Abstract
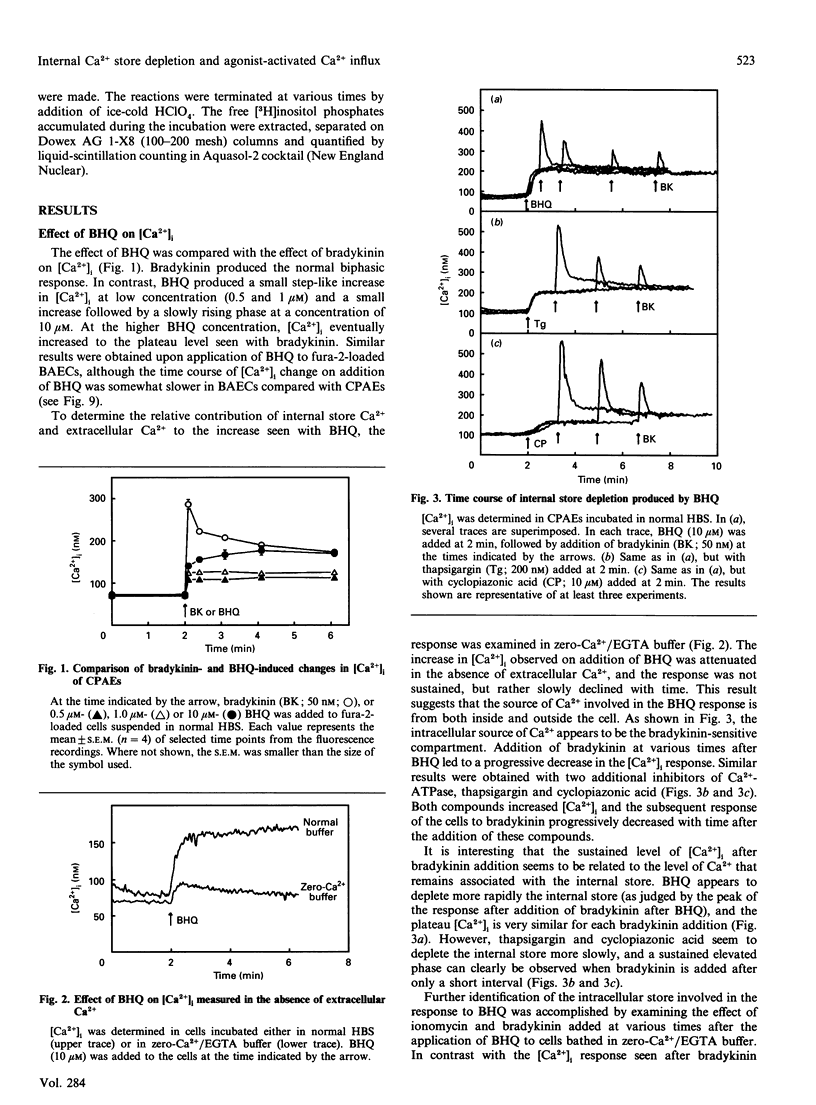
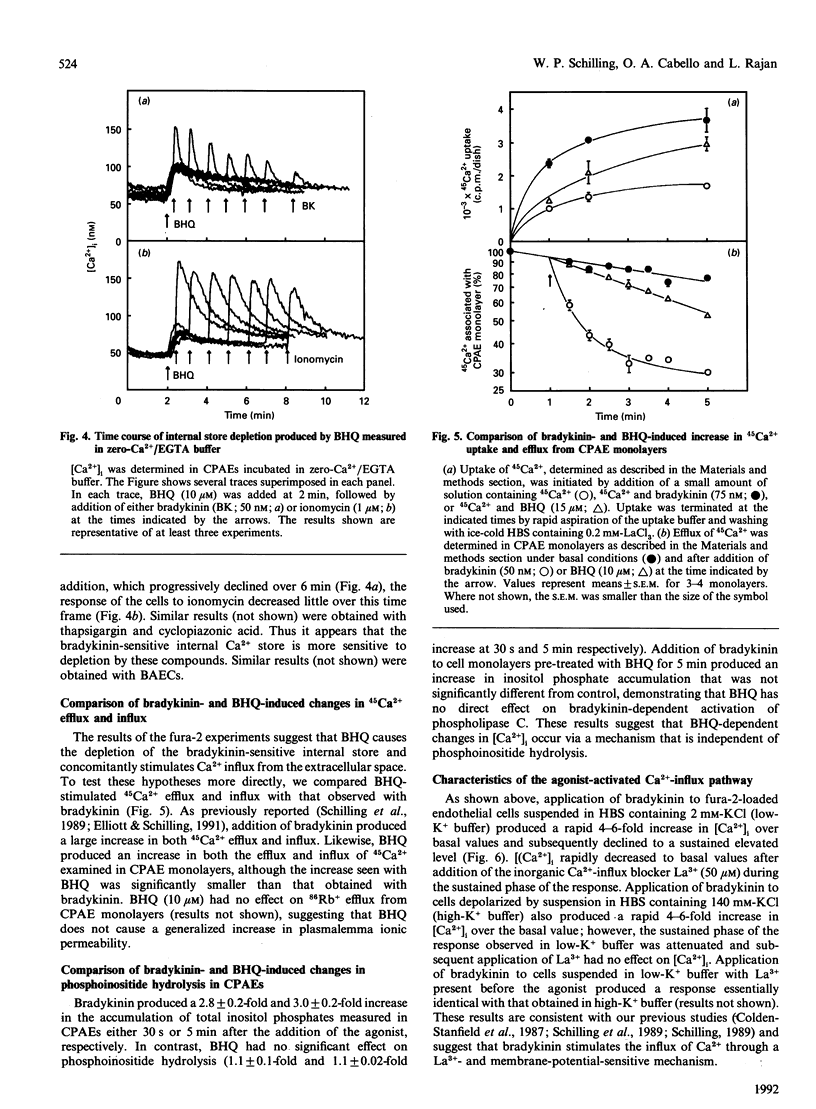
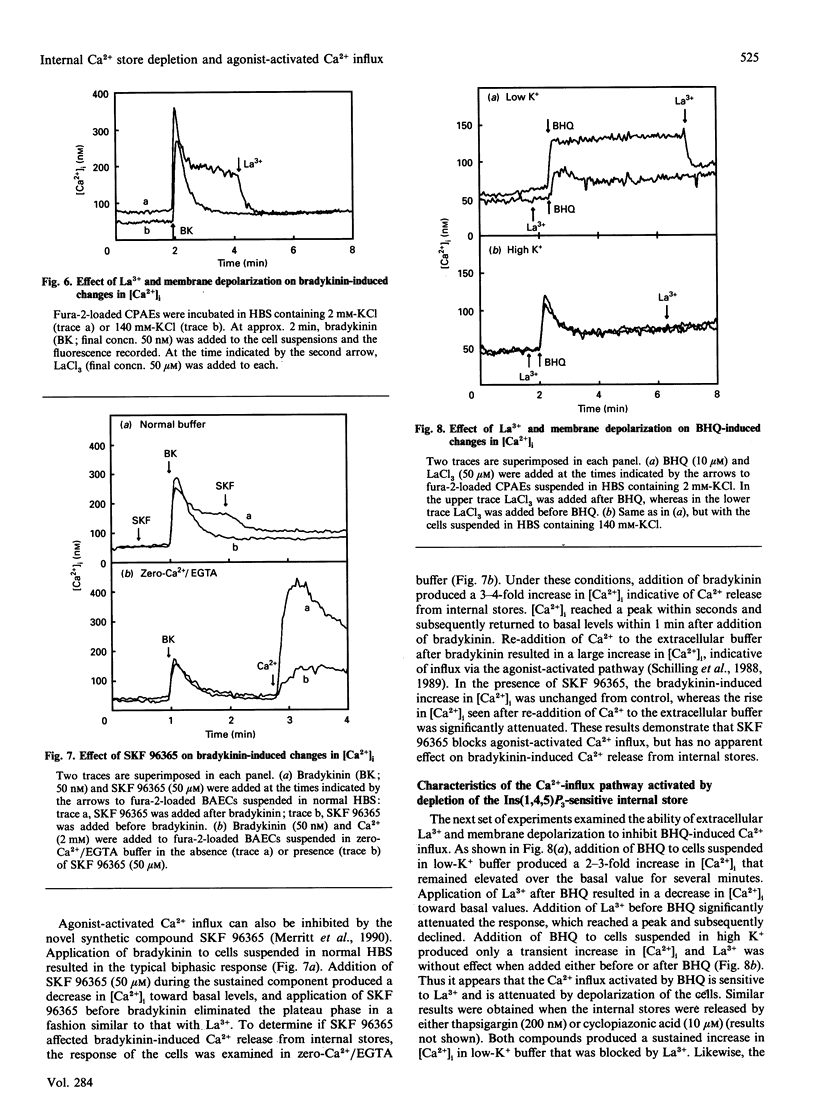
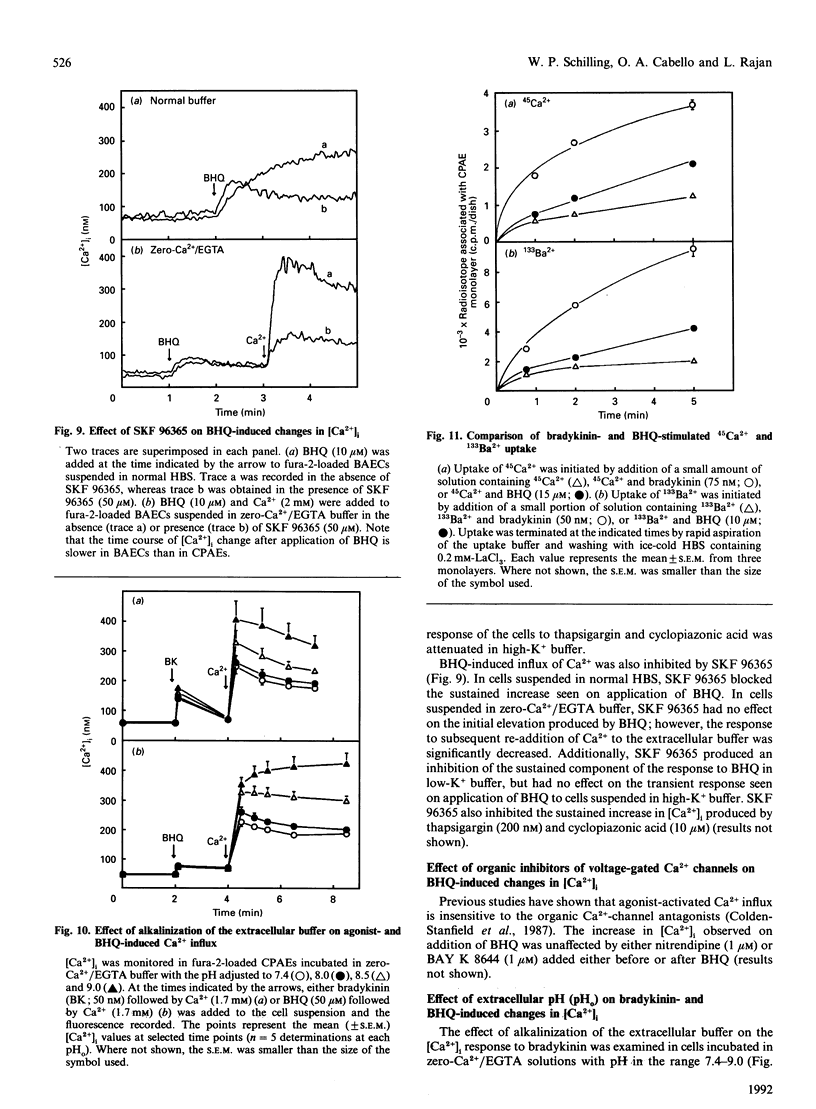
Previous studies in non-excitable cells have suggested that depletion of internal Ca2+ stores activates Ca2+ influx from the extracellular space via a mechanism that does not require stimulation of phosphoinositide hydrolysis. To test this hypothesis in vascular endothelial cells, the effect of the Ca(2+)-ATPase/pump inhibitor 2,5-di-t-butylhydroquinone (BHQ) on cytosolic free Ca2+ concentration ([Ca2+]i) was examined. BHQ produced a dose-dependent increase in [Ca2+]i, which remained elevated over basal values for several minutes and was substantially inhibited in the absence of extracellular Ca2+. Application of bradykinin after BHQ demonstrated that the BHQ-sensitive compartment partially overlapped the bradykinin-sensitive store. Similar results were obtained with thapsigargin and cyclopiazonic acid, two other Ca(2+)-ATPase inhibitors. Although BHQ had no effect on phosphoinositide hydrolysis, both 45Ca2+ influx and efflux were stimulated by this agent. These results suggest that depletion of the agonist-sensitive Ca2+ store is sufficient for activation of Ca2+ influx. Several characteristics of the Ca(2+)-influx pathway activated by internal store depletion were compared with those of the agonist-activated pathway. Bradykinin-stimulated Ca2+ influx was increased at alkaline extracellular pH (pHo), and was inhibited by extracellular La3+, by depolarization of the membrane, and by the novel Ca(2+)-influx blocker 1-(beta-[3-(4-methoxyphenyl)propoxy]-4- methoxyphenethyl)-1H-imidazole hydrochloride (SKF 96365). Additionally, bradykinin stimulated influx of both 45Ca2+ and 133Ba2+, consistent with the hypothesis that the agonist-activated influx pathway is permeable to both of these bivalent cations. Likewise, activation of Ca2+ influx by BHQ, thapsigargin and cyclopiazonic acid was blocked by La3+, membrane depolarization and SKF 96365, but was unaffected by nitrendipine or BAY K 8644. Furthermore, Ca2+ influx stimulated by BHQ was increased at alkaline pHo and BHQ stimulated the influx of both 45Ca2+ and 133Ba2+ to the same extent. These results demonstrate that the agonist-activated Ca(2+)-influx pathway and the pathway activated by depletion of the agonist-sensitive internal Ca2+ store are indistinguishable.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altin J. G., Biden T. J., Karjalainen A., Bygrave F. L. Exposure to depolarizing concentrations of K+ inhibits hormonally-induced calcium influx in rat liver. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1282–1289. doi: 10.1016/s0006-291x(88)81367-6. [DOI] [PubMed] [Google Scholar]
- Buchan K. W., Martin W. Bradykinin induces elevations of cytosolic calcium through mobilisation of intracellular and extracellular pools in bovine aortic endothelial cells. Br J Pharmacol. 1991 Jan;102(1):35–40. doi: 10.1111/j.1476-5381.1991.tb12128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colden-Stanfield M., Schilling W. P., Ritchie A. K., Eskin S. G., Navarro L. T., Kunze D. L. Bradykinin-induced increases in cytosolic calcium and ionic currents in cultured bovine aortic endothelial cells. Circ Res. 1987 Nov;61(5):632–640. doi: 10.1161/01.res.61.5.632. [DOI] [PubMed] [Google Scholar]
- Dehlinger-Kremer M., Zeuzem S., Schulz I. Interaction of caffeine-, IP3- and vanadate-sensitive Ca2+ pools in acinar cells of the exocrine pancreas. J Membr Biol. 1991 Jan;119(1):85–100. doi: 10.1007/BF01868543. [DOI] [PubMed] [Google Scholar]
- Dhalla N. S., Pierce G. N., Panagia V., Singal P. K., Beamish R. E. Calcium movements in relation to heart function. Basic Res Cardiol. 1982 Mar-Apr;77(2):117–139. doi: 10.1007/BF01908167. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Lew P. D., Andersson T., Pozzan T. Plasma membrane potential modulates chemotactic peptide-stimulated cytosolic free Ca2+ changes in human neutrophils. J Biol Chem. 1987 Apr 5;262(10):4574–4579. [PubMed] [Google Scholar]
- Elliott S. J., Eskin S. G., Schilling W. P. Effect of t-butyl-hydroperoxide on bradykinin-stimulated changes in cytosolic calcium in vascular endothelial cells. J Biol Chem. 1989 Mar 5;264(7):3806–3810. [PubMed] [Google Scholar]
- Elliott S. J., Schilling W. P. Carmustine augments the effects of tert-butyl hydroperoxide on calcium signaling in cultured pulmonary artery endothelial cells. J Biol Chem. 1990 Jan 5;265(1):103–107. [PubMed] [Google Scholar]
- Elliott S. J., Schilling W. P. Oxidative stress inhibits bradykinin-stimulated 45Ca2+ flux in pulmonary vascular endothelial cells. Am J Physiol. 1991 Feb;260(2 Pt 2):H549–H556. doi: 10.1152/ajpheart.1991.260.2.H549. [DOI] [PubMed] [Google Scholar]
- Foder B., Scharff O., Thastrup O. Ca2+ transients and Mn2+ entry in human neutrophils induced by thapsigargin. Cell Calcium. 1989 Oct;10(7):477–490. doi: 10.1016/0143-4160(89)90025-0. [DOI] [PubMed] [Google Scholar]
- Goeger D. E., Riley R. T., Dorner J. W., Cole R. J. Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles. Biochem Pharmacol. 1988 Mar 1;37(5):978–981. doi: 10.1016/0006-2952(88)90195-5. [DOI] [PubMed] [Google Scholar]
- Goeger D. E., Riley R. T. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 1989 Nov 15;38(22):3995–4003. doi: 10.1016/0006-2952(89)90679-5. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986 Oct 20;207(1):95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D., Needham L. A. Thrombin-stimulated elevation of human endothelial-cell cytoplasmic free calcium concentration causes prostacyclin production. Biochem J. 1988 Apr 1;251(1):243–249. doi: 10.1042/bj2510243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry P. D., Schuchleib R., Davis J., Weiss E. S., Sobel B. E. Myocardial contracture and accumulation of mitochondrial calcium in ischemic rabbit heart. Am J Physiol. 1977 Dec;233(6):H677–H684. doi: 10.1152/ajpheart.1977.233.6.H677. [DOI] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990 Feb;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass G. E., Duddy S. K., Moore G. A., Orrenius S. 2,5-Di-(tert-butyl)-1,4-benzohydroquinone rapidly elevates cytosolic Ca2+ concentration by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. J Biol Chem. 1989 Sep 15;264(26):15192–15198. [PubMed] [Google Scholar]
- Kass G. E., Llopis J., Chow S. C., Duddy S. K., Orrenius S. Receptor-operated calcium influx in rat hepatocytes. Identification and characterization using manganese. J Biol Chem. 1990 Oct 15;265(29):17486–17492. [PubMed] [Google Scholar]
- Kwan C. Y., Putney J. W., Jr Uptake and intracellular sequestration of divalent cations in resting and methacholine-stimulated mouse lacrimal acinar cells. Dissociation by Sr2+ and Ba2+ of agonist-stimulated divalent cation entry from the refilling of the agonist-sensitive intracellular pool. J Biol Chem. 1990 Jan 15;265(2):678–684. [PubMed] [Google Scholar]
- Kwan C. Y., Takemura H., Obie J. F., Thastrup O., Putney J. W., Jr Effects of MeCh, thapsigargin, and La3+ on plasmalemmal and intracellular Ca2+ transport in lacrimal acinar cells. Am J Physiol. 1990 Jun;258(6 Pt 1):C1006–C1015. doi: 10.1152/ajpcell.1990.258.6.C1006. [DOI] [PubMed] [Google Scholar]
- Liao C. F., Schilling W. P., Birnbaumer M., Birnbaumer L. Cellular responses to stimulation of the M5 muscarinic acetylcholine receptor as seen in murine L cells. J Biol Chem. 1990 Jul 5;265(19):11273–11284. [PubMed] [Google Scholar]
- Llopis J., Chow S. B., Kass G. E., Gahm A., Orrenius S. Comparison between the effects of the microsomal Ca(2+)-translocase inhibitors thapsigargin and 2,5-di-(t-butyl)-1,4-benzohydroquinone on cellular calcium fluxes. Biochem J. 1991 Jul 15;277(Pt 2):553–556. doi: 10.1042/bj2770553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Activators of potassium channels enhance calcium influx into endothelial cells as a consequence of potassium currents. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jul;342(1):94–99. doi: 10.1007/BF00178979. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990 May;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Increased free calcium in endothelial cells under stimulation with adenine nucleotides. J Cell Physiol. 1986 Mar;126(3):414–420. doi: 10.1002/jcp.1041260312. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Refilling of endothelial calcium stores without bypassing the cytosol. FEBS Lett. 1990 Dec 10;276(1-2):108–110. doi: 10.1016/0014-5793(90)80519-o. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Mahaut-Smith M. P., Grinstein S. The role of intracellular Ca2+ in the regulation of the plasma membrane Ca2+ permeability of unstimulated rat lymphocytes. J Biol Chem. 1991 Jun 15;266(17):10872–10879. [PubMed] [Google Scholar]
- Merritt J. E., Armstrong W. P., Benham C. D., Hallam T. J., Jacob R., Jaxa-Chamiec A., Leigh B. K., McCarthy S. A., Moores K. E., Rink T. J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990 Oct 15;271(2):515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J. E., Rink T. J. Regulation of cytosolic free calcium in fura-2-loaded rat parotid acinar cells. J Biol Chem. 1987 Dec 25;262(36):17362–17369. [PubMed] [Google Scholar]
- Mertz L. M., Baum B. J., Ambudkar I. S. Refill status of the agonist-sensitive Ca2+ pool regulates Mn2+ influx into parotid acini. J Biol Chem. 1990 Sep 5;265(25):15010–15014. [PubMed] [Google Scholar]
- Mohr F. C., Fewtrell C. Depolarization of rat basophilic leukemia cells inhibits calcium uptake and exocytosis. J Cell Biol. 1987 Mar;104(3):783–792. doi: 10.1083/jcb.104.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. A., McConkey D. J., Kass G. E., O'Brien P. J., Orrenius S. 2,5-Di(tert-butyl)-1,4-benzohydroquinone--a novel inhibitor of liver microsomal Ca2+ sequestration. FEBS Lett. 1987 Nov 30;224(2):331–336. doi: 10.1016/0014-5793(87)80479-9. [DOI] [PubMed] [Google Scholar]
- Morgan-Boyd R., Stewart J. M., Vavrek R. J., Hassid A. Effects of bradykinin and angiotensin II on intracellular Ca2+ dynamics in endothelial cells. Am J Physiol. 1987 Oct;253(4 Pt 1):C588–C598. doi: 10.1152/ajpcell.1987.253.4.C588. [DOI] [PubMed] [Google Scholar]
- Muallem S., Pandol S. J., Beeker T. G. Modulation of agonist-activated calcium influx by extracellular pH in rat pancreatic acini. Am J Physiol. 1989 Dec;257(6 Pt 1):G917–G924. doi: 10.1152/ajpgi.1989.257.6.G917. [DOI] [PubMed] [Google Scholar]
- Muallem S., Schoeffield M. S., Fimmel C. J., Pandol S. J. Agonist-sensitive calcium pool in the pancreatic acinar cell. I. Permeability properties. Am J Physiol. 1988 Aug;255(2 Pt 1):G221–G228. doi: 10.1152/ajpgi.1988.255.2.G221. [DOI] [PubMed] [Google Scholar]
- Oettgen H. C., Terhorst C., Cantley L. C., Rosoff P. M. Stimulation of the T3-T cell receptor complex induces a membrane-potential-sensitive calcium influx. Cell. 1985 Mar;40(3):583–590. doi: 10.1016/0092-8674(85)90206-5. [DOI] [PubMed] [Google Scholar]
- Oldershaw K. A., Taylor C. W. 2,5-Di-(tert-butyl)-1,4-benzohydroquinone mobilizes inositol 1,4,5-trisphosphate-sensitive and -insensitive Ca2+ stores. FEBS Lett. 1990 Nov 12;274(1-2):214–216. doi: 10.1016/0014-5793(90)81366-v. [DOI] [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S., Fimmel C. J., Muallem S. The agonist-sensitive calcium pool in the pancreatic acinar cell. Activation of plasma membrane Ca2+ influx mechanism. J Biol Chem. 1987 Dec 15;262(35):16963–16968. [PubMed] [Google Scholar]
- Papp B., Enyedi A., Kovács T., Sarkadi B., Wuytack F., Thastrup O., Gárdos G., Bredoux R., Levy-Toledano S., Enouf J. Demonstration of two forms of calcium pumps by thapsigargin inhibition and radioimmunoblotting in platelet membrane vesicles. J Biol Chem. 1991 Aug 5;266(22):14593–14596. [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Formation and actions of calcium-mobilizing messenger, inositol 1,4,5-trisphosphate. Am J Physiol. 1987 Feb;252(2 Pt 1):G149–G157. doi: 10.1152/ajpgi.1987.252.2.G149. [DOI] [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. Effects of ionic substitution on [Ca2+]i rises evoked by thrombin and PAF in human platelets. Eur J Pharmacol. 1986 Aug 22;128(1-2):99–107. doi: 10.1016/0014-2999(86)90563-7. [DOI] [PubMed] [Google Scholar]
- Sauve R., Parent L., Simoneau C., Roy G. External ATP triggers a biphasic activation process of a calcium-dependent K+ channel in cultured bovine aortic endothelial cells. Pflugers Arch. 1988 Oct;412(5):469–481. doi: 10.1007/BF00582535. [DOI] [PubMed] [Google Scholar]
- Schilling W. P. Effect of membrane potential on cytosolic calcium of bovine aortic endothelial cells. Am J Physiol. 1989 Sep;257(3 Pt 2):H778–H784. doi: 10.1152/ajpheart.1989.257.3.H778. [DOI] [PubMed] [Google Scholar]
- Schilling W. P., Rajan L., Strobl-Jager E. Characterization of the bradykinin-stimulated calcium influx pathway of cultured vascular endothelial cells. Saturability, selectivity, and kinetics. J Biol Chem. 1989 Aug 5;264(22):12838–12848. [PubMed] [Google Scholar]
- Schilling W. P., Ritchie A. K., Navarro L. T., Eskin S. G. Bradykinin-stimulated calcium influx in cultured bovine aortic endothelial cells. Am J Physiol. 1988 Aug;255(2 Pt 2):H219–H227. doi: 10.1152/ajpheart.1988.255.2.H219. [DOI] [PubMed] [Google Scholar]
- Seidler N. W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Oct 25;264(30):17816–17823. [PubMed] [Google Scholar]
- Shine K. I., Douglas A. M., Ricchiuti N. V. Calcium, strontium, and barium movements during ischemia and reperfusion in rabbit ventricle. Implications for myocardial preservation. Circ Res. 1978 Nov;43(5):712–720. doi: 10.1161/01.res.43.5.712. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Sam L. M., Justen J. M., Bundy G. L., Bala G. A., Bleasdale J. E. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther. 1990 May;253(2):688–697. [PubMed] [Google Scholar]
- Steiger G. J., Brady A. J., Tan S. T. Intrinsic regulatory properties of contractility in the myocardium. Circ Res. 1978 Mar;42(3):339–350. doi: 10.1161/01.res.42.3.339. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Hanley M. R., Thastrup O., Christensen S. B., Snyder S. H. Inositol trisphosphate and thapsigargin discriminate endoplasmic reticulum stores of calcium in rat brain. Biochem Biophys Res Commun. 1990 Oct 30;172(2):811–816. doi: 10.1016/0006-291x(90)90747-b. [DOI] [PubMed] [Google Scholar]
- Walker P. M. Pathophysiology of acute arterial occlusion. Can J Surg. 1986 Sep;29(5):340–342. [PubMed] [Google Scholar]


