |
| 35/M | LE Trauma | 35 × 12 (420) | 4 | None |
| 37/M | UE Trauma | 8 × 30 (240) | 2 | Superficial dehiscence |
F, female; H&N, head and neck; LE, lower extremity; M, male; SCC, squamous cell carcinoma; UE, upper extremity.
DISCUSSION
The concept of microsurgical engineering, in which the native vasculature is reorganized within and between flaps, has been previously described for use in both head and neck and autologous breast reconstruction to mitigate donor site morbidity and to optimize flap perfusion.4,5 Here, the use of intraflap perforator exchange in ALT flaps, also referred to as turbocharging by some authors, mimics the abdominal perforator exchange (APEX) technique for deep inferior epigastric perforator flaps.4,6,7 APEX was developed to optimize both flap perfusion and to maintain abdominal wall integrity. In some deep inferior epigastric perforator patients, dissection of multiple perforators may violate interposed rectus abdominus muscle. To avoid this, select perforators can be independently divided and re-anastomosed to the main pedicle. APEX thereby “exchanges” the native vasculature into a workable configuration; vascular continuity is deconstructed and restored while muscular continuity is preserved.4,8 Similarly, this technique seeks to maximize perfusion in flaps with perceived tissue volume-perforasome mismatch. This occurs when a flap is too large to be adequately supplied by a single perforator, when the primary perforator is too small or eccentric, or when a significant degree of intramuscular dissection would be required to preserve secondary perforators.
Vascular studies of ALT flaps have demonstrated that one to four usable perforators typically emerge between the anterior superior iliac spine and the lateral patellar border.9 However, significant discrepancies in perforator course (septocutaneous, musculoseptocutaneous, musculocutaneous) or origin may complicate the intraoperative dissection. Although most commonly sourced from the dLCFA, perforators may arise from the ascending, transverse, or oblique branches, or superficial or profunda femoris arteries.2 Dissection of multiple pedicles may be tedious, particularly if long intramuscular courses are present, and may increase operative time and morbidity.
The relationship between flap size and perforator count among ALT flaps remains a topic of continuing investigation.10 The inclusion of additional perforators to improve flap perfusion has been suggested as a means for avoiding partial necrosis, with one recent study recommending multiple cutaneous perforators whenever possible.10 ALT flaps supplied by a single perforator have been reported as large as 250 to 650 cm2.10–12 Nevertheless, surgeons may minimize flap size when only a single cutaneous perforator is available.
CONCLUSIONS
TPEX is a technique that optimizes the perfusion of larger ALT flaps when length or location of the dominant perforator is a concern with a negligible increase in operative time. Future studies examining flap flow dynamics with TPEX should be considered.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOWLEDGMENTS
Dr. Stalder was a faculty member with Louisiana State University Health Sciences Center, Division of Plastic Surgery throughout the care of all patients included in this article as well as during project development and data collection. He has since transitioned into the community during writing of the final article.
Footnotes
Published online 16 August 2024.
Disclosure statements are at the end of this article, following the correspondence information.
REFERENCES
- 1.Collins J, Ayeni O, Thoma A. A systematic review of anterolateral thigh flap donor site morbidity. Can J Plast Surg. 2012;20:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LakhianiI C, Lee MR, Saint-Cyr M. Vascular anatomy of the anterolateral thigh flap: a systematic review. Plast Reconstr Surg. 2012;130:1254–1268. [DOI] [PubMed] [Google Scholar]
- 3.Koshima I, Fukuda H, Yamamoto H, et al. Free anterolateral thigh flaps for reconstruction of head and neck defects. Plast Reconstr Surg. 1993;92:421–428; discussion 429. [PubMed] [Google Scholar]
- 4.Dellacroce FJ, Dellacroce HC, Blum CA, et al. Myth-busting the DIEP flap and an introduction to the abdominal perforator exchange (APEX) breast reconstruction technique: a single-surgeon retrospective review. Plast Reconstr Surg. 2019;143:992–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wisecarver I, Mundinger G, Tarakji M, et al. Microsurgical engineering: Bilateral deep inferior epigastric artery perforator flap with flow-through intraflap anastomosis. Plast Reconstr Surg Glob Open. 2018;6:e1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeong S, Koo D, Moon K, et al. The turbocharged wide anterolateral thigh perforator flap to reconstruct massive soft tissue defects in traumatized lower extremities: a case series. Front Surg. 2022;9:991094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YJ, Lee YJ, Oh DY, et al. Reconstruction of wide soft tissue defects with extended anterolateral thigh perforator flap turbocharged technique with anteromedial thigh perforator. Microsurgery. 2019;40:440–446. [DOI] [PubMed] [Google Scholar]
- 8.Andejani D, AlThubaiti G. Intersection-splitting deep inferior epigastric perforator flap. Plast Reconstr Surg Glob Open. 2019;7:e2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Beule T, Van Deun W, Vranckx J, et al. Anatomical variations and pre-operative imaging technique concerning the anterolateral thigh flap: guiding the surgeon. Br J Radiol. 2016;89:20150920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang K, Tsai C, Chang C, et al. Comparison of flap outcomes between single‐ and multiple‐perforator‐based free anterolateral thigh flap in head and neck reconstruction. Microsurgery. 2019;39:150–155. [DOI] [PubMed] [Google Scholar]
- 11.Saint-Cyr M, Schaverien M, Wong C, et al. The extended anterolateral thigh flap: anatomical basis and clinical experience. Plast Reconstr Surg. 2009;123:1245–1255. [DOI] [PubMed] [Google Scholar]
- 12.Nojima K, Brown SA, Acikel C, et al. Defining vascular supply and territory of thinned perforator flaps: part I. anterolateral thigh perforator flap. Plast Reconstr Surg. 2005;116:182–193. [DOI] [PubMed] [Google Scholar]