Abstract
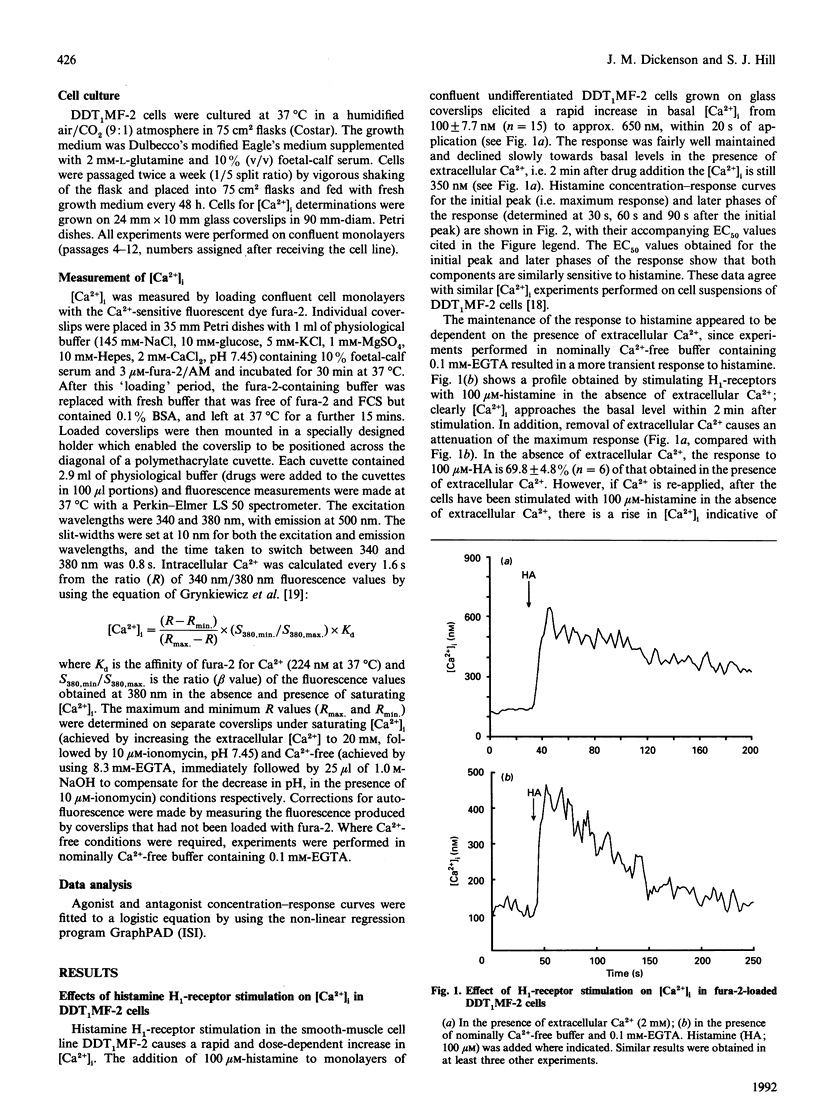
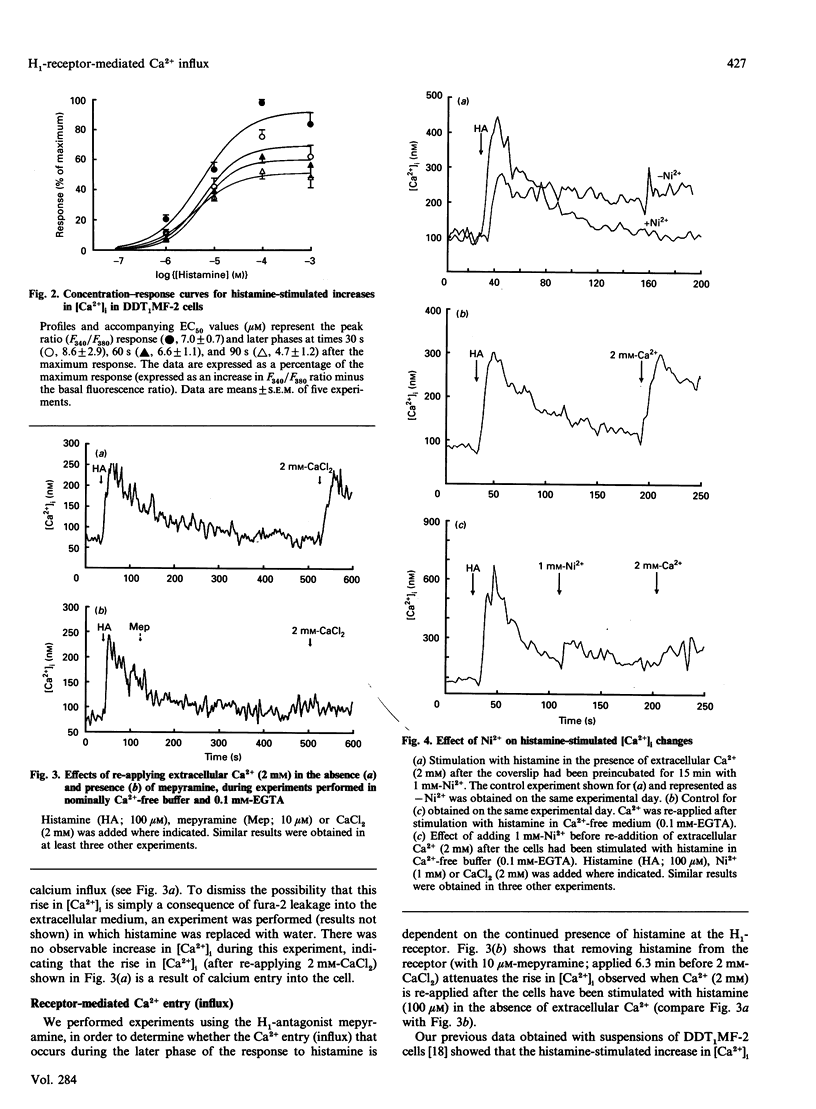
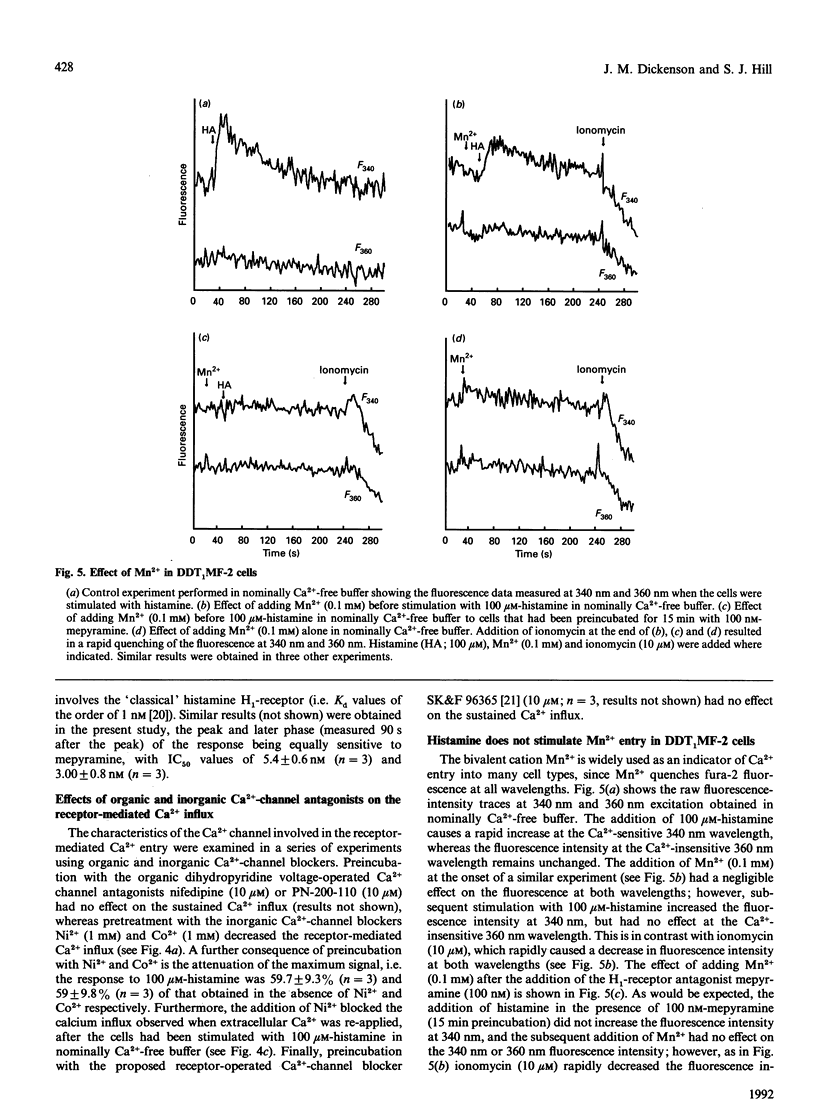
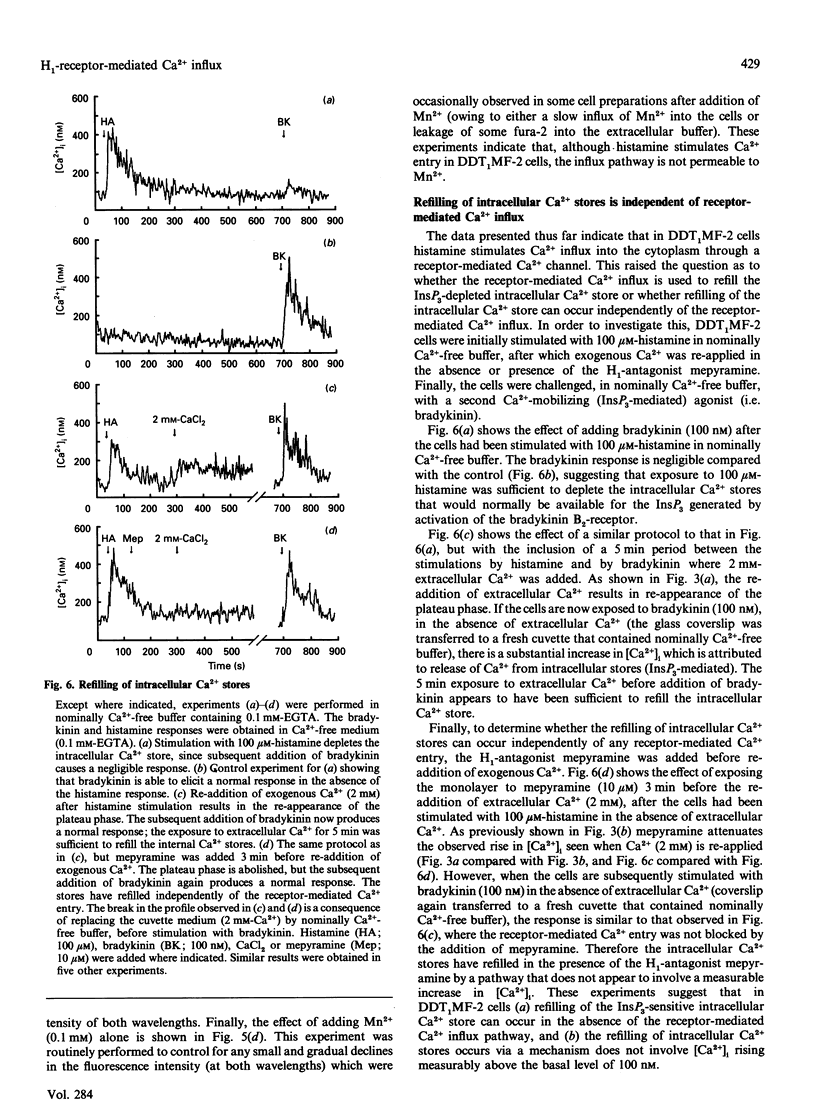
Undifferentiated monolayers of the hamster vas deferens smooth-muscle cell line, DDT1MF-2, were grown on glass coverslips and loaded with the Ca(2+)-sensitive fluorescent dye fura-2. Stimulation with histamine produced a rapid and maintained increase in intracellular free Ca2+ ([Ca2+]i), with an EC50 of 7.0 +/- 0.7 microM. The initial rise in [Ca2+]i can be attributed to Ca2+ release from intracellular stores, whereas the maintained or plateau phase is due to influx of extracellular Ca2+. The Ca2+ influx associated with the plateau phase required the continued presence of histamine on the receptor, since the H1-antagonist mepyramine (10 microM) attenuated the rise in [Ca2+]i observed when extracellular Ca2+ was re-applied after the cells had been stimulated with histamine, in experiments performed in nominally Ca(2+)-free buffer. Pretreatment with the inorganic Ca(2+)-channel blockers Ni2+ (1 mM) and Co2+ (1 mM) inhibited the influx component, whereas the organic voltage-operated Ca(2+)-channel antagonists nifedipine (10 microM) and PN-200-110 (10 microM) had no effect. These data suggest that histamine stimulates Ca2+ influx through an H1-receptor-activated Ca2+ channel. Experiments with Mn2+ indicated that the receptor-mediated Ca(2+)-influx pathway(s) is impermeable to Mn2+. Furthermore, the refilling of Ca2+ stores can occur independently of H1-receptor-mediated influx, since store refilling can be demonstrated even when the receptor-mediated Ca2+ entry is blocked by mepyramine. In conclusion, H1-receptor activation in the smooth-muscle cell line DDT1MF-2 stimulates both release of Ca2+ from intracellular stores [inositol 1,4,5-triphosphate (InsP3)-mediated] and Ca2+ influx through a receptor-activated Ca2+ channel. The subsequent refilling of the InsP3-sensitive intracellular Ca2+ store is independent of histamine H1-receptor stimulation (mepyramine-insensitive) and occurs without an observable rise in cytosolic free Ca2+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barritt G. J., Hughes B. P. The nature and mechanism of activation of the hepatocyte receptor-activated Ca2+ inflow system. Cell Signal. 1991;3(4):283–292. doi: 10.1016/0898-6568(91)90056-z. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bian J. H., Ghosh T. K., Wang J. C., Gill D. L. Identification of intracellular calcium pools. Selective modification by thapsigargin. J Biol Chem. 1991 May 15;266(14):8801–8806. [PubMed] [Google Scholar]
- Blayney L. M., Gapper P. W., Newby A. C. Inhibition of a receptor-operated calcium channel in pig aortic microsomes by cyclic GMP-dependent protein kinase. Biochem J. 1991 Feb 1;273(Pt 3):803–806. doi: 10.1042/bj2730803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blayney L. M., Newby A. C. Histamine and a guanine nucleotide increase calcium permeability in pig aortic microsomal fractions. Biochem J. 1990 Apr 1;267(1):105–109. doi: 10.1042/bj2670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson J. M., Hill S. J. Histamine-stimulated increases in intracellular calcium in the smooth muscle cell line, DDT1MF-2. Biochem Pharmacol. 1991 Sep 27;42(8):1545–1550. doi: 10.1016/0006-2952(91)90423-3. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Evidence that agonists stimulate bivalent-cation influx into human endothelial cells. Biochem J. 1988 Oct 1;255(1):179–184. doi: 10.1042/bj2550179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam T. J., Jacob R., Merritt J. E. Influx of bivalent cations can be independent of receptor stimulation in human endothelial cells. Biochem J. 1989 Apr 1;259(1):125–129. doi: 10.1042/bj2590125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. J. Distribution, properties, and functional characteristics of three classes of histamine receptor. Pharmacol Rev. 1990 Mar;42(1):45–83. [PubMed] [Google Scholar]
- Irvine R. F. 'Quantal' Ca2+ release and the control of Ca2+ entry by inositol phosphates--a possible mechanism. FEBS Lett. 1990 Apr 9;263(1):5–9. doi: 10.1016/0014-5793(90)80692-c. [DOI] [PubMed] [Google Scholar]
- Johnson C. L., Johnson C. G., Bazan E., Garver D., Gruenstein E., Ahluwalia M. Histamine receptors in human fibroblasts: inositol phosphates, Ca2+, and cell growth. Am J Physiol. 1990 Mar;258(3 Pt 1):C533–C543. doi: 10.1152/ajpcell.1990.258.3.C533. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Kanaide H., Nishimura J., Kuga T., Kobayashi S., Nakamura M. Histamine-induced calcium transients in vascular smooth muscle cells: effects of verapamil and diltiazem. Am J Physiol. 1989 Aug;257(2 Pt 2):H563–H570. doi: 10.1152/ajpheart.1989.257.2.H563. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Kanaide H., Nishimura J., Shogakiuchi Y., Kobayashi S., Nakamura M. Histamine activates H1-receptors to induce cytosolic free calcium transients in cultured vascular smooth muscle cells from rat aorta. Biochem Biophys Res Commun. 1986 Feb 26;135(1):172–177. doi: 10.1016/0006-291x(86)90958-7. [DOI] [PubMed] [Google Scholar]
- McDonough P. M., Eubanks J. H., Brown J. H. Desensitization and recovery of muscarinic and histaminergic Ca2+ mobilization in 1321N1 astrocytoma cells. Biochem J. 1988 Jan 1;249(1):135–141. doi: 10.1042/bj2490135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldolesi J., Clementi E., Fasolato C., Zacchetti D., Pozzan T. Ca2+ influx following receptor activation. Trends Pharmacol Sci. 1991 Aug;12(8):289–292. doi: 10.1016/0165-6147(91)90577-f. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Armstrong W. P., Benham C. D., Hallam T. J., Jacob R., Jaxa-Chamiec A., Leigh B. K., McCarthy S. A., Moores K. E., Rink T. J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990 Oct 15;271(2):515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Rink T. J. Rapid increases in cytosolic free calcium in response to muscarinic stimulation of rat parotid acinar cells. J Biol Chem. 1987 Apr 15;262(11):4958–4960. [PubMed] [Google Scholar]
- Mitsuhashi M., Payan D. G. Characterization of functional histamine H1 receptors on a cultured smooth muscle cell line. J Cell Physiol. 1988 Mar;134(3):367–375. doi: 10.1002/jcp.1041340307. [DOI] [PubMed] [Google Scholar]
- Molleman A., Hoiting B., Duin M., van den Akker J., Nelemans A., Den Hertog A. Potassium channels regulated by inositol 1,3,4,5-tetrakisphosphate and internal calcium in DDT1 MF-2 smooth muscle cells. J Biol Chem. 1991 Mar 25;266(9):5658–5663. [PubMed] [Google Scholar]
- Murray R. K., Kotlikoff M. I. Receptor-activated calcium influx in human airway smooth muscle cells. J Physiol. 1991 Apr;435:123–144. doi: 10.1113/jphysiol.1991.sp018501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes S. G., Iaizzo P. A., Richelson E., Powis G. Histamine-induced intracellular free Ca++, inositol phosphates and electrical changes in murine N1E-115 neuroblastoma cells. J Pharmacol Exp Ther. 1988 Oct;247(1):114–121. [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield-Payne M. S. Cyclic GMP mediates the agonist-stimulated increase in plasma membrane calcium entry in the pancreatic acinar cell. J Biol Chem. 1990 Aug 5;265(22):12846–12853. [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Sjölander A., Grönroos E., Hammarström S., Andersson T. Leukotriene D4 and E4 induce transmembrane signaling in human epithelial cells. Single cell analysis reveals diverse pathways at the G-protein level for the influx and the intracellular mobilization of Ca2+. J Biol Chem. 1990 Dec 5;265(34):20976–20981. [PubMed] [Google Scholar]


