Abstract
Objective:
Characterize the incidence, associated clinical factors, timing of infection, microbiology, and incidence of concordant blood culture of urinary tract infections (UTIs) in very low birth weight (VLBW, <1500g) infants.
Design:
Multicenter cohort study of VLBW infants with gestational age (GA) ≤32 weeks, still hospitalized on postnatal day 7, and discharged 2010–2018 from Pediatrix Medical Group neonatal intensive care units. Demographic and clinical characteristics of infants with and without UTI were compared. Multivariable logistic regression evaluated adjusted odds of UTI diagnosis.
Results:
Of 86,492 included infants, 5,988 (7%) had a UTI. The most common pathogen was Enterococcus spp. (20%), followed by Escherichia coli (19%) and Klebsiella spp. (18%). Candida spp. (6%) was the most common non-bacterial pathogen. Concordant positive blood culture was present in 8% of infants with a UTI diagnoses. UTI was associated with lower GA, male sex, vaginal delivery, prenatal steroid exposure, and longer length of hospitalization.
Conclusion:
UTI is a common cause of infection in VLBW infants, especially among the smallest, most premature, male infants, and those with longer length of hospitalization. Neonatal clinicians should consider obtaining urine culture in the setting of late-onset sepsis evaluations in VLBW infants.
Keywords: very low birth weight infants, urinary tract infection, small for gestational age, concordant blood culture
Urinary tract infection (UTI) is a common cause of late-onset sepsis (LOS) in very low birth weight infants (VLBW, birth weight <1500g) and is responsible for substantial morbidity in this population.1 The signs of UTI in VLBW infants are non-specific, including increased apnea, bradycardia and desaturation episodes, feeding intolerance, temperature instability, and lethargy.2–4 Given the non-specific signs, awareness of associated clinical factors may raise clinical suspicion and accelerate timely diagnosis and treatment. Clinical decompensation secondary to UTI in VLBW infants may result in need for increased respiratory support, interruption of enteral feeding, and escalation of other medical therapies. UTI can also coincide with bacteremia and necrotizing enterocolitis (NEC) in this population.5,6 UTI may lead to sepsis, and scarring can lead to renal damage, which in turn may result in renal insufficiency, proteinuria, and hypertension later in life.7 UTI in premature infants may further compound increased risk for renal impairment in the setting of postnatal nephrogenesis, exposure to nephrotoxic medications, and hyperoxia.8 A recent study of 18 premature infants born at mean gestational age (GA) 29.4 weeks with a single episode of UTI and structurally normal kidneys showed no increased risk for recurrent UTI or kidney disease in the first two decades of life; however, this pilot study was limited by small sample size.9 In older infants in the outpatient setting, UTI may be a harbinger of major urinary tract anatomical abnormalities; however, severe structural anomalies requiring further treatment are present in less than 5% of VLBW infants with UTIs.10
The incidence of UTI in VLBW infants is reported to be between 3–8.5%11,12; however, estimates vary widely in published literature.13 Earlier studies have shown a higher UTI incidence with lower birth weight, lower GA, and presence of renal anomalies.11,12 Male VLBW infants appear to be at higher risk compared to females.13 Other reported associated factors for UTI in VLBW infants include mechanical ventilation, central line access, older postnatal age, and duration of hospitalization.11,12,14 Prior studies of the epidemiology of UTI in VLBW infants are limited to single-center studies and relatively small sample sizes. Despite the higher incidence of UTI in premature infants, multicenter studies are lacking and are critical to standardize guidelines for diagnosis, treatment, and management in the VLBW population. Our objective was to evaluate the incidence, microbiology, and associated clinical factors of UTI in a large, multicenter cohort of VLBW infants to inform risk stratification and management of UTI in this population. Our hypothesis was that lower birthweight, lower GA, male sex, higher severity of initial illness (with 5-minute Apgar score as a marker), and vaginal delivery would be associated with increased risk of UTI.
Methods
Study design and data source
We conducted a multicenter cohort study of infants discharged from the neonatal intensive care unit (NICU) from 2010 to 2018. Deidentified data were obtained from the Pediatrix Clinical Data Warehouse (Sunrise, FL USA), a multicenter clinical database including patients from nearly 400 NICU sites in 35 states and Puerto Rico. NICUs included in the Pediatrix Clinical Data Warehouse are community and academic centers throughout North America and ranged from Level I through IV. Clinicians in these NICUs generate infant data daily including admission history and physicals, daily progress notes, and discharge summaries with demographics, administered medications, laboratory results, microbiologic culture results, limited dosing information, and diagnoses available. From the daily notes, data are extracted and consolidated into the Pediatrix Clinical Data Warehouse.15 Infants with birth weight ≤1500g, gestational age ≤32 weeks, and still hospitalized on postnatal day 7 were included. In order to focus this analysis on typical VLBW infants, those with severe and uncommon urinary tract abnormalities including diagnosis of neurogenic bladder, cystic kidney disease, bilateral renal agenesis, horseshoe kidney, ectopic kidney, renal dysplasia, renal hypoplasia, multicystic dysplastic kidney, megaureter, ureter duplication, ureter agenesis, ectopic ureter, ureteropelvic junction obstruction, congenital mesoblastic nephroma, exstrophy of urinary bladder, and Prune Belly syndrome were excluded. Infants diagnosed with hydronephrosis or pelviectasis were not excluded. We extracted information on prenatal characteristics, demographics, exposure to medications and interventions while in the hospital, and in-hospital clinical outcomes. Permission to conduct this analysis was provided by the Duke University Institutional Review Board (Durham, NC USA).
Definitions and participants
The following data were collected from birth until discharge or death: GA at birth; exposure to prenatal steroids; Apgar score at 5 minutes of life; sex; birth weight; small for GA (SGA) status; mode of delivery; postnatal age (PNA) at time of positive urine culture; microbiology of urine culture; and microbiology of blood cultures drawn within 3 days of UTI. UTI was defined as isolation of a single pathogenic organism from urine culture collected on or after postnatal day 3. Urine cultures with isolation of more than one organism were considered a contaminant. Multiple positive urine cultures for the same organism within 21 days were considered collectively as a single UTI. Biochemical urine studies, such as urinalysis, were not available for analysis. Method of urine culture collection and colony counts of positive urine cultures were not available for analysis. We excluded UTI occurrences for organisms typically considered contaminants, including non-speciated Streptococci, Bacillus spp., Corynebacterium spp., Gram-positive rods (not including Listeria sp.), Lactobacillus spp., Micrococcus spp., Stomatococcus spp., and Bacteroides spp. Coagulase-negative Staphylococcus (CoNS) infections were determined to be definite, probable, or possible.16 Definite and probable blood and urine CoNS infections were used in the analysis. We defined a definite CoNS infection as 2 positive cultures drawn on the same day; probable CoNS infection as 2 positive cultures within a 4-day period, 3 positive cultures within a 7-day period, or 4 positive cultures within a 10-day period; and possible CoNS infection as a culture positive for CoNS that did not meet criteria for definite or probable CoNS infection. Concordant blood culture was defined as isolation of the same pathogenic organism in blood within 3 days before or after collection of positive urine culture. SGA status was defined as birth weight less than tenth percentile for GA as determined by Olsen growth chart.17
Statistical analysis
Summary statistics for the variables of interest, including sex, GA, birth weight, prenatal steroid exposure, SGA status, 5-minute Apgar score, mode of delivery, and length of hospitalization were calculated among infants who were diagnosed with at least one UTI, those with at least one urine culture obtained without a UTI, and those without a urine culture obtained during hospitalization. Microbiologic pathogens of first positive urine cultures were tabulated. Postnatal age at first positive urine culture was described. We calculated the incidence of concordant positive blood culture within 3 days of positive urine culture. To evaluate differences between groups, Chi-square tests were used for categorical variables, and Wilcoxon rank sum tests were used for continuous variables. The unadjusted odds of UTI diagnosis were calculated for all variables of interest, including birth weight, GA, 5-minute Apgar, SGA status, sex, prenatal steroid exposure, and mode of delivery. Multivariable logistic regression was used to evaluate the adjusted odds of UTI diagnosis among all infants in the cohort, regardless of whether a urine culture was obtained during hospitalization. All significant variables in univariable analysis were considered for inclusion in the multivariable model; site was included as a random effect. Birth weight and GA were collinear in the multivariable logistic regression; therefore, only GA was used in the multivariable logistic regression to avoid multicollinearity. Olsen growth curves for infants born at 22 weeks GA are not currently defined; therefore, SGA status was not used in the final multivariable model. Length of hospitalization in days was added to the multivariable model to account for variable lengths of hospitalization. Standard assumption diagnostics were conducted prior to selection of the final model. From the final multivariable model, odds ratios (ORs) with 95% confidence intervals (CIs) were determined. A second multivariable logistic regression was constructed using the same method as above and included only infants with at least one urine culture obtained during hospitalization. STATA 16.1 (College Station, TX) was used to perform the statistical analysis. A p<0.05 was considered statistically significant for all tests.
Results
The median GA of the entire cohort was 28 weeks (quarter 1 [Q1], quarter 3 [Q3]: 26, 30) with median birth weight of 1.078kg (Q1, Q3: 0.820, 1.300). Of 86,492 included infants (Figure 1), 5988 (7%) infants had a UTI (Table 1). The group with UTI had a higher proportion of males, lower GAs, and lower birth weights (Table 1). Positive urine cultures were obtained on median postnatal day 36 (Q1, Q3: 21, 60) (Figure 2). Enterococcus spp. was the most common pathogen (20%), followed by Escherichia coli (E. coli) (19%) and Klebsiella spp. (18%) (Table 3). Candida spp. (6%) was the most common non-bacterial pathogen. Regarding LOS evaluations during hospitalization, 25% of infants had urine and blood cultures, 2% of infants had urine but no blood culture, 24% of infants had blood but no urine culture, and 49% had neither obtained. Concordant positive blood culture was present in 8% of infants with UTI diagnosis (n=474). Six percent (300/5283) of infants with at least one UTI died, while 5% (3854/74,162) of infants without a UTI died before discharge. In the multivariable analysis of the entire cohort, infants ≤24 weeks GA had the highest risk of UTI (Table 2). Compared to infants 29–32 weeks GA, infants ≤24 weeks GA had an increased adjusted odds ratio (aOR) of UTI of 2.03 (95% CI 1.83–2.26) and infants 25–28 weeks GA had aOR 1.76 (95% CI 1.63–1.90). Other factors associated with UTI included male sex (aOR 2.53; 95% CI 2.38–2.70), vaginal delivery (aOR 1.12; 95% CI 1.05–1.20), prenatal steroid exposure (aOR 1.15; 95% CI 1.06–1.25), and longer length of hospitalization (aOR 1.02; 95% CI 1.02–1.02). A multivariable analysis including only infants with at least one urine culture obtained during hospitalization had similar results (Supplemental Material).
Figure 1. Flow Diagram.
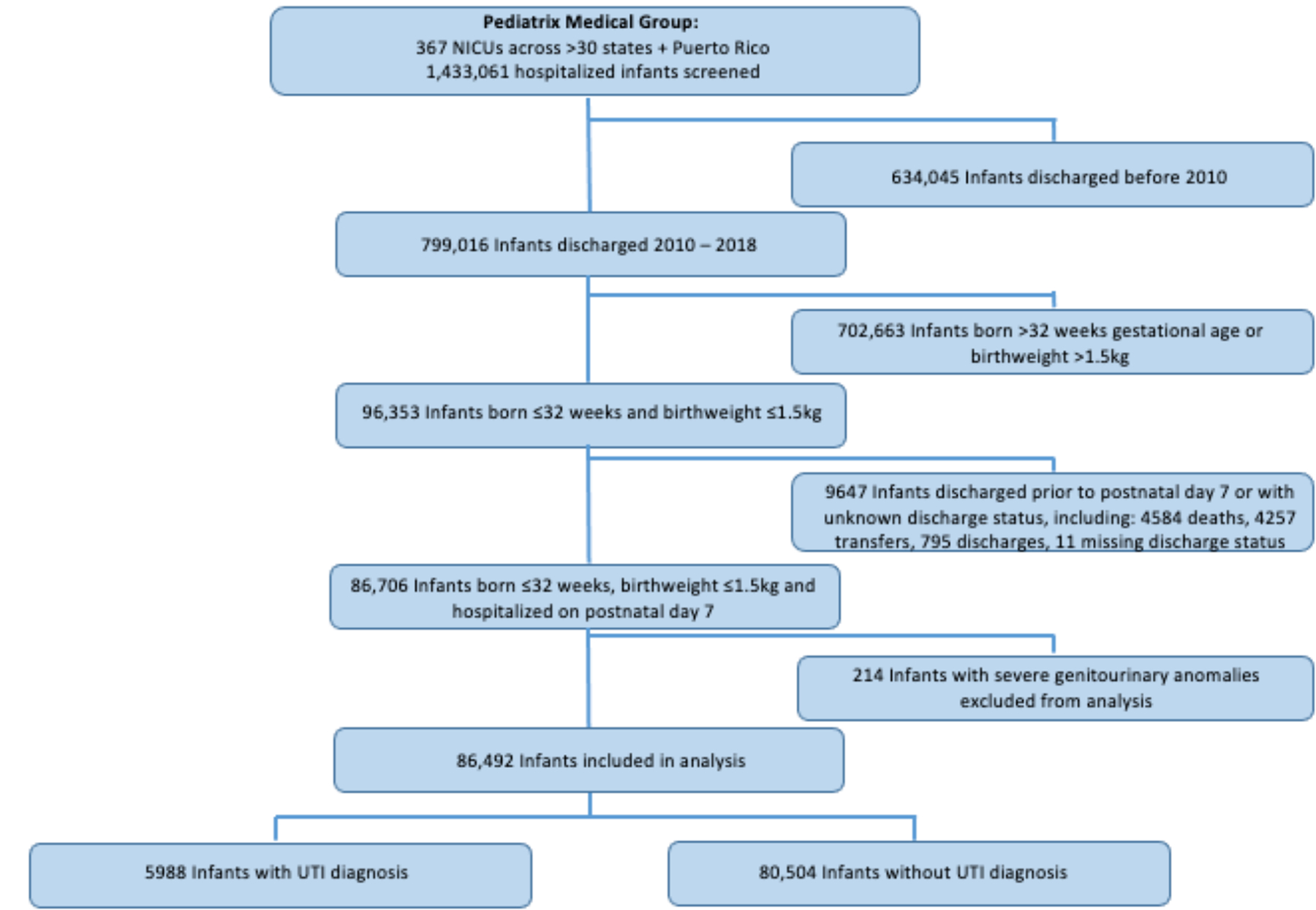
This figure displays a flow diagram of the initial study population, exclusions, and the final number of infants included in the analysis.
ELBW = extremely low birth weight; NICU = neonatal intensive care unit; UTI = urinary tract infection; VLBW = very low birth weight
Table 1.
Demographics and Clinical Characteristics of Infants with Positive Urine Culture, Negative Urine Culture, and No Urine Culture Collected during Hospitalization
| Characteristics | Positive Urine Culture(s) N=5988 (%) |
Negative Urine Culture(s) N=16,786 (%) |
No Urine Culture N=63,718 (%) |
|---|---|---|---|
| Male | 4190 (70) | 8637 (51) | 30,808 (48) |
| Gestational age (weeks) | |||
| ≤24 | 1294 (22) | 2733 (16) | 5153 (8) |
| 25–28 | 3427 (57) | 8931 (53) | 25,687 (40) |
| 29–32 | 1267 (21) | 5122 (31) | 32,878 (52) |
| Small for gestational age | 1020 (17) | 4062 (24) | 10,986 (17) |
| 5-minute Apgar score | |||
| 0–3 | 490 (8) | 1225 (7) | 2944 (5) |
| 4–6 | 1375 (24) | 3558 (22) | 10,081 (16) |
| 7–10 | 3978 (68) | 11,571 (71) | 48,920 (79) |
| Birth weight (grams) | |||
| <500 | 191 (3) | 631 (4) | 899 (1) |
| 500–999 | 3622 (60) | 9469 (56) | 21,352 (34) |
| 1,000–1500 | 2175 (36) | 6686 (40) | 41,467 (65) |
| Vaginal delivery | 1719 (29) | 4169 (25) | 15,335 (24) |
| Prenatal steroid exposure | 5001 (84) | 13,776 (82) | 53,415 (84) |
| Length of hospitalization (days), median (IQR) | 91 [68–120] | 78 [53–106] | 54 [39–76] |
UTI = Urinary Tract Infection; IQR = interquartile range
Figure 2. Postnatal Day of First Positive Urine Culture.
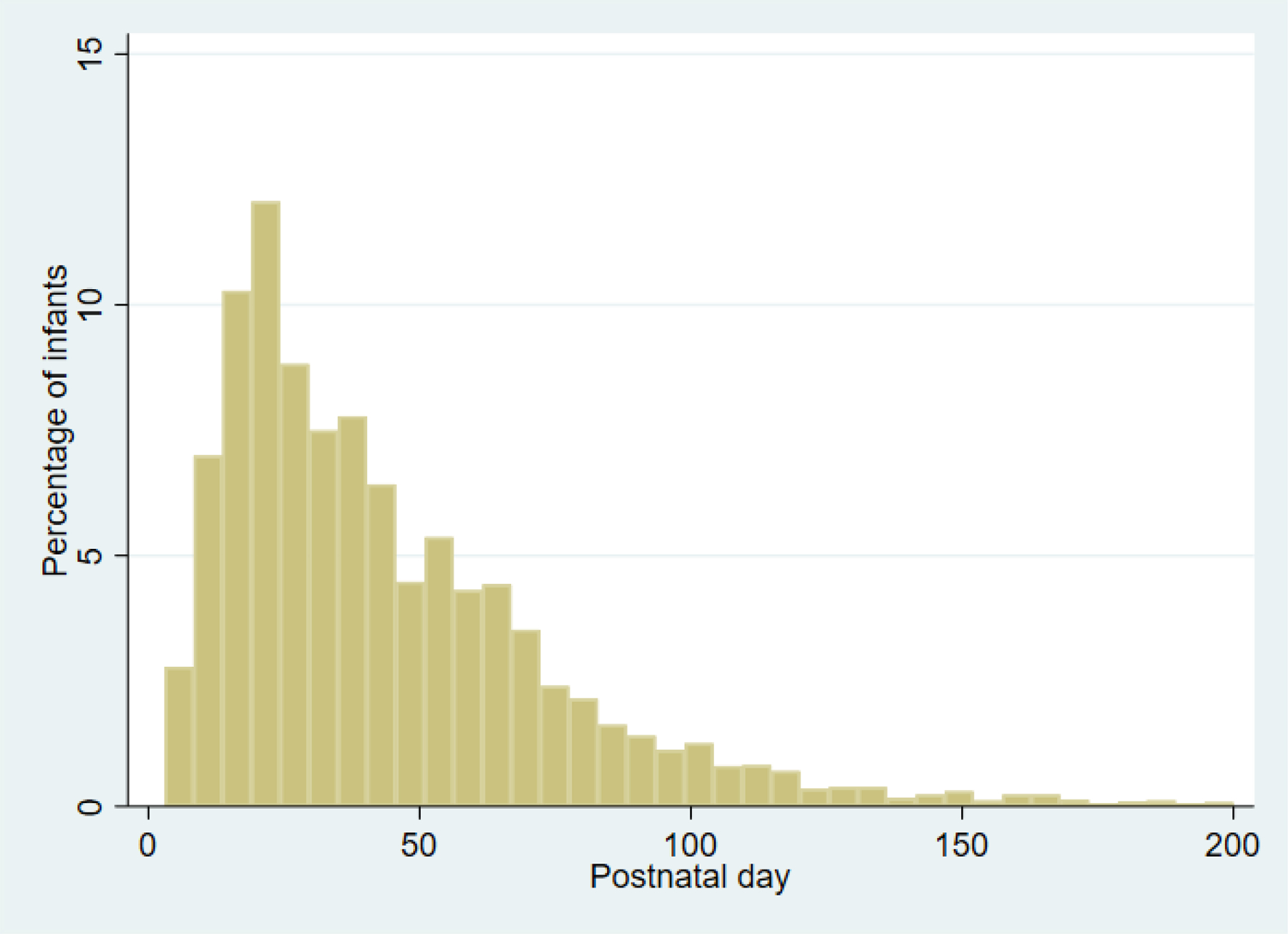
This figure displays the percentage of infants with first positive urine culture on which postnatal day. Positive urine cultures were most common in infants postnatal age 8–30 days.
Table 3.
Pathogens Causing First Urinary Tract Infection *
| Organism | Frequency | Percent |
|---|---|---|
| Enterococcus spp. | 1201 | 20 |
| Escherichia coli | 1167 | 19 |
| Klebsiella spp. | 1081 | 18 |
| Enterobacter | 723 | 12 |
| Candida spp. | 375 | 6 |
| Other | 265 | 4 |
| CoNS | 220 | 4 |
| Other GNR | 178 | 3 |
| GBS | 148 | 2 |
| Staphylococcus aureus | 140 | 2 |
| Citrobacter spp. | 138 | 2 |
| Serratia spp. | 136 | 2 |
| Proteus spp. | 110 | 2 |
| Pseudomonas spp. | 84 | 1 |
All pathogens with percentage of cultures ≥1% are shown.
CoNS = Coagulase-negative Staphylococcus; GBS = group B Streptococcus; GNR = gram-negative rods, not otherwise specified
Table 2.
Multivariable Logistic Regression Analysis of Demographic and Clinical Characteristics associated with UTI among All Infants in the Cohort
| Characteristics | OR (95% CI) | p-value |
|---|---|---|
| Sex | ||
| Female | (Reference) | — |
| Male | 2.53 (2.38–2.70) | <0.001 |
| Gestational age (weeks) | ||
| ≤24 | 2.03 (1.83–2.26) | <0.001 |
| 25–28 | 1.76 (1.63–1.90) | <0.001 |
| 29–32 | (Reference) | — |
| 5-minute Apgar Score | ||
| 0–3 | 1.09 (0.98–1.23) | 0.13 |
| 4–6 | 1.08 (1.00–1.16) | 0.05 |
| 7–10 | (Reference) | — |
| Vaginal delivery | 1.12 (1.05–1.20) | 0.001 |
| Prenatal steroids | 1.15 (1.06–1.25) | 0.001 |
| Length of hospitalization (days) | 1.02 (1.02–1.02) | <0.001 |
CI = confidence interval; OR = odds ratio; UTI = urinary tract infection
Discussion
In this large cohort study of VLBW infants, UTI was associated with lower GA, male sex, vaginal delivery, prenatal steroid exposure, and longer length of hospitalization. Seven percent of infants had a UTI diagnosis, which is similar to published literature, with incidences of UTI in VLBW infants ranging from 3.2% to 8.5%.2,4,12,13 This seven percent is the same as the prevalence of UTI in febrile infants in the general population.18 The relatively high incidence of UTI in VLBW infants highlights the importance of obtaining urine cultures in LOS evaluations in this population. Timing of UTI in our cohort, with highest incidence postnatal days 8 to 30, is similar to findings in other previous studies.2,4,6,19
Enterococcus spp., E. coli, Klebsiella spp., and Enterobacter spp. were the most common cause pathogens grown in urine culture in this cohort. Other studies have also frequently noted Gram negative pathogens as the most common pathogens in the premature infant population. In a single-center study of 1127 VLBW infants with 60 UTIs identified between 1995–2003, Klebsiella spp. was the most common at 43% of positive urine cultures.4 Klebsiella spp. was also the most common pathogen in a single-center study of 762 VLBW infants with 66 UTIs identified between 1990–200113 and another single-center study of 232 VLBW infants with 32 UTIs identified between 2005–2015.12 Other studies have shown E. coli to be the most common pathogen, including a single-center study of 572 VBLW infants with 20 UTIs identified conducted between 2004–2006.2 The rate of Enterococcus spp. positive urine cultures in this cohort was similar to previous studies completed in premature infants, which showed Enterococcus spp. growth in 7.9 to 17.9% of positive urine cultures.6,10–12,14,19 Enterococcus spp. appears to be more common in the hospitalized premature population compared to older children in the outpatient setting.20 This may in part be due to the preponderance of males with UTIs in the VLBW population, as Enterococcus spp. appears to be more common in male infants and children of all age groups compared to females.20 However, E. coli remains the most common UTI pathogen in male children overall.20 It is possible that some Enterococcus spp. positive cultures were not true UTI, as non-catheterized urine specimens included in the analysis may have contributed to contaminated urine cultures. Our study showed a relatively low incidence of coagulase-negative Staphylococcus (CoNS) at 4% of positive urine cultures, whereas other studies of UTIs in infants in the NICU showed CoNS in 10% to 28% of positive urine cultures.2,5,10,19,21 This relatively low incidence of CoNS in this cohort may be due to including only definite and probable CoNS infections, while excluding possible CoNS infections. The rate of Candida spp. positive urine cultures in this cohort is similar to other studies of UTIs in preterm infants with percentages ranging from 7.3–15%.5,13,22 The choice of empiric coverage should be based on local susceptibility patterns for the most common pathogens outlined in this study. Understanding the spectrum of pathogens causing UTI in VLBW is necessary to ensure proper antimicrobial coverage, especially in the setting of increasing proportion of antibiotic-resistant organisms causing infections in the NICU.23–25
Concordant positive blood culture in 8% of infants with a UTI diagnoses in this cohort is similar to prior studies, with the incidence of concordant positive blood cultures ranging from 8.8–13%.4,5,13 The incidence of concordant positive blood cultures has been reported as high as 38% in a retrospective single-center study of 189 VLBW infants, although the incidence of UTI 48/189 (25%) was high in that study.26 Concordant positive blood and urine cultures may have resulted from primary bacteremia with hematogenous seeding of the urinary system, or from primary UTI with resultant bacteremia.
This study identified several associated clinical factors for UTI, including lower GA, male sex, vaginal delivery, prenatal steroid exposure, and longer length of hospitalization. Lower GA and male sex have previously been shown to be associated with increased risk of UTI in smaller and single-center observation studies, which are confirmed here.13,14 Premature infants are at increased risk for many infections, including UTI, likely due to immature immune systems, along with increased need for invasive procedures. As GA is strongly associated with all causes of late onset sepsis, GA alone does not differentiate risk among UTI, bacteremia, and meningitis, although this association does reinforce the increased risk of infection in low GA infants. The OR for UTI in males compared to females in this cohort was 2.53 (95% CI 2.38–2.70); this finding is similar to a single-center study conducted 1995–2003 that included 1127 VLBW infants and showed OR 2.96 (95% CI 1.28–6.85).4 While reliable data regarding circumcision was not available in this dataset, given the low GAs examined in this study, very few male infants would have been eligible for circumcision at time of first UTI. Vaginal delivery was associated with increased odds of UTI compared to C-section, which may be due to exposure to vaginal bacterial flora during the birthing process. Prenatal steroid exposure was associated with increased odds for UTI compared to no prenatal steroid exposure, which has not been studied previously. While steroid exposure may alter immune responses,27,28 this increased risk of UTI in the setting of prenatal steroid exposure could be due to increased survival among infants with prenatal steroid exposure and, therefore, greater opportunity to develop a UTI. Longer length of hospitalization has been shown to be associated with UTI diagnosis, which is confirmed here.11,12 Infants with longer lengths of hospitalization have greater opportunity to develop and be diagnosed with a UTI, and likely have greater severity of illness, which may also be associated with increased UTI risk.
A strength of this cohort study is the large sample size derived from 367 community and academic NICUs across the United States. While the regression model examining associated clinical factors for UTI presented here includes all infants in the cohort regardless of whether urine culture was collected during hospitalization, a similar model including only infants with at least one urine culture collected during hospitalization had similar results (Supplemental Material).
This study has several limitations. There is likely misclassification of some positive urine isolates as UTI, since additional diagnostic criteria for UTI, including biochemical urine studies (e.g., urinalysis), were not analyzed and colony counts were not available for analysis. We expect that any differential misclassification due to our less specific (but not less sensitive) definition of UTI would bias OR’s in the direction of the null. Therefore, we may currently underestimate the factors associated with UTI in VLBW infants than would a more specific definition of UTI. Additionally, method of urine culture collection was not available for analysis; therefore, non-catheterized or indwelling catheter urine specimens included in the analysis may have contributed to contaminated urine cultures. There may be clinical and demographic factors associated with UTI in VLBW infants that were not examined in this study. Variations in clinical practice and unit policies on thresholds to obtain urine culture may have influenced our study, as UTIs may have gone undiagnosed if urine culture was not obtained.
Conclusion
In this large cohort study of VLBW infants, clinical factors associated with UTI diagnosis included lower GA, male sex, vaginal delivery, prenatal steroid exposure, and longer length of hospitalization. The most common pathogen was Enterococcus spp., followed by E. coli, Klebsiella spp., Enterobacter spp., and Candida spp. Concordant positive blood culture was present in 8% of these UTI diagnoses. UTI remains a common cause of LOS in VLBW infants, especially among the smallest, most premature, male infants, and those with greater severity of illness. Neonatal clinicians should consider obtaining urine culture in the setting of LOS evaluations in VLBW infants. Results from this study may increase awareness of UTI risk in this population and aid in risk stratification to improve diagnosis and outcomes of UTI in VLBW infants. Next steps should include study of preventative measures that may reduce the incidence of UTI in this population.
Supplementary Material
Sources of funding:
This work was funded under National Institute of Child Health and Human Development (NICHD) contract (HHSN275201 000003I) for the Pediatric Trials Network (PI Daniel K. Benjamin Jr.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by Duke Clinical Research Institute’s R25 Summer Training in Academic Research (STAR) Program (grant #5R25HD076475-09).
Conflict of interest disclosures:
RGG has received support from industry for research services (https://dcri.org/about-us/conflict-of-interest/). The authors have no other conflicts of interest relevant to this article to disclose.
Data availability statement:
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Mohseny AB, van Velze V, Steggerda SJ, Smits-Wintjens VEHJ, Bekker V, Lopriore E. Late-onset sepsis due to urinary tract infection in very preterm neonates is not uncommon. Eur J Pediatr, 2018;177:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke D, Gowrishankar M, Etches P, Lee BE, Robinson JL. Management and outcome of positive urine cultures in a neonatal intensive care unit. J Infect Public Health. 2010;3:152–8. [DOI] [PubMed] [Google Scholar]
- 3.Griffin MP, Lake DE, Bissonette EA, Harrell FE Jr., O’Shea TM, Moorman JR. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics. 2005;116:1070–4. [DOI] [PubMed] [Google Scholar]
- 4.Levy I, Comarsca J, Davidovits M, Klinger G, Sirota L, Linder N. Urinary tract infection in preterm infants: the protective role of breastfeeding. Pediatr Nephrol. 2009;24:527–31. [DOI] [PubMed] [Google Scholar]
- 5.Downey LC, Benjamin DK Jr., Clark RH, et al. Urinary tract infection concordance with positive blood and cerebrospinal fluid cultures in the neonatal intensive care unit. J Perinatol. 2013;33:302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pineda LC, Hornik CP, Seed PC, et al. Association between positive urine cultures and necrotizing enterocolitis in a large cohort of hospitalized infants. Early Hum Dev. 2015;91:583–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wennerström M, Hansson S, Jodal U, Stokland E. Primary and acquired renal scarring in boys and girls with urinary tract infection. J Pediatr. 2000;136:30–4. [DOI] [PubMed] [Google Scholar]
- 8.Gubhaju L, Sutherland MR, Black MJ. Preterm birth and the kidney: implications for long-term renal health. Reprod Sci. 2011;18:322–33. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg L, Borovitz Y, Sokolover N, Lebel A, Davidovits M. Long-term follow-up of premature infants with urinary tract infection. Eur J Pediatr. 2021;180:3059–3066. [DOI] [PubMed] [Google Scholar]
- 10.Nowell L, Moran C, Smith PB, et al. Prevalence of renal anomalies after urinary tract infections in hospitalized infants less than 2 months of age. J Perinatol. 2010;30:281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruangkit C, Satpute A, Vogt BA, Hoyen C, Viswanathan S. Incidence and risk factors of urinary tract infection in very low birth weight infants. J Neonatal Perinatal Med. 2016;9:83–90. [DOI] [PubMed] [Google Scholar]
- 12.Drumm CM, Siddiqui JN, Desale S, Ramasethu J. Urinary tract infection is common in VLBW infants. J Perinatol. 2019;39:80–85. [DOI] [PubMed] [Google Scholar]
- 13.Bauer S, Eliakim A, Pomeranz A, et al. Urinary tract infection in very low birth weight preterm infants. Pediatr Infect Dis J. 2003;22:426–30. [DOI] [PubMed] [Google Scholar]
- 14.Foglia EE, Lorch SA. Clinical predictors of urinary tract infection in the neonatal intensive care unit. J Neonatal Perinatal Med. 2012;5:327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system--tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70. [DOI] [PubMed] [Google Scholar]
- 16.Hornik CP, Fort P, Clark RH, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88 Suppl 2(Suppl 2):S69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–24. [DOI] [PubMed] [Google Scholar]
- 18.Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008; 27: 302–308. [DOI] [PubMed] [Google Scholar]
- 19.Flannery DD, Brandsma E, Saslow J, Mackley AB, Paul DA, Aghai ZH. Do infants in the neonatal intensive care unit diagnosed with urinary tract infection need a routine voiding cystourethrogram? J Matern Fetal Neonatal Med. 2019;32:1749–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edlin RS, Shapiro DJ, Hersh AL, Copp HL. Antibiotic resistance patterns of outpatient pediatric urinary tract infections. J Urol. 2013;190:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asghar AM, Leong T, Cooper CS, Arlen AM. Hospital-acquired urinary tract infections in neonatal ICU patients: is voiding cystourethrogram necessary? Urology. 2017;105:163–6. [DOI] [PubMed] [Google Scholar]
- 22.Weems MF, Wei D, Ramanathan R, Barton L, Vachon L, Sardesai S. Urinary tract infections in a neonatal intensive care unit. Am J Perinatol. 2015;32:695–702. [DOI] [PubMed] [Google Scholar]
- 23.Stoll BJ, Puopolo KM, Hansen NI, et al. Early-onset neonatal sepsis 2015 to 2017, the rise of Escherichia coli, and the need for novel prevention strategies. JAMA Pediatr. 2020;174:e200593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bizzarro MJ, Dembry L-M, Baltimore RS, Gallagher PG. Changing patterns in neonatal Escherichia coli sepsis and ampicillin resistance in the era of intrapartum antibiotic prophylaxis. Pediatrics. 2008;121: 689–96. [DOI] [PubMed] [Google Scholar]
- 25.Puopolo KM, Eichenwald EC. No change in the incidence of ampicillin-resistant, neonatal, early-onset sepsis over 18 years. Pediatrics. 2010;125:e1031–8. [DOI] [PubMed] [Google Scholar]
- 26.Tamim MM, Alesseh H, Aziz H. Analysis of the efficacy of urine culture as part of sepsis evaluation in the premature infant. Pediatr Infect Dis J. 2003;22:805–8. [DOI] [PubMed] [Google Scholar]
- 27.Kavelaars A, van der Pompe G, Bakker JM, et al. Altered immune function in human newborns after prenatal administration of betamethasone: enhanced natural killer cell activity and decreased T cell proliferation in cord blood. Pediatr Res. 1999;45(3):306–312. [DOI] [PubMed] [Google Scholar]
- 28.Diepenbruck I, Much CC, Krumbholz A, et al. Effect of prenatal steroid treatment on the developing immune system. J Mol Med (Berl). 2013;91(11):1293–1302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


