Abstract
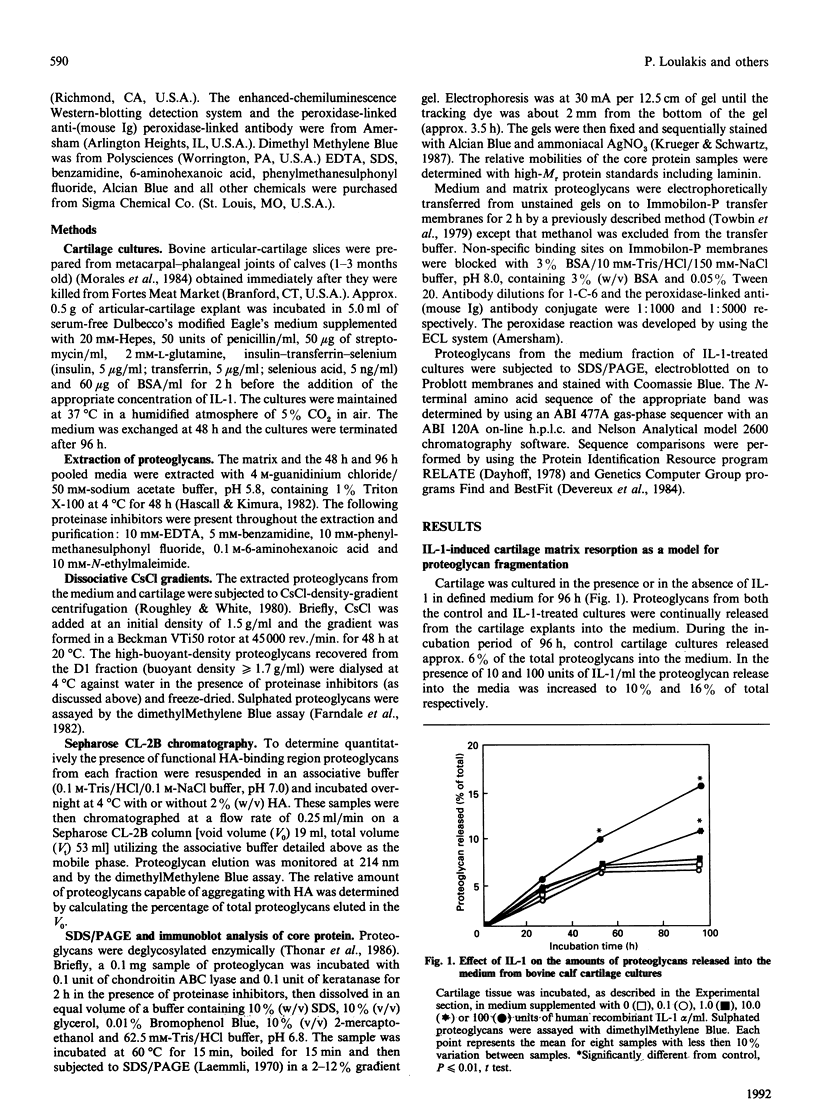
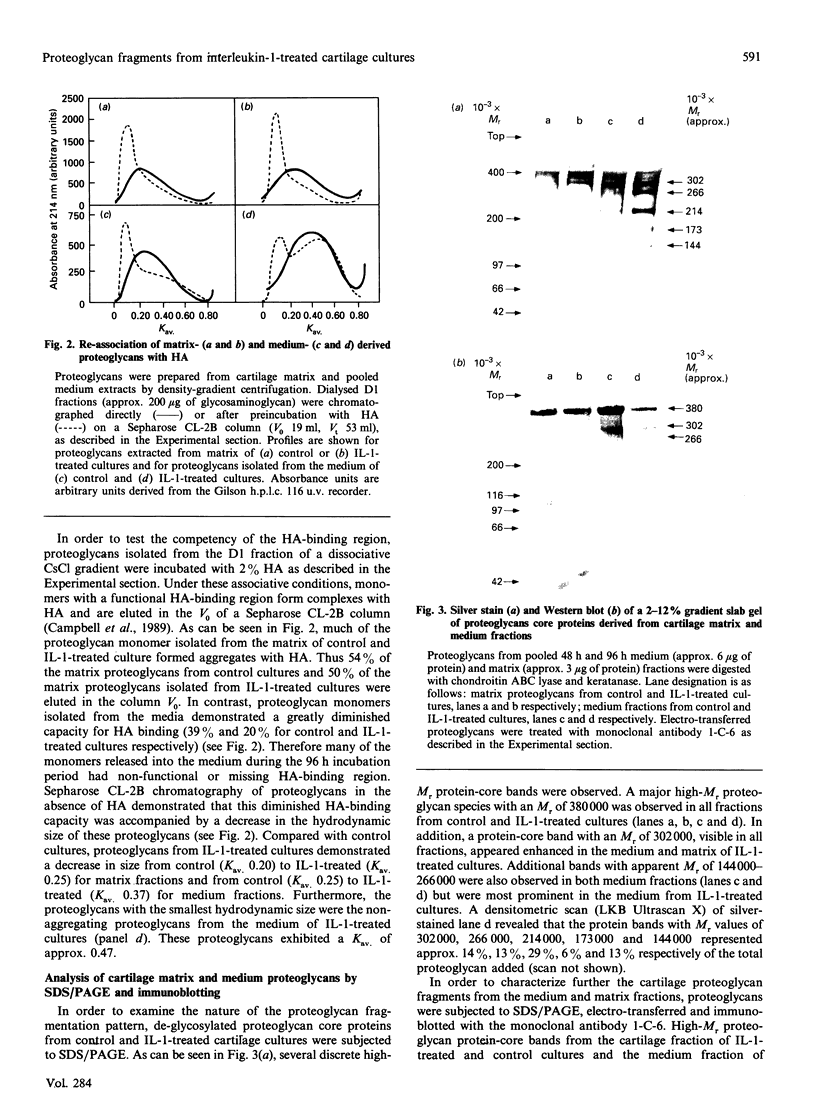
Bovine articular cartilage was cultured both in the presence and in the absence of human recombinant interleukin-1 alpha (IL-1) (100 units/ml). Addition of this cytokine stimulated matrix degradation approx. 3-fold. This increased degradation permitted characterization of the large chondroitin sulphate proteoglycan (aggrecan) fragments accumulating in the media. When compared with controls, the proteoglycans isolated from the medium of cultures treated with IL-1 exhibited a decrease in the Kav. (control 0.25; IL-1-treated 0.37), determined by Sepharose CL-2B chromatography. This decrease in proteoglycan size was accompanied by a decreased ability of these monomers to associate with hyaluronic acid. Thus only 20% of the proteoglycans isolated from the medium of IL-1-treated cultures, compared with 39% for control cultures, had the capacity to form high-M(r) aggregates with hyaluronic acid. SDS/PAGE analysis of the proteoglycans from the media of IL-1-treated cultures demonstrated several large proteoglycan protein-core bands (M(r) 144,000-380,000). The protein-core bands with M(r) 144,000-266,000 exhibited a significantly decreased reactivity with monoclonal antibody 1-C-6 (specific for domains G1 and G2). The N-terminal amino acid sequence of four of these protein-core bands (M(r) 144,000, 173,000, 214,000 and 266,000) yielded sequences LGQRPPV-Y-PQLF(E), AGEGP(S)GILEL-GAP(S)-AP(D)M, GLG-VEL-LPGE and (A)RGSVIL-AKPDFEV-P-A. A comparison of these N-terminal amino acid sequences with the published proteoglycan sequence for bovine nasal cartilage [Oldberg, Antonsson & Heinegård (1987) Biochem. J. 243, 255-259], rat chondrosarcoma [Doege, Sasaki, Horigan, Hassell & Yamada (1987) J. Biol. Chem. 262, 17757-17769] and human articular cartilage [Doege, Sasaki, Kimura & Yamada (1991) J. Biol. Chem. 266, 894-902] permitted assignment of their relative positions on the core protein. Furthermore, on the basis of this similarity to published sequence, putative sites of enzymic cleavage were constructed. These theoretical cleavage sites revealed a glutamic acid residue in the P1 position and an uncharged polar or non-polar residue in the P1' position.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arner E. C., Kirkland J. J. Effect of interleukin-1 on the size distribution of cartilage proteoglycans as determined by sedimentation field flow fractionation. Biochim Biophys Acta. 1989 Oct 13;993(1):100–107. doi: 10.1016/0304-4165(89)90148-7. [DOI] [PubMed] [Google Scholar]
- Benton H. P., Tyler J. A. Inhibition of cartilage proteoglycan synthesis by interleukin I. Biochem Biophys Res Commun. 1988 Jul 15;154(1):421–428. doi: 10.1016/0006-291x(88)90703-6. [DOI] [PubMed] [Google Scholar]
- Campbell I. K., Piccoli D. S., Butler D. M., Singleton D. K., Hamilton J. A. Recombinant human interleukin-1 stimulates human articular cartilage to undergo resorption and human chondrocytes to produce both tissue- and urokinase-type plasminogen activator. Biochim Biophys Acta. 1988 Nov 17;967(2):183–194. doi: 10.1016/0304-4165(88)90008-6. [DOI] [PubMed] [Google Scholar]
- Campbell I. K., Roughley P. J., Mort J. S. The action of human articular-cartilage metalloproteinase on proteoglycan and link protein. Similarities between products of degradation in situ and in vitro. Biochem J. 1986 Jul 1;237(1):117–122. doi: 10.1042/bj2370117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Handley C. J., D'Souza S. E. Turnover of proteoglycans in articular-cartilage cultures. Characterization of proteoglycans released into the medium. Biochem J. 1989 Apr 1;259(1):21–25. doi: 10.1042/bj2590021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Handley C. J., Hascall V. C., Campbell R. A., Lowther D. A. Turnover of proteoglycans in cultures of bovine articular cartilage. Arch Biochem Biophys. 1984 Oct;234(1):275–289. doi: 10.1016/0003-9861(84)90350-3. [DOI] [PubMed] [Google Scholar]
- Campbell M. A., Handley C. J. The effect of retinoic acid on proteoglycan turnover in bovine articular cartilage cultures. Arch Biochem Biophys. 1987 Oct;258(1):143–155. doi: 10.1016/0003-9861(87)90331-6. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doege K. J., Sasaki M., Kimura T., Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991 Jan 15;266(2):894–902. [PubMed] [Google Scholar]
- Doege K., Sasaki M., Horigan E., Hassell J. R., Yamada Y. Complete primary structure of the rat cartilage proteoglycan core protein deduced from cDNA clones. J Biol Chem. 1987 Dec 25;262(36):17757–17767. [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Handley C. J., McQuillan D. J., Hascall G. K., Robinson H. C., Lowther D. A. The effect of serum on biosynthesis of proteoglycans by bovine articular cartilage in culture. Arch Biochem Biophys. 1983 Jul 1;224(1):206–223. doi: 10.1016/0003-9861(83)90205-9. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Kimura J. H. Proteoglycans: isolation and characterization. Methods Enzymol. 1982;82(Pt A):769–800. doi: 10.1016/0076-6879(82)82102-2. [DOI] [PubMed] [Google Scholar]
- Hubbard J. R., Steinberg J. J., Bednar M. S., Sledge C. B. Effect of purified human interleukin-1 on cartilage degradation. J Orthop Res. 1988;6(2):180–187. doi: 10.1002/jor.1100060204. [DOI] [PubMed] [Google Scholar]
- Kempson G. E., Tuke M. A., Dingle J. T., Barrett A. J., Horsfield P. H. The effects of proteolytic enzymes on the mechanical properties of adult human articular cartilage. Biochim Biophys Acta. 1976 May 28;428(3):741–760. doi: 10.1016/0304-4165(76)90205-1. [DOI] [PubMed] [Google Scholar]
- Krueger R. C., Jr, Schwartz N. B. An improved method of sequential alcian blue and ammoniacal silver staining of chondroitin sulfate proteoglycan in polyacrylamide gels. Anal Biochem. 1987 Dec;167(2):295–300. doi: 10.1016/0003-2697(87)90167-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maroudas A., Ziv I., Weisman N., Venn M. Studies of hydration and swelling pressure in normal and osteoarthritic cartilage. Biorheology. 1985;22(2):159–169. doi: 10.3233/bir-1985-22206. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., Pelletier J. P., Cloutier J. M., Howell D. S., Ghandur-Mnaymneh L., Woessner J. F., Jr Neutral proteases capable of proteoglycan digesting activity in osteoarthritic and normal human articular cartilage. Arthritis Rheum. 1984 Mar;27(3):305–312. doi: 10.1002/art.1780270310. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., Pelletier J. P. Neutral metalloproteases and age related changes in human articular cartilage. Ann Rheum Dis. 1987 May;46(5):363–369. doi: 10.1136/ard.46.5.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales T. I., Wahl L. M., Hascall V. C. The effect of bacterial lipopolysaccharides on the biosynthesis and release of proteoglycans from calf articular cartilage cultures. J Biol Chem. 1984 Jun 10;259(11):6720–6729. [PubMed] [Google Scholar]
- Nguyen Q., Murphy G., Roughley P. J., Mort J. S. Degradation of proteoglycan aggregate by a cartilage metalloproteinase. Evidence for the involvement of stromelysin in the generation of link protein heterogeneity in situ. Biochem J. 1989 Apr 1;259(1):61–67. doi: 10.1042/bj2590061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima T., Towle C. A., Mankin H. J., Treadwell B. V. Secretion of higher levels of active proteoglycanases from human osteoarthritic chondrocytes. Arthritis Rheum. 1986 Feb;29(2):292–295. doi: 10.1002/art.1780290219. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Antonsson P., Heinegård D. The partial amino acid sequence of bovine cartilage proteoglycan, deduced from a cDNA clone, contains numerous Ser-Gly sequences arranged in homologous repeats. Biochem J. 1987 Apr 1;243(1):255–259. doi: 10.1042/bj2430255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Howell D. S., Ghandur-Mnaymneh L., Enis J. E., Woessner J. F., Jr Collagenase and collagenolytic activity in human osteoarthritic cartilage. Arthritis Rheum. 1983 Jan;26(1):63–68. doi: 10.1002/art.1780260110. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Malemud C. J. Canine osteoarthritis: effects of endogenous neutral metalloproteoglycanases on articular cartilage proteoglycans. J Orthop Res. 1988;6(3):379–388. doi: 10.1002/jor.1100060309. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe A., Tyler J. A., Hardingham T. E. Articular cartilage cultured with interleukin 1. Increased release of link protein, hyaluronate-binding region and other proteoglycan fragments. Biochem J. 1986 Sep 1;238(2):571–580. doi: 10.1042/bj2380571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J Biol Chem. 1980 Jan 10;255(1):217–224. [PubMed] [Google Scholar]
- Roughley P. J., White R. J., Poole A. R. Identification of a hyaluronic acid-binding protein that interferes with the preparation of high-buoyant-density proteoglycan aggregates from adult human articular cartilage. Biochem J. 1985 Oct 1;231(1):129–138. doi: 10.1042/bj2310129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., White R. J., Poole A. R., Mort J. S. The inability to prepare high-buoyant-density proteoglycan aggregates from extracts of normal adult human articular cartilage. Biochem J. 1984 Aug 1;221(3):637–644. doi: 10.1042/bj2210637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J., Pilsworth L. M., Sarsfield S. J., Gavrilovic J., Heath J. K. Pig catabolin is a form of interleukin 1. Cartilage and bone resorb, fibroblasts make prostaglandin and collagenase, and thymocyte proliferation is augmented in response to one protein. Biochem J. 1984 Dec 1;224(2):461–466. doi: 10.1042/bj2240461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Neame P. J., Boynton R. E., Flannery C. R. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991 May 15;266(14):8683–8685. [PubMed] [Google Scholar]
- Smith R. L., Allison A. C., Schurman D. J. Induction of articular cartilage degradation by recombinant interleukin 1 alpha and 1 beta. Connect Tissue Res. 1989;18(4):307–316. doi: 10.3109/03008208909019079. [DOI] [PubMed] [Google Scholar]
- Stevens J. W., Oike Y., Handley C., Hascall V. C., Hampton A., Caterson B. Characteristics of the core protein of the aggregating proteoglycan from the Swarm rat chondrosarcoma. J Cell Biochem. 1984;26(4):247–259. doi: 10.1002/jcb.240260405. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
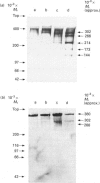
- Tyler J. A. Chondrocyte-mediated depletion of articular cartilage proteoglycans in vitro. Biochem J. 1985 Jan 15;225(2):493–507. doi: 10.1042/bj2250493. [DOI] [PMC free article] [PubMed] [Google Scholar]