Abstract
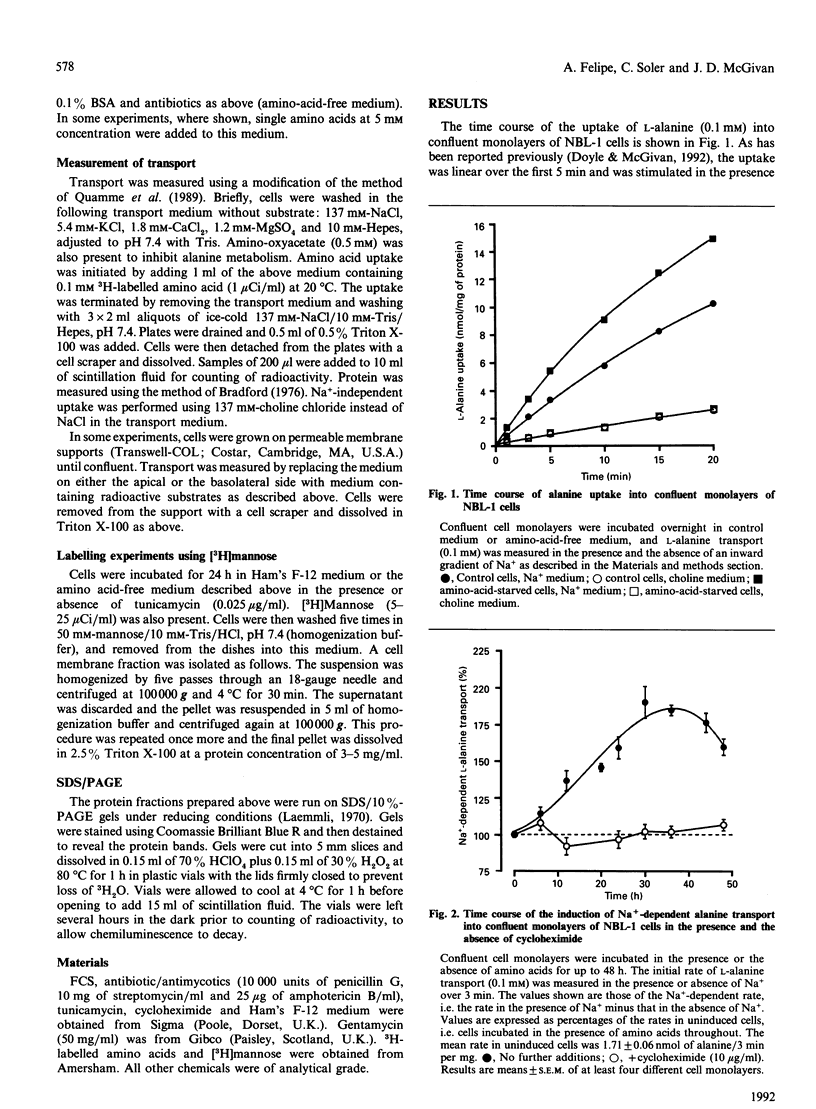
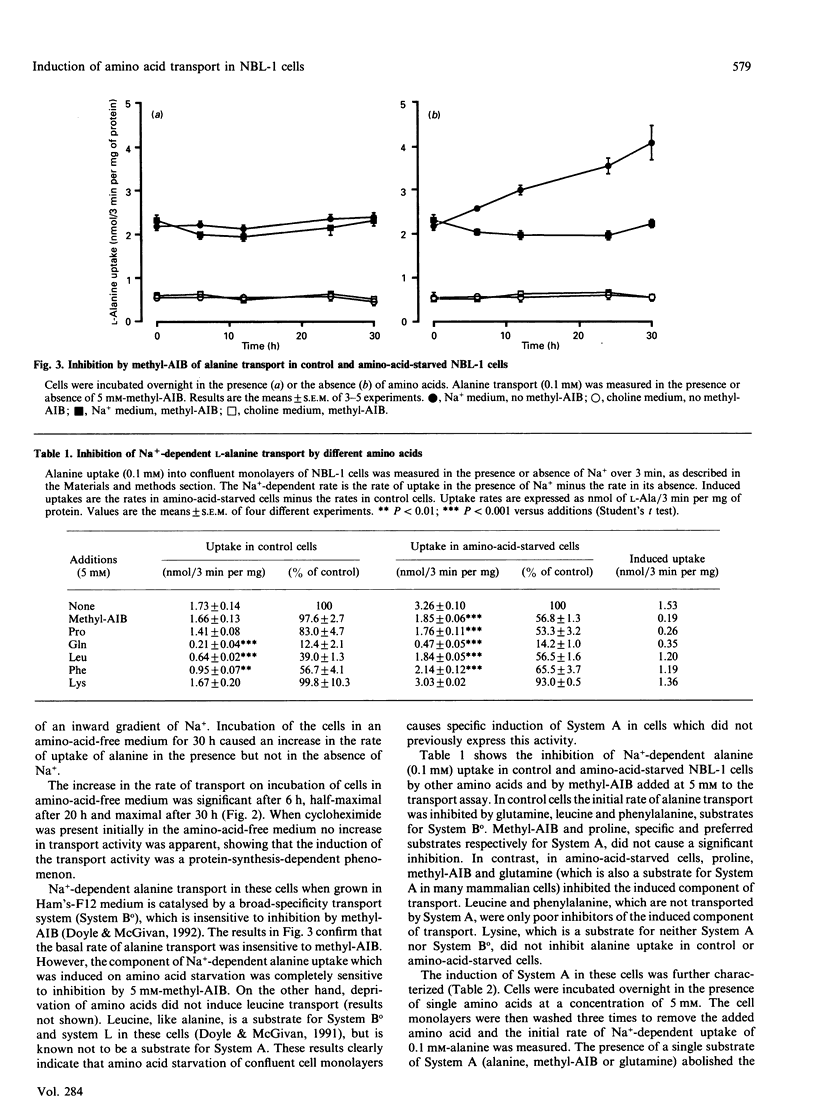
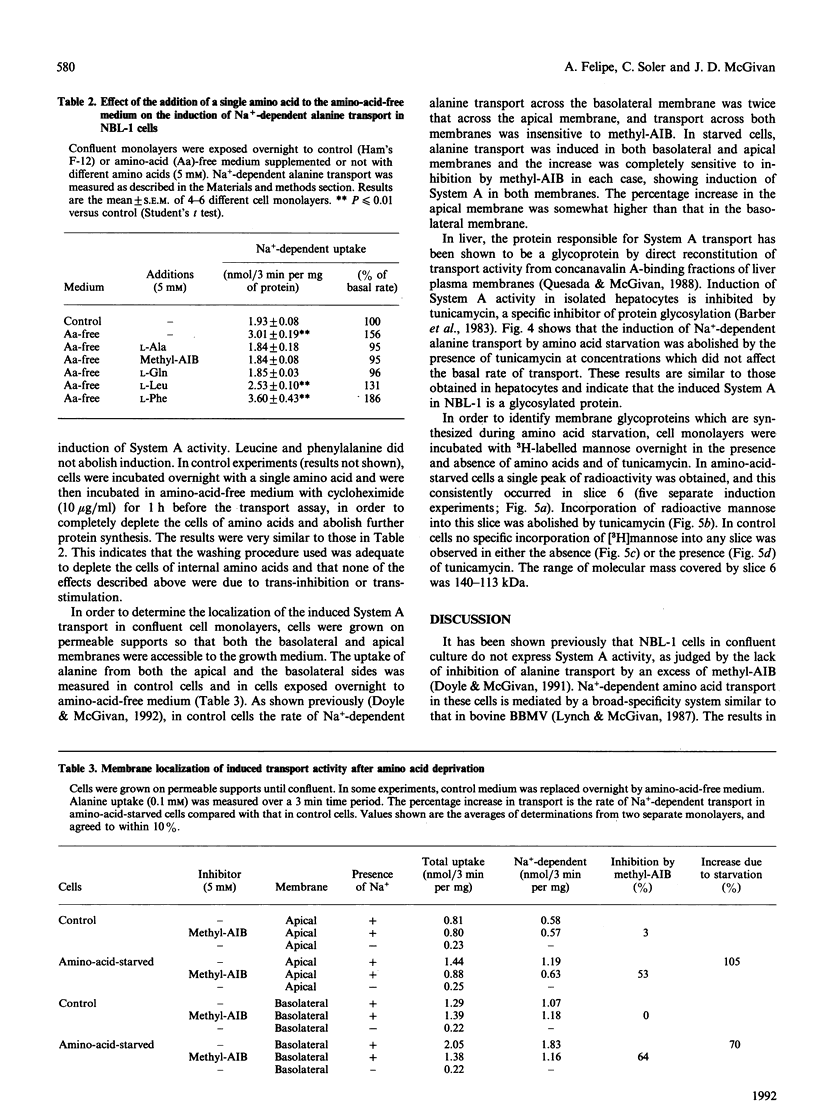
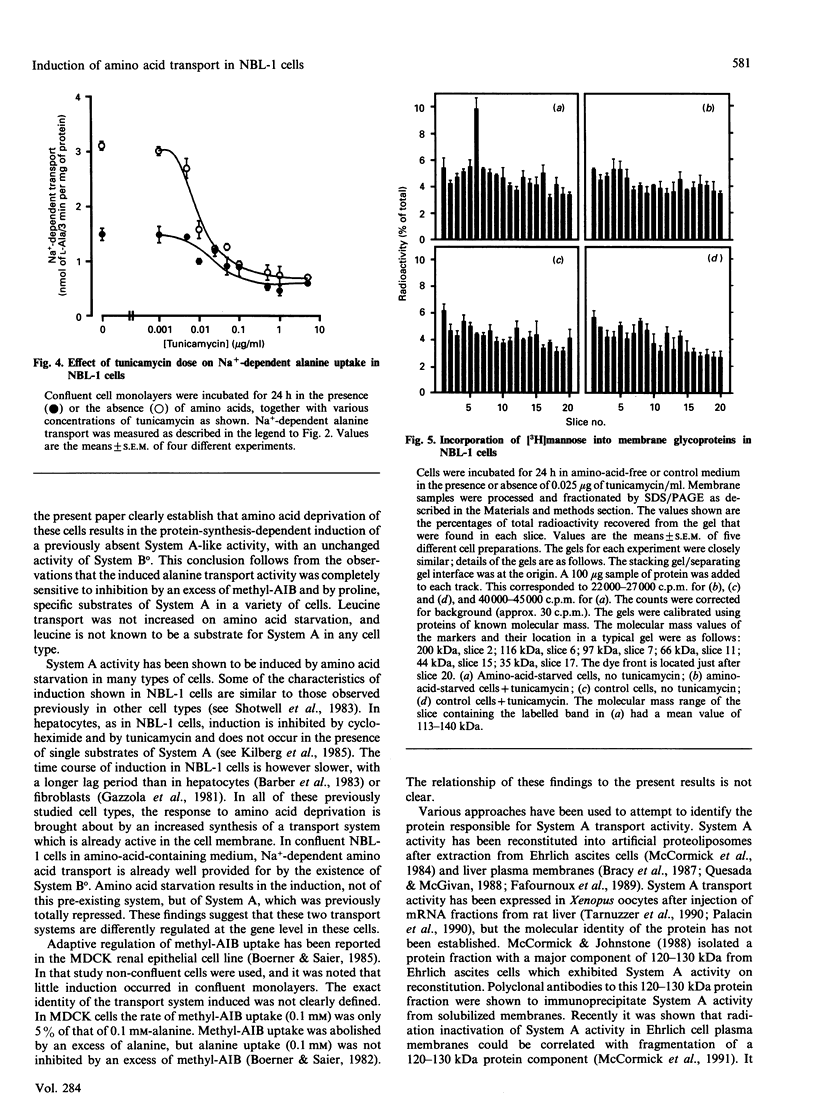
1. Amino acid deprivation of confluent monolayers of the bovine renal epithelial cell line NBL-1 causes a stimulation of Na(+)-dependent alanine transport. 2. This stimulation is mediated by a protein-synthesis-dependent induction of 2-(methylamino)isobutyric acid (methyl-AIB)-sensitive alanine transport activity (System A), which was not previously present in these cells. 3. Induction was prevented by the addition of methyl-AIB, alanine or glutamine. 4. Tunicamycin prevented the induction of alanine transport activity. 5. Induction of System A activity was accompanied by incorporation of [3H]mannose into a single membrane protein band of molecular mass 113-140 kDa. 6. These results are consistent with the possibility that induced System A activity in confluent NBL-1 cells is mediated by the synthesis of a 113-140 kDa membrane glycoprotein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber E. F., Handlogten M. E., Kilberg M. S. Induction of amino acid transport system A in rat hepatocytes is blocked by tunicamycin. J Biol Chem. 1983 Oct 10;258(19):11851–11855. [PubMed] [Google Scholar]
- Boerner P., Saier M. H., Jr Adaptive regulatory control of System A transport activity in a kidney epithelial cell line (MDCK) and in a transformed variant (MDCK-T1). J Cell Physiol. 1985 Feb;122(2):308–315. doi: 10.1002/jcp.1041220221. [DOI] [PubMed] [Google Scholar]
- Boerner P., Saier M. H., Jr Growth regulation and amino acid transport in epithelial cells: influence of culture conditions and transformation on A, ASC, and L transport activities. J Cell Physiol. 1982 Nov;113(2):240–246. doi: 10.1002/jcp.1041130209. [DOI] [PubMed] [Google Scholar]
- Bracy D. S., Schenerman M. A., Kilberg M. S. Solubilization and reconstitution of hepatic System A-mediated amino acid transport. Preparation of proteoliposomes containing glucagon-stimulated transport activity. Biochim Biophys Acta. 1987 May 12;899(1):51–58. doi: 10.1016/0005-2736(87)90238-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990 Jan;70(1):43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Doyle F. A., McGivan J. D. The bovine renal epithelial cell line NBL-1 expresses a broad specificity Na(+)-dependent neutral amino acid transport system (System Bo) similar to that in bovine renal brush border membrane vesicles. Biochim Biophys Acta. 1992 Feb 17;1104(1):55–62. doi: 10.1016/0005-2736(92)90131-5. [DOI] [PubMed] [Google Scholar]
- Fafournoux P., Dudenhausen E. E., Kilberg M. S. Solubilization and reconstitution characteristics of hepatic system A-mediated amino acid transport. J Biol Chem. 1989 Mar 25;264(9):4805–4811. [PubMed] [Google Scholar]
- Gazzola G. C., Dall'Asta V., Guidotti G. G. Adaptive regulation of amino acid transport in cultured human fibroblasts. Sites and mechanism of action. J Biol Chem. 1981 Apr 10;256(7):3191–3198. [PubMed] [Google Scholar]
- Guidotti G. G., Borghetti A. F., Gazzola G. C. The regulation of amino acid transport in animal cells. Biochim Biophys Acta. 1978 Dec 15;515(4):329–366. doi: 10.1016/0304-4157(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Helps C. R., McGivan J. Adaptive regulation of Na(+)-dependent phosphate transport in the bovine renal epithelial cell line NBL-1. Identification of the phosphate transporter as a 55-kDa glycoprotein. Eur J Biochem. 1991 Sep 15;200(3):797–803. doi: 10.1111/j.1432-1033.1991.tb16247.x. [DOI] [PubMed] [Google Scholar]
- Kilberg M. S., Barber E. F., Handlogten M. E. Characteristics and hormonal regulation of amino acid transport system A in isolated rat hepatocytes. Curr Top Cell Regul. 1985;25:133–163. doi: 10.1016/b978-0-12-152825-6.50009-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lynch A. M., McGivan J. D. A rapid method for the reconstitution of Na+-dependent neutral amino acid transport from bovine renal brush-border membranes. Biochem J. 1987 Jun 15;244(3):503–508. doi: 10.1042/bj2440503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J. I., Jetté M., Potier M., Béliveau R., Johnstone R. M. Molecular size of a Na(+)-dependent amino acid transporter in Ehrlich ascites cell plasma membranes estimated by radiation inactivation. Biochemistry. 1991 Apr 16;30(15):3704–3709. doi: 10.1021/bi00229a016. [DOI] [PubMed] [Google Scholar]
- McCormick J. I., Johnstone R. M. Simple and effective purification of a Na+-dependent amino acid transport system from Ehrlich ascites cell plasma membrane. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7877–7881. doi: 10.1073/pnas.85.21.7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J. I., Tsang D., Johnstone R. M. A simple and efficient method for reconstitution of amino acid and glucose transport systems from Ehrlich ascites cells. Arch Biochem Biophys. 1984 Jun;231(2):355–365. doi: 10.1016/0003-9861(84)90398-9. [DOI] [PubMed] [Google Scholar]
- Palacin M., Werner A., Dittmer J., Murer H., Biber J. Expression of rat liver Na+/L-alanine co-transport in Xenopus laevis oocytes. Effect of glucagon in vivo. Biochem J. 1990 Aug 15;270(1):189–195. doi: 10.1042/bj2700189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quamme G., Biber J., Murer H. Sodium-phosphate cotransport in OK cells: inhibition by PTH and "adaptation" to low phosphate. Am J Physiol. 1989 Dec;257(6 Pt 2):F967–F973. doi: 10.1152/ajprenal.1989.257.6.F967. [DOI] [PubMed] [Google Scholar]
- Quesada A. R., McGivan J. D. A rapid method for the functional reconstitution of amino acid transport systems from rat liver plasma membranes. Partial purification of System A. Biochem J. 1988 Nov 1;255(3):963–969. doi: 10.1042/bj2550963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh O., Kudo Y., Shikata H., Yamada K., Kawasaki T. Characterization of amino-acid transport systems in guinea-pig intestinal brush-border membrane. Biochim Biophys Acta. 1989 Oct 16;985(2):120–126. doi: 10.1016/0005-2736(89)90355-6. [DOI] [PubMed] [Google Scholar]
- Schwegler J. S., Heuner A., Silbernagl S. Electrogenic transport of neutral and dibasic amino acids in a cultured opossum kidney cell line (OK). Pflugers Arch. 1989 Sep;414(5):543–550. doi: 10.1007/BF00580989. [DOI] [PubMed] [Google Scholar]
- Sepúlveda F. V., Pearson J. D. Characterization of neutral amino acid uptake by cultured epithelial cells from pig kidney. J Cell Physiol. 1982 Aug;112(2):182–188. doi: 10.1002/jcp.1041120205. [DOI] [PubMed] [Google Scholar]
- Shotwell M. A., Kilberg M. S., Oxender D. L. The regulation of neutral amino acid transport in mammalian cells. Biochim Biophys Acta. 1983 May 24;737(2):267–284. doi: 10.1016/0304-4157(83)90003-5. [DOI] [PubMed] [Google Scholar]
- Stevens B. R., Kaunitz J. D., Wright E. M. Intestinal transport of amino acids and sugars: advances using membrane vesicles. Annu Rev Physiol. 1984;46:417–433. doi: 10.1146/annurev.ph.46.030184.002221. [DOI] [PubMed] [Google Scholar]
- Tarnuzzer R. W., Campa M. J., Qian N. X., Englesberg E., Kilberg M. S. Expression of the mammalian system A neutral amino acid transporter in Xenopus oocytes. J Biol Chem. 1990 Aug 15;265(23):13914–13917. [PubMed] [Google Scholar]


