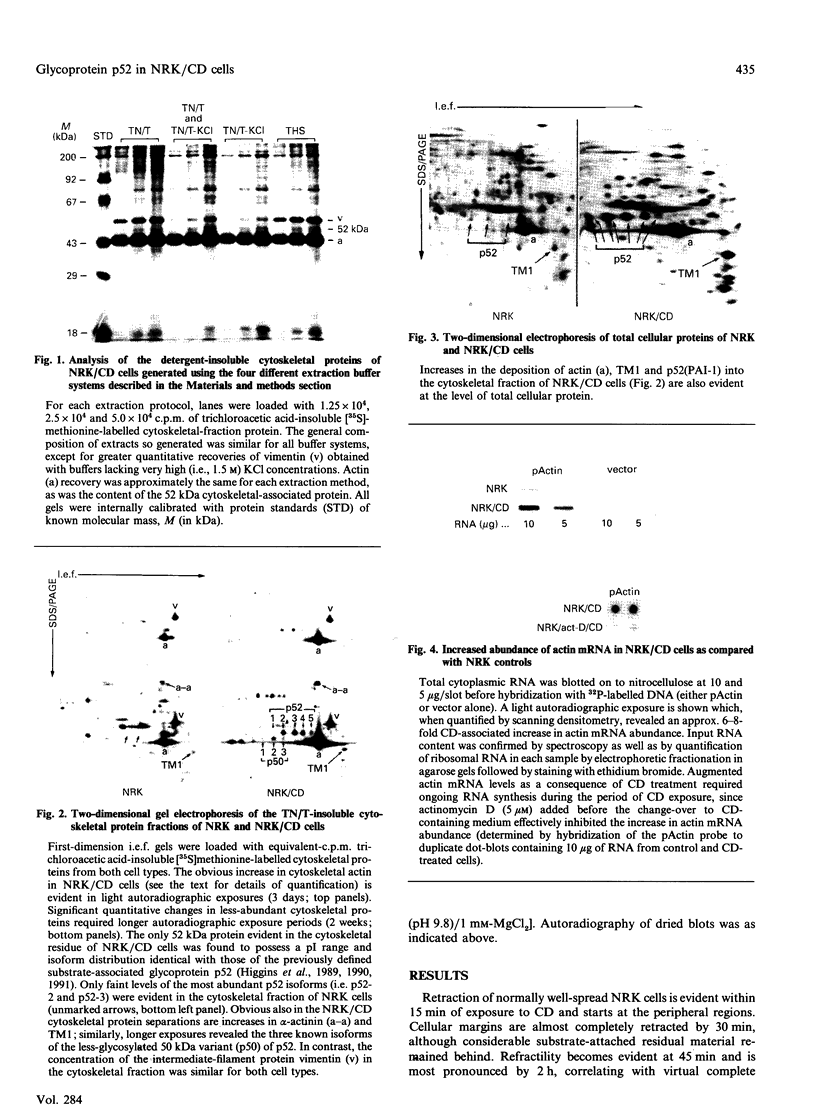
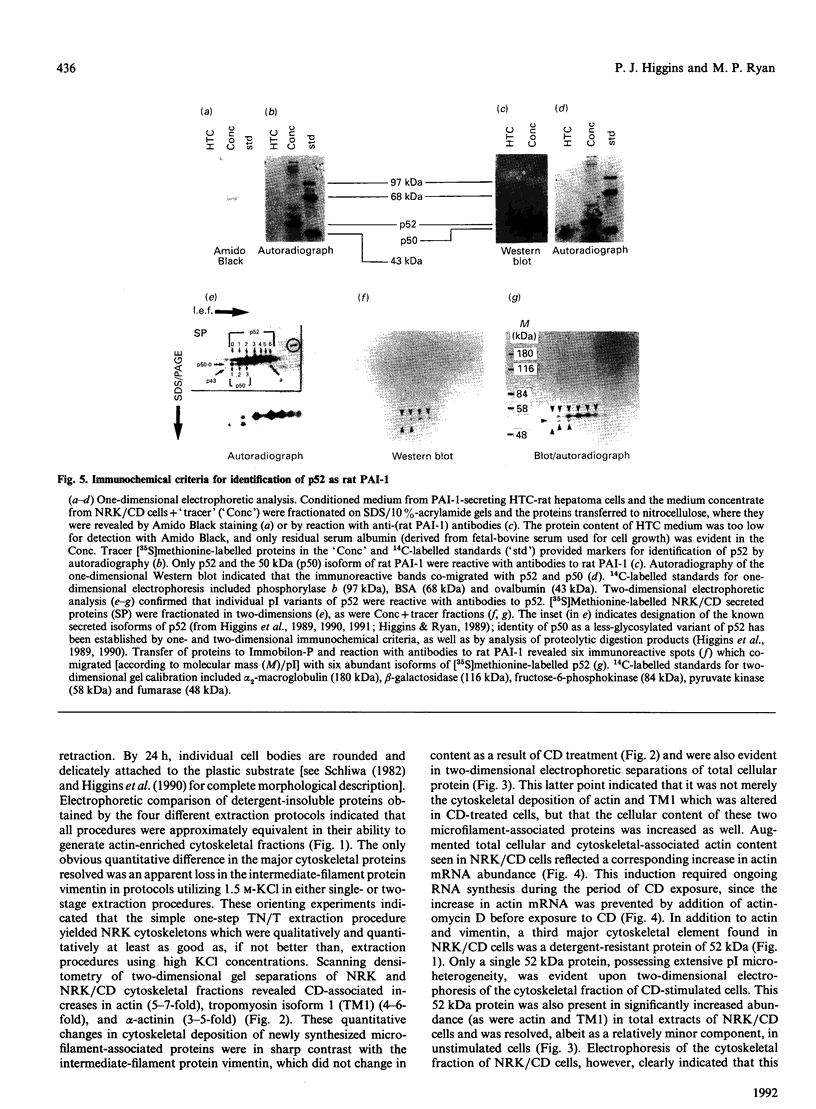
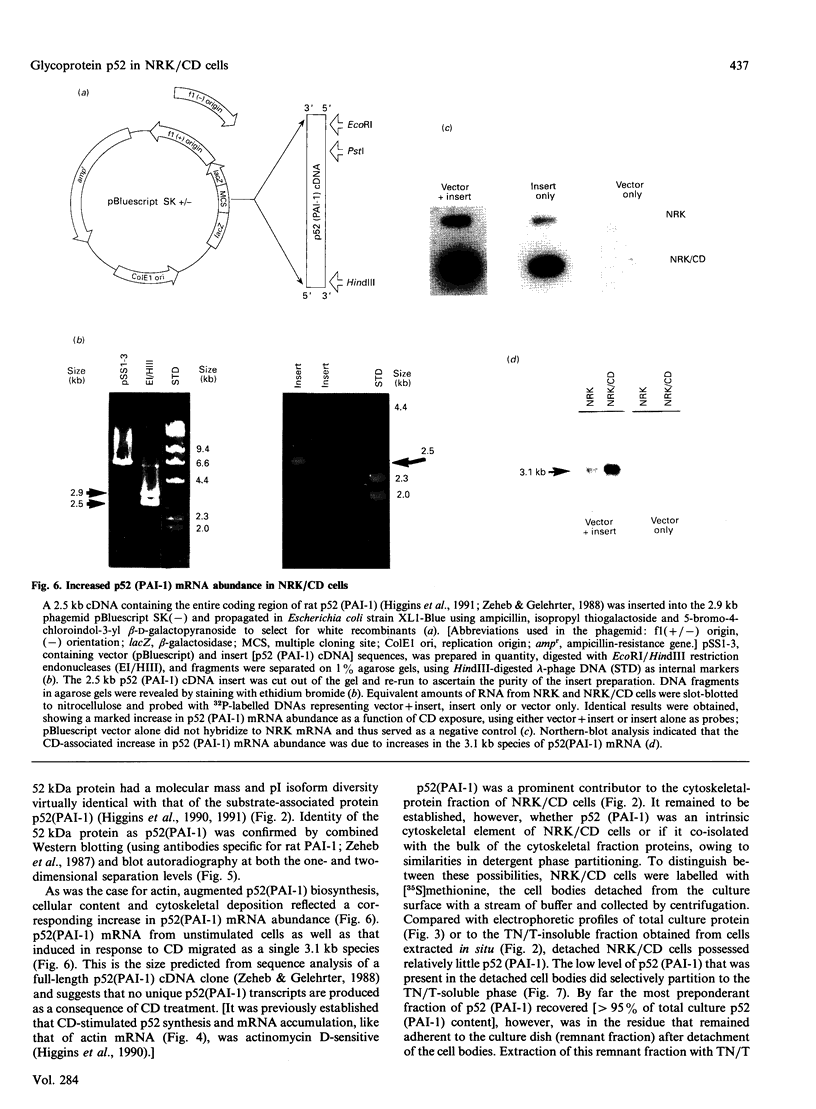
Abstract
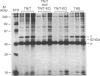
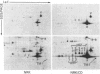
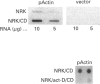
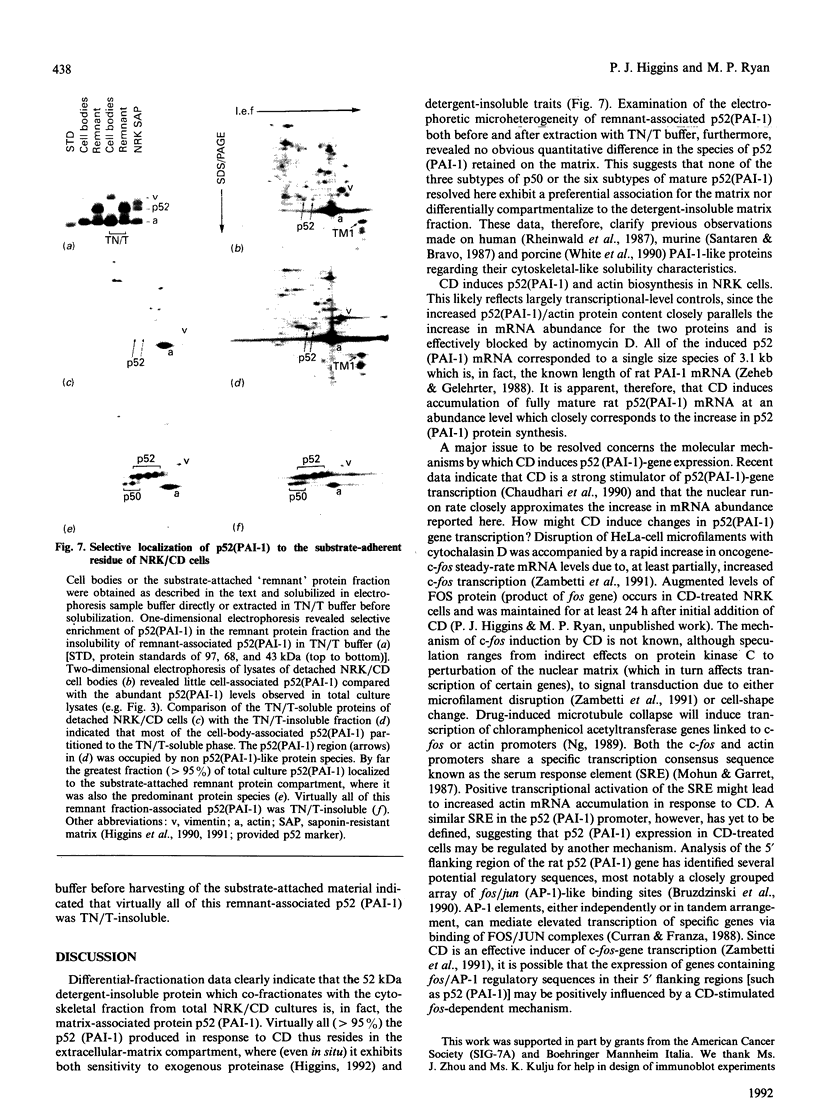
Cell shape profoundly affects cellular metabolic activity, protein and nucleic acid synthesis, and cytoskeletal organization. To examine the influence of cell shape on protein expression, normal rat kidney (NRK) cells were exposed to the microfilament-disrupting drug cytochalasin D (CD), labelled with [35S]methionine, and newly synthesized cellular and cytoskeletal proteins examined by two-dimensional gel electrophoresis. CD produced dramatic changes in cell shape (from a flat to round phenotype) with concomitant 3-7-fold increases in the cellular content and cytoskeletal deposition of the microfilament-associated proteins actin, alpha-actinin, and tropomyosin isoform 1. Augmented actin protein content in NRK/CD cells was paralleled by a corresponding increase in actin mRNA abundance and was inhibited by prior addition of actinomycin D. A detergent-insoluble protein of 52 kDa was also detected at high levels in the cytoskeletal fraction of NRK/CD cells. Two-dimensional electrophoretic mapping of total cellular and cytoskeletal proteins revealed this 52 kDa protein to be the previously described glycoprotein p52 [Higgins & Ryan (1989) Biochem. J. 257, 173-182]. By using electrophoretic and immunochemical criteria, p52 was identified as plasminogen-activator inhibitor type-1 (PAI-1). Like actin, CD-induced p52(PAI-1) synthesis, cellular content, and partitioning to the detergent-insoluble cytoskeletal compartment reflected a corresponding increase in p52(PAI-1) mRNA. Such induction was similarly inhibited by actinomycin D. p52(PAI-1) expression in the NRK-cell system is thus responsive to CD-mediated shape changes and requires ongoing RNA synthesis for its induction. Differential extraction of detached cell bodies and the substrate-adherent 'remnant' fraction of NRK/CD cultures, furthermore, indicated that p52(PAI-1) was not an intrinsic internal cytoskeletal element but, rather, selectively localized to the extracellular residue. p52(PAI-1) retained its detergent-insoluble characteristics even in this isolated 'remnant' fraction, where it was also the predominant protein species resolved.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruzdzinski C. J., Riordan-Johnson M., Nordby E. C., Suter S. M., Gelehrter T. D. Isolation and characterization of the rat plasminogen activator inhibitor-1 gene. J Biol Chem. 1990 Feb 5;265(4):2078–2085. [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Garrels J. I., Franza B. R., Jr The REF52 protein database. Methods of database construction and analysis using the QUEST system and characterizations of protein patterns from proliferating and quiescent REF52 cells. J Biol Chem. 1989 Mar 25;264(9):5283–5298. [PubMed] [Google Scholar]
- Garrels J. I. The QUEST system for quantitative analysis of two-dimensional gels. J Biol Chem. 1989 Mar 25;264(9):5269–5282. [PubMed] [Google Scholar]
- Godman G. C., Miranda A. F. Cellular contractility and the visible effects of cytochalasin. Front Biol. 1978;46:277–429. [PubMed] [Google Scholar]
- Higgins P. J., Chaudhari P., Ryan M. P. Cell-shape regulation and matrix protein p52 content in phenotypic variants of ras-transformed rat kidney fibroblasts. Functional analysis and biochemical comparison of p52 with proteins implicated in cell-shape determination. Biochem J. 1991 Feb 1;273(Pt 3):651–658. doi: 10.1042/bj2730651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins P. J., Ryan M. P. Biochemical localization of the transformation-sensitive 52 kDa (p52) protein to the substratum contact regions of cultured rat fibroblasts. Butyrate induction, characterization, and quantification of p52 in v-ras transformed cells. Biochem J. 1989 Jan 1;257(1):173–182. doi: 10.1042/bj2570173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins P. J., Ryan M. P., Chaudhari P. Cytochalasin D-mediated hyperinduction of the substrate-associated 52-kilodalton protein p52 in rat kidney fibroblasts. J Cell Physiol. 1989 May;139(2):407–417. doi: 10.1002/jcp.1041390225. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Ryan M. P., Zeheb R., Gelehrter T. D., Chaudhari P. p52 induction by cytochalasin D in rat kidney fibroblasts: homologies between p52 and plasminogen activator inhibitor type-1. J Cell Physiol. 1990 May;143(2):321–329. doi: 10.1002/jcp.1041430216. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Ryan M. P. p52(PAI-1) and actin expression in butyrate-induced flat revertants of v-ras-transformed rat kidney cells. Biochem J. 1991 Nov 1;279(Pt 3):883–890. doi: 10.1042/bj2790883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohun T. J., Garrett N. An amphibian cytoskeletal-type actin gene is expressed exclusively in muscle tissue. Development. 1987 Oct;101(2):393–402. doi: 10.1242/dev.101.2.393. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Jorgensen J. L., Hahn W. C., Terpstra A. J., O'Connell T. M., Plummer K. K. Mesosecrin: a secreted glycoprotein produced in abundance by human mesothelial, endothelial, and kidney epithelial cells in culture. J Cell Biol. 1987 Feb;104(2):263–275. doi: 10.1083/jcb.104.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. P., Borenfreund E., Higgins P. J. Cytoarchitectural analysis of epithelial sheets formed in vitro by hepatic tumor cells possessing defined intermediate-sized filament cytoskeletal abnormalities. Am J Pathol. 1989 Feb;134(2):447–456. [PMC free article] [PubMed] [Google Scholar]
- Ryan M. P., Higgins P. J. Cytoarchitecture of Kirsten sarcoma virus-transformed rat kidney fibroblasts: butyrate-induced reorganization within the actin microfilament network. J Cell Physiol. 1988 Oct;137(1):25–34. doi: 10.1002/jcp.1041370104. [DOI] [PubMed] [Google Scholar]
- Ryan M. P., Higgins P. J. Discrimination between the nuclear lamin and intermediate filament (cytokeratin/vimentin) proteins of rat hepatic tumor cells by differential solubility and electrophoretic criteria. Int J Biochem. 1987;19(12):1187–1192. doi: 10.1016/0020-711x(87)90101-7. [DOI] [PubMed] [Google Scholar]
- Ryan M. P., Higgins P. J. Sodium-n-butyrate induces secretion and substrate accumulation of p52 in Kirsten sarcoma virus-transformed rat kidney fibroblasts. Int J Biochem. 1989;21(1):31–37. doi: 10.1016/0020-711x(89)90024-4. [DOI] [PubMed] [Google Scholar]
- Santarén J. F., Bravo R. Immediate induction of a 45 K secreted glycoprotein by serum and growth factors in quiescent mouse 3T3 cells. Two-dimensional gel analysis. Exp Cell Res. 1987 Feb;168(2):494–506. doi: 10.1016/0014-4827(87)90022-x. [DOI] [PubMed] [Google Scholar]
- Schliwa M. Action of cytochalasin D on cytoskeletal networks. J Cell Biol. 1982 Jan;92(1):79–91. doi: 10.1083/jcb.92.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson C. J., Geoghegan T. E. Actin gene expression in murine erythroleukemia cells treated with cytochalasin D. Exp Cell Res. 1990 Jul;189(1):28–32. doi: 10.1016/0014-4827(90)90252-6. [DOI] [PubMed] [Google Scholar]
- Tannenbaum J. Cytochalasin D alters the rate of synthesis of some HEp-2 cytoskeletal proteins. Examination by two-dimensional gel electrophoresis. Eur J Biochem. 1986 Mar 17;155(3):533–542. doi: 10.1111/j.1432-1033.1986.tb09521.x. [DOI] [PubMed] [Google Scholar]
- White J. E., Tsan M. F., Phillips P. G., Higgins P. J. The substrate-associated protein p45 of porcine endothelial cells: multiple isoforms, cytoskeletal-like properties and induction by hyperoxic stress. Int J Biochem. 1990;22(10):1159–1164. doi: 10.1016/0020-711x(90)90115-j. [DOI] [PubMed] [Google Scholar]
- Zeheb R., Gelehrter T. D. Cloning and sequencing of cDNA for the rat plasminogen activator inhibitor-1. Gene. 1988 Dec 20;73(2):459–468. doi: 10.1016/0378-1119(88)90510-0. [DOI] [PubMed] [Google Scholar]
- Zeheb R., Rafferty U. M., Rodriguez M. A., Andreasen P., Gelehrter T. D. Immunoaffinity purification of HTC rat hepatoma cell plasminogen activator-inhibitor-1. Thromb Haemost. 1987 Dec 18;58(4):1017–1023. [PubMed] [Google Scholar]