Abstract
Background
In 2018 the World Health Organization recommended a switch to an all oral bedaquiline-based second-line regimen for treatment of drug-resistant tuberculosis (DR-TB). How these new second-line regimens fare in comparison to first-line regimens for treatment of drug-sensitive tuberculosis (DS-TB) is not well known.
Methods
In this study, we contemporaneously enrolled subjects with DS-TB (n = 31) or DR-TB (n = 23) and assessed their response to therapy with first-line (rifampin, isoniazid, ethambutol, pyrazinamide) or second-line (bedaquiline, pyrazinamide, levofloxacin, linezolid, clofazimine) regimens, respectively.
Results
We found that the early bactericidal activity of first- and second-line regimens was similar during the first 2 weeks of therapy as determined by BACTEC MGIT, colony-forming units, and a liquid limiting dilution assay capable of detecting differentially detectable/culturable Mycobacterium tuberculosis. Furthermore, an identical percentage (77.8%) of subjects from the DS-TB and DR-TB cohorts converted to culture negative after 2 months of therapy.
Conclusions
Despite presenting with more advanced disease at time of treatment, subjects with DR-TB receiving an all oral bedaquiline-based second-line treatment regimen displayed a similar microbiological response to therapy as subjects with DS-TB receiving a first-line treatment regimen.
Keywords: EBA, MDR, fluoroquinolone, diarylquinoline, treatment shortening
This study reports that the early bactericidal activity of a bedaquiline-based second-line regimen for drug-resistant tuberculosis (TB) is similar to that of the standard rifampin-based first-line regimen for drug-sensitive TB.
In 2021, an estimated 10.6 million individuals were diagnosed with tuberculosis (TB) and 1.6 million died of the disease [1]. Approximately 4% of these infections consisted of drug-resistant TB (DR-TB; defined as rifampin or multidrug resistance), which accounted for a disproportionate 12% of TB-related deaths [1]. DR-TB requires longer treatment regimens with second-line drugs that are more toxic and expensive, which strains public health resources and promotes treatment noncompliance, further driving drug resistance. Troublingly, rates of DR-TB are on the rise globally (up 3.1% from 2020 rates), which poses a threat for TB control and elimination efforts [1].
A major advancement in the treatment of DR-TB came with the approval of bedaquiline, a new diarylquinoline drug that is the current backbone of second-line regimens [2]. Clinical trials have repeatedly demonstrated that addition of bedaquiline to existing second-line regimens improves culture conversion rates and decreases mortality in patients with DR-TB [3–9]. Furthermore, replacement of second-line injectable aminoglycoside drugs with bedaquiline resulted in comparably high treatment success rates but with less toxicity [10–12]. These findings led to the decision in 2018 by the World Health Organization (WHO) to recommend a switch to all oral bedaquiline-based second-line regimens for treatment of DR-TB [13]. How these new second-line regimens fare in comparison to first-line regimens for the treatment of drug-sensitive TB (DS-TB), however, has not been thoroughly studied.
In this study, we contemporaneously enrolled subjects with DS- or DR-TB at the GHESKIO Centers in Port-au-Prince, Haiti, and monitored their response to first- or second-line therapy, respectively. The early bactericidal activity (EBA) of these 2 regimens, along with their corresponding effects on clinical signs and symptoms, during the first 2 months of therapy is presented.
METHODS
Study Population and Design
This was a prospective observational study conducted at the Groupe Haïtien d’Étude du Sarcome de Kaposi et des Infectieuses Opportuniste (GHESKIO) Centers in Haiti, and was approved by both the Weill Cornell Medical College and GHESKIO institutional review boards. All participants provided written informed consent.
All aspects about the study design, including exclusion/inclusion criteria and participant characteristics, have been previously reported [14–16]. In short, the following enrollment criteria were used for the DS-TB cohort: ≥18 years of age; diagnosis of active pulmonary TB based on clinical symptoms and signs, chest radiography, and Xpert MTB/RIF (Cepheid, Sunnyvale, California) positivity, without indication of rifampin resistance; no indication of extrapulmonary manifestations of TB; and no history of previous TB treatment. The DR-TB cohort met the same criteria, except participants were not excluded if they had previously been treated for DS-TB; Xpert indicated rifampin resistance; and drug susceptibility testing confirmed resistance to rifampin. Drug susceptibility testing for isoniazid, rifampin, and ethambutol was performed using the BACTEC MGIT SIRE kit and on 7H10 based on standard methods [17], and for pyrazinamide using the BACTEC MGIT Z kit.
Subjects with DS-TB were followed in the GHESKIO outpatient clinic for the duration of their directly observed therapy (DOT). These participants received isoniazid, rifampin, ethambutol, and pyrazinamide for 2 months and then isoniazid and rifampin for 4 months. Subjects with DR-TB were hospitalized in GHESKIO's inpatient multidrug-resistant TB hospital for approximately the first 4 months of therapy with DOT regimens comprised of bedaquiline (400 mg/day for 2 weeks then 200 mg 3 times per week for 22 weeks), levofloxacin (20 mg/kg daily for duration of treatment), linezolid (600 mg/day for 12 months), clofazimine (100 mg/day for duration of treatment), and pyrazinamide (30–40 mg/kg/day for duration of treatment). Of note, bedaquiline was discontinued after 6 months and linezolid after 12 months, with the remaining drugs continued to complete 20 months of therapy. Subjects who demonstrated pyrazinamide resistance continued to receive pyrazinamide for the duration of their therapy. TB signs and symptoms (cough, dyspnea, hemoptysis, fever, and pleuritic chest pain) were graded according to the Division of AIDS grading system [18].
Sputum Processing and Microbiological Assays
Overnight sputum was collected from both cohorts prior to initiation of therapy, and at 2 weeks and 2 months after initiation of therapy in a cool box with ice packs (4°C). Some sample collections were missed because of the coronavirus disease 2019 (COVID-19) pandemic, some samples resulted in contamination during culture or did not yield enough sputum to perform culture, resulting in a variable number of sputum samples available for analysis at each timepoint.
Experimental work took place in a biosafety level 3 laboratory at GHESKIO with appropriate safety guidelines and personal protective equipment. Decontamination of sputum, preparation of culture filtrate (CF), and colony-forming unit (CFU), limiting dilution (LD), and BACTEC MGIT assays were conducted as previously reported [14, 15, 19, 20]. There are Mycobacterium tuberculosis (Mtb) populations in human sputum that are not detected by standard culture methods and these Mtb are not enumerated by CFU on solid agar [14, 19, 20]. These Mtb can be detected using an LD assay (with or without CF) and are called differentially detectable/culturable Mtb (DD Mtb) [19]. Positivity for DD Mtb was defined as previously reported [14, 20], namely when the CFU value was less than the lower bound of the 95% confidence interval (CI) of the most probable number (MPN) estimation from the LD assay with or without CF, which promotes recovery of DD Mtb populations from certain sputum samples [14, 15, 20]. LD assays performed with and without CF are referred to as MPN+CF and MPN−CF, respectively. To quantify viable Mtb from participant sputa, the highest Mtb number per milliliter obtained from CFU, MPN−CF, or MPN+CF was used (referred to as MtbMax). Rate of Mtb killing during the first 2 weeks of therapy was only calculated for subjects who had data available at both timepoints. Lack of culture conversion to negative at month 2 was defined as positivity by at least 1 culture method (BACTEC MGIT, CFU, MPN−CF, or MPN+CF).
Data Analysis
Data were uploaded to REDCap [21, 22], a secure web-based software platform designed to support data capture for research studies. Data were summarized and analyzed using R software 4.2.2. Mtb count obtained by either CFU or MPN-LD assays are presented as log10 values per milliliter of sputum. Continuous variables were summarized by median and interquartile range and categorical variables were summarized by count and percentage. Continuous measures were compared using the Mann–Whitney test and categorical measures were compared by the χ2 test between groups. Rate of Mtb killing during early treatment is defined by the absolute difference in the Mtb counts quantified at baseline and after 2 weeks of therapy. For factors associated with continuous measures such as the rate of Mtb killing during early treatment, we performed univariate linear regression and reported the regression coefficients and 95% CIs.
RESULTS
Study Population
We enrolled 31 subjects with DS-TB and 23 subjects with DR-TB at the GHESKIO Centers in Haiti from 2018 to 2021 (Supplementary Table 1). Subjects with DS-TB received a standard first-line regimen consisting of isoniazid, rifampin, ethambutol, and pyrazinamide for 2 months and then isoniazid and rifampin for 4 months. Subjects with DR-TB received a second-line regimen consisting of bedaquiline, pyrazinamide, levofloxacin, linezolid, and clofazimine for the first 6 months of therapy, with bedaquiline discontinued after 6 months and linezolid after 12 months (for a total of 20 months of therapy).
Subjects with DS-TB were all treatment naive, whereas 82.6% of subjects with DR-TB had previously received treatment for DS-TB (Table 1). All subjects with DR-TB were resistant to rifampin, and 21 of 23 were also resistant to isoniazid (Supplementary Table 1).
Table 1.
Participant Characteristics for the Drug-Sensitive and Drug-Resistant Tuberculosis Cohorts at Time of Enrollment
| Characteristic | DS-TB (n = 31) | DR-TB (n = 23) | P Value |
|---|---|---|---|
| Age, y, median (IQR) | 31.0 (25.0–39.0) | 33.0 (27.5–42.5) | .575 |
| Male sex | 19 (61.3) | 13 (56.5) | .942 |
| HIV positive | 2 (6.5) | 4 (17.4) | .408 |
| Xpert positivity | .528 | ||
| High | 13 (41.9) | 10 (43.5) | |
| Medium | 12 (38.7) | 11 (47.8) | |
| Low/very low | 6 (19.4) | 2 (8.7) | |
| Bilateral disease | 9 (30.0) | 14 (60.9) | .049 |
| Presence of cavities | 14 (46.7) | 10 (43.5) | 1.000 |
| Creatinine, mg/dL, median (IQR) | 0.70 (0.60–0.80) | 0.60 (0.55–0.70) | .169 |
| Hemoglobin, g/dL, median (IQR) | 10.7 (9.57–11.35) | 10.6 (9.98–11.45) | .840 |
| Marital status | .585 | ||
| Married | 5 (16.1) | 3 (13.0) | |
| Partnered | 7 (22.6) | 7 (30.4) | |
| Single/separated | 19 (61.3) | 13 (56.5) | |
| Daily income, USD, median (IQR) | 1.18 (0.0–5.36) | 1.78 (0.0–6.25) | .887 |
| Highest education | .861 | ||
| None | 4 (12.9) | 4 (17.4) | |
| Primary | 8 (25.8) | 8 (34.8) | |
| Secondary | 17 (54.8) | 9 (39.1) | |
| University/professional | 2 (6.5) | 2 (8.7) | |
| Previously treated for tuberculosis | 0 (0.0) | 19 (82.6) | <.001 |
P-values less than 0.05 have been bolded. Data are presented as No. (%) unless otherwise indicated.
Abbreviations: DR-TB, drug-resistant tuberculosis; DS-TB, drug-sensitive tuberculosis; HIV, human immunodeficiency virus; IQR, interquartile range; USD, United States dollars.
Clinical Response to Therapy
At time of enrollment, subjects with DR-TB had a 2-fold higher rate of bilateral disease (P = .049) and a 50% higher rate of dyspnea (P = .046) in comparison to subjects with DS-TB (Tables 1 and 2). After 2 months of therapy, the 2 cohorts were comparable in terms of clinical signs and symptoms, except for a modestly higher median pulse oximetry value for subjects with DR-TB (99% for DR-TB vs 98% for DS-TB, P = .003) (Table 2, Supplementary Table 2).
Table 2.
Clinical Characteristics Prior to Therapy and at 2 Months Post–Initiation of Therapy for the Drug-Sensitive and Drug-Resistant Tuberculosis Cohorts
| Characteristic | Day 0 (Pretreatment) | Month 2 (Post–Initiation of Therapy) | ||||
|---|---|---|---|---|---|---|
| DS-TB (n = 31) | DR-TB (n = 23) | P Value | DS-TB (n = 21–24)a | DR-TB (n = 19–21)a | P Value | |
| Dyspnea | 18 (58.1) | 20 (87.0) | .046 | 12 (50.0) | 7 (35.0) | .487 |
| Cough | 31 (100.0) | 22 (95.7) | .880 | 19 (79.2) | 13 (65.0) | .477 |
| Fever | 10 (32.3) | 5 (22.7) | .653 | 0 (0.0) | 0 (0.0) | NA |
| Blood-tinged sputum | 0 (0.0) | 2 (8.7) | .345 | 1 (4.2) | 1 (5.3) | 1.00 |
| Frank hemoptysis | 0 (0.0) | 0 (0.0) | NA | 0 (0.0) | 0 (0.0) | NA |
| Pleuritic chest pain | 10 (32.3) | 13 (56.5) | .132 | 10 (41.7) | 10 (50.0) | .804 |
| BMI, kg/m2, median (IQR) | 19.0 (17.3–21.0) | 17.9 (17.0–19.6) | .407 | 19.6 (18.4–21.9) | 18.7 (17.4–20.9) | .289 |
| Pulse oximetry, %, median (IQR) | 97.00 (95.2–98.0) | 97.00 (96.0–98.5) | .386 | 98.0 (96.0–98.0) | 99.0 (97.8–99.0) | .003 |
P-values less than 0.05 have been bolded. Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; DR-TB, drug-resistant tuberculosis; DS-TB, drug-sensitive tuberculosis; IQR, interquartile range; NA, not applicable; USD, United States dollars.
aNot all participant characteristics were recorded for all participants at month 2, resulting in the variable n values.
Early Bactericidal Activity of First- and Second-line Regimens
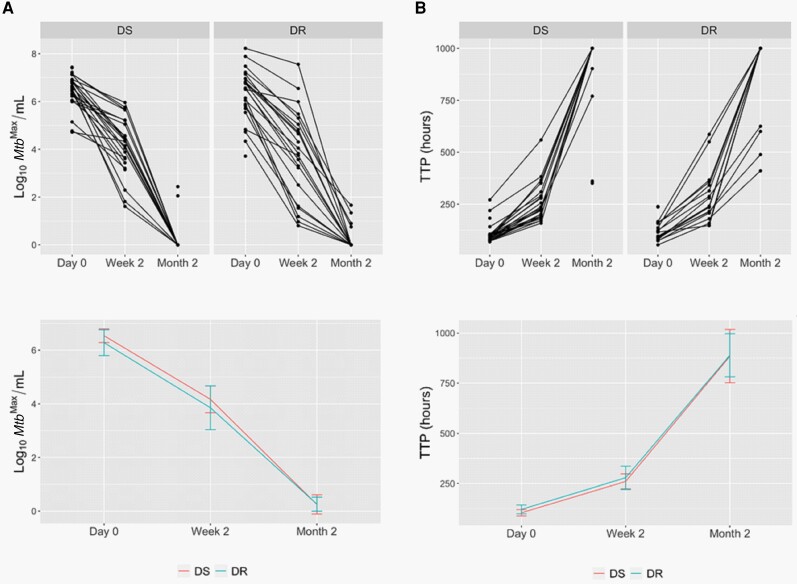
We next assessed the EBA of first and second-line regimens during the first 2 months of therapy. We used 3 assays to quantitate viable Mtb: (1) the BACTEC MGIT automated liquid culture system; (2) CFU on solid agar; and (3) liquid LD assay with and without CF that is capable of detecting DD Mtb, which are Mtb persister populations that fail to grow either on solid medium or in liquid medium unless the suspension has been extensively diluted. Of note, the rate of Mtb killing during the first 2 weeks of therapy was similar for first- and second-line regimens as determined by all microbiological assays (Figure 1, Table 3). Furthermore, an identical percentage (77.8%) of subjects from the 2 cohorts converted to culture negative after 2 months of therapy (Supplementary Table 3). Therefore, the 2 regimens exhibit similar bactericidal activity against Mtb during the first 2 months of therapy.
Figure 1.
Quantification of viable Mycobacterium tuberculosis (Mtb) from the sputa of subjects with drug-sensitive (DS) or drug-resistant (DR) tuberculosis during the first 2 months of therapy with first-line or second-line regimens, respectively. A, Maximum Mtb count (MtbMax) obtained by colony-forming units or most probable number from the liquid limiting dilution assay performed with or without culture filtrate. B, Time to positivity (TTP) by BACTEC MGIT. Top panels show data per subject and bottom panels show median values per cohort, with error bars representing 95% confidence intervals.
Table 3.
Quantification of Viable Mycobacterium tuberculosis (Mtb) by Different Microbiological Methods Demonstrates That the Drug-Sensitive and Drug-Resistant Cohorts Display Similar Mtb Counts in Their Sputa Prior to Therapy (Day 0) and at 2 Weeks Post–Initiation of Therapy
| Method | Day 0 (Pretreatment) | Week 2 (Post–Initiation of Therapy) | Δ (Week 2 vs Day 0) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DS-TB (n = 30–31)a | DR-TB (n = 22–23)a | P Value | DS-TB (n = 24–25)a | DR-TB (n = 18–22)a | P Value | DS-TB (n = 24–25)a | DR-TB (n = 18–22)a | P Value | |
| Log10 CFU, Mtb/mL | 6.5 (6.1–6.8) | 6.2 (5.2–6.7) | .24 | 3.6 (3.1–4.4) | 3.7 (1.8–4.7) | .73 | 2.3 (2.0–3.1) | 2.4 (2.0–3.2) | .96 |
| Log10 MPN−CF, Mtb/mL | 6.6 (6.2–6.9) | 6.3 (5.6–6.8) | .21 | 4.0 (3.2–5.1) | 3.7 (1.4–4.7) | .45 | 2.2 (1.2–3.0) | 2.5 (2.1–3.4) | .26 |
| Log10 MPN+CF, Mtb/mL | 6.4 (6.1–6.7) | 6.5 (5.6–6.9) | .62 | 4.2 (3.4–5.1) | 4.0 (3.2–5.1) | .80 | 2.0 (1.3–2.8) | 2.2 (1.5–3.3) | .41 |
| Log10MtbMax, Mtb/mLb | 6.7 (6.3–6.9) | 6.5 (5.8–6.9) | .33 | 4.3 (3.6–5.1) | 3.9 (2.7–5.0) | .55 | 2.1 (1.1–2.9) | 2.3 (1.8–3.2) | .30 |
| TTP, h | 93.5 (79.0–102.5) | 105.7 (90.1–152.2) | .13 | 230.5 (193.0–290.0) | 238.0 (210.2–327.5) | .73 | 148.5 (118.5–178.0) | 150.2 (127.6–199.5) | .47 |
Data are presented as median (interquartile range) unless otherwise indicated.
Abbreviations: CFU, colony-forming units; DR-TB, drug-resistant tuberculosis; DS-TB, drug-sensitive tuberculosis; MPN–CF, most probable number from the liquid limiting dilution assay performed without culture filtrate; MPN+CF, most probable number from the liquid limiting dilution assay performed with culture filtrate; Mtb, Mycobacterium tuberculosis; MtbMax, maximum Mycobacterium tuberculosis count; TTP, time to positivity.
aVariable n value at each timepoint is a consequence of contamination events, missed collection timepoints, or insufficient sample quantity to perform microbiological assays.
bThe maximum Mtb count obtained by CFU, MPN−CF, or MPN+CF.
Multivariate regression analyses did not identify differences in Mtb killing during the first 2 weeks of therapy when adjusting for differences in baseline characteristics between the 2 cohorts. However, when limiting analyses only to subjects with bilateral disease, the DR-TB cohort did show improved rates of Mtb killing during the first 2 weeks of therapy in comparison to the DS-TB cohort (P < .05) (Supplementary Table 4). This difference, however, was not statistically significant when extended to the first 2 months of therapy (Supplementary Table 4).
Participant Characteristics That Correlate With Culture Conversion at Month 2
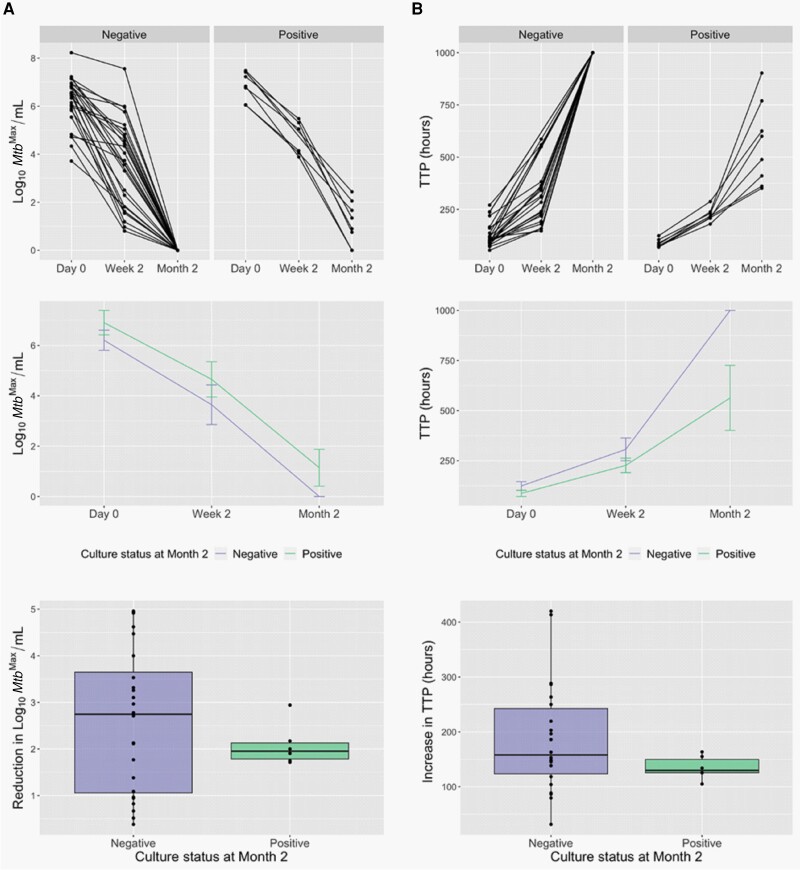
Next, we examined whether any participant characteristics correlated with lack of culture conversion to negative at month 2. Given the small number of subjects positive by culture at this timepoint, we combined the cohorts for these analyses. Notably, subjects who were culture positive at month 2 all had unresolved dyspnea (P < .001) and demonstrated a >20-fold lower gain in weight (P = .043) in comparison to subjects who were culture negative (Supplementary Table 5). Consistent with previous reports [23–25], high Mtb load (P < .05) at time of enrollment significantly correlated with culture positivity at month 2 (Figure 2, Supplementary Table 6), though the rate of Mtb killing during the first 2 weeks of therapy did not (Figure 2, Supplementary Table 7). Low BMI (P < .05) at time of enrollment and dyspnea (P = .001), pleuritic chest pain (P = .019), and low BMI (P = .013) at 2 months of therapy (Supplementary Tables 8 and 9) also correlated with lack of culture conversion to negative.
Figure 2.
Culture positivity at month 2 is associated with high Mycobacterium tuberculosis (Mtb) load prior to initiation of therapy as determined by the maximum Mtb count (MtbMax) obtained by colony-forming units or most probable number from the liquid limiting dilution assay performed with or without culture filtrate (A, P = .043) or time to positivity (TTP) by BACTEC MGIT (B, P = .027). However, the rate of Mtb killing during the first 2 weeks of therapy is similar for the 2 groups. Top panels show data per subject, middle panels show median values per cohort ± 95% confidence interval, and bottom panels show box plots for the reduction in Mtb counts during the first 2 weeks of therapy based on culture status at month 2. Drug-sensitive and drug-resistant cohorts are combined in this analysis.
Participant Characteristics That Correlate With Rate of Mtb Killing With Therapy
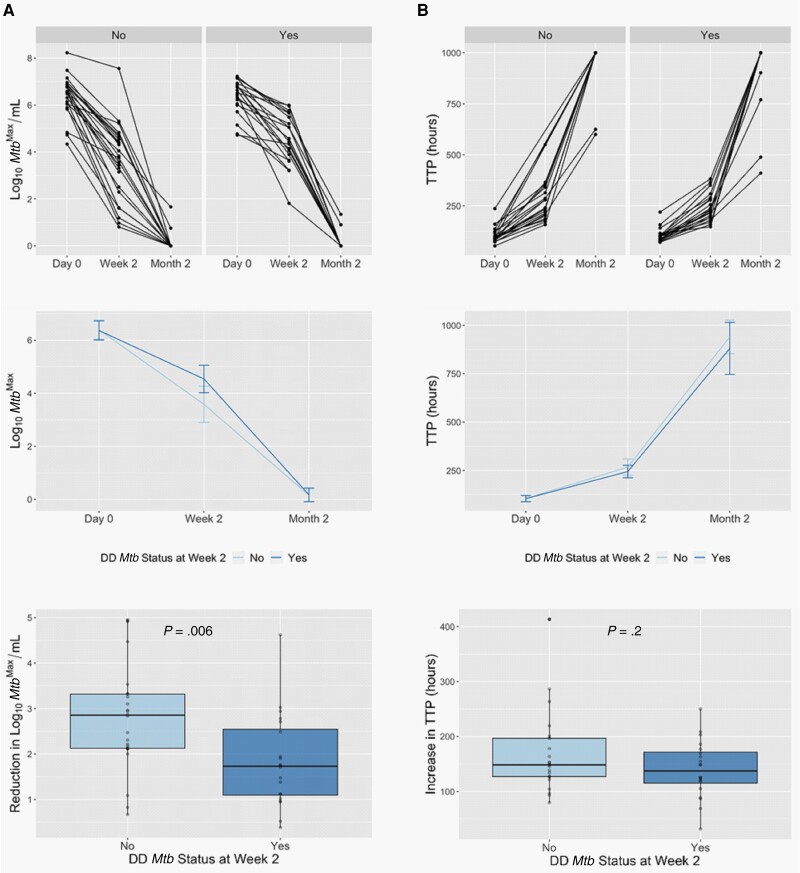
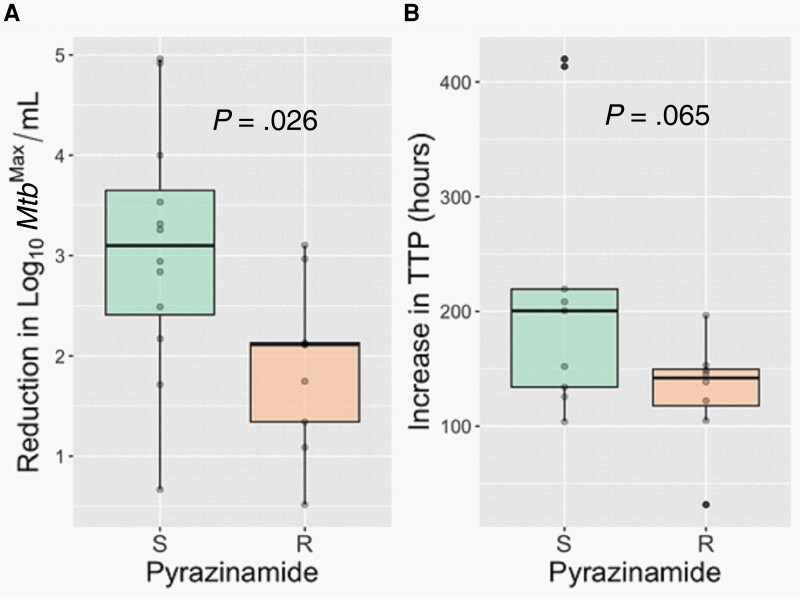
Finally, we assessed whether any participant characteristics correlated with the rate of Mtb killing during the first 2 weeks of therapy. As before, we combined the cohorts for these analyses. Consistent with a recent report [26], positivity for DD Mtb at week 2 correlated with a slower rate of Mtb killing during the first 2 weeks of therapy (P = .006; Figure 3, Supplementary Table 10). Subjects who were married or living with a partner had higher rates of Mtb killing during the first 2 weeks of therapy in comparison to those who were single, divorced, or separated (Supplementary Table 11) (P < .05). Finally, in the DR-TB cohort, resistance to pyrazinamide (a drug that is part of their second-line regimen) correlated with a slower rate of Mtb killing during the first 2 weeks of therapy (Figure 4;P = .026). While the sample size was small, pyrazinamide resistance did not correlate with lack of culture conversion at month 2 (Supplementary Table 12).
Figure 3.
Positivity for differentially detectable/culturable Mycobacterium tuberculosis (DD Mtb) at week 2 (as determined by the most probable number from the liquid limiting dilution assay performed with culture filtrate [MPN+CF] to colony-forming units [CFU] ratio) is associated with a slower rate of Mtb killing during the first 2 weeks of therapy as determined by the maximum Mtb count (MtbMax) obtained by CFU, MPN−CF, or MPN+CF (A). A similar trend is observed by time to positivity (TTP) by BACTEC MGIT (B). Top panels show data per subject, middle panels show median values per cohort ± 95% confidence intervals, and bottom panels show box plots for the reduction in Mtb counts during the first 2 weeks of therapy based on DD Mtb status.
Figure 4.
Subjects with drug-resistant tuberculosis who have resistance (R) to pyrazinamide (a drug that is part of their second-line regimen) show a slower rate of Mycobacterium tuberculosis (Mtb) killing during the first 2 weeks of therapy as determined by the maximum Mtb count (MtbMax) obtained by colony-forming units or most probable number from the liquid limiting dilution assay performed with or without culture filtrate (A) and time to positivity (TTP) by BACTEC MGIT (B). Abbreviations: S, sensitive; R, resistant.
DISCUSSION
The 2018 WHO recommendation of an all oral bedaquiline-based drug regimen for treatment of DR-TB represented a major advancement in TB care [13]. In these new regimens, bedaquiline replaced the injectable aminoglycosides, which decreased toxicity and simplified DR-TB treatment regimens. In the present study, we examined how a bedaquiline-based second-line regimen for DR-TB performed in comparison to the standard rifampin-based first-line regimen for DS-TB.
Our data indicate that the EBA of a second-line regimen consisting of bedaquiline, levofloxacin, linezolid, clofazimine, and pyrazinamide for DR-TB is comparable to a first-line regimen consisting of rifampin, isoniazid, ethambutol, and pyrazinamide for DS-TB (Figure 1). This was evident by similar rates of Mtb killing during the first 2 weeks of therapy (Table 3) and identical rates of conversion to culture negative at 2 months of therapy (Supplementary Table 3). These similarities were accompanied by similar signs and symptoms for the 2 cohorts after 2 months of therapy (Table 2, Supplementary Table 2). Therefore, despite the fact that subjects with DR-TB had more advanced disease at time of treatment, and >80% had previously received treatment for DS-TB, they displayed a similar microbiological response to therapy as their DS-TB counterparts.
Our data further indicate that despite its potent sterilizing activity, bedaquiline is likely not solely responsible for these effects. Although receiving a total of 5 drugs, subjects with DR-TB who showed resistance to pyrazinamide had a slower rate of Mtb killing during the first 2 weeks of therapy than those who were sensitive (Figure 4). This finding is consistent with studies showing that inclusion of pyrazinamide in other second-line regimens improved culture conversion rates and treatment outcomes only in participants who retained sensitivity to the drug [27–29]. These data provide further support for the utility of drug susceptibility testing for pyrazinamide whenever possible to better tailor DR-TB treatment regimens.
Finally, given the similarities observed for the 2 treatment regimens, it raises the possibility that subjects receiving the second-line regimen examined here may benefit from a shorter course of therapy. A recent trial of a similar regimen used in this study (containing bedaquiline, levofloxacin, linezolid, and pyrazinamide) found a ≥12-month relapse-free cure rate of 75% after 6 months of therapy; similar to what was observed for the older 24-month regimen (70%) that included injectables [30]. This study was limited by small sample size and frequent drug substitutions for linezolid in the intervention arm. Nonetheless, these findings are consistent with bedaquiline's potent EBA—comparable to that of isoniazid and rifampin [31]—and recent studies showing that the combination of bedaquiline with pyrazinamide and a fluoroquinolone acts as one of the most powerful therapeutic backbones for TB [29, 32, 33]. These findings, however, need to be tempered with rising rates of pyrazinamide resistance (41% found in this study), which was associated with decreased EBA despite the fact that 4 other drugs were coadministered (Figure 4). Recent trials indicate that bedaquiline-based regimens may also hold promise for shortening treatment regimens for DS-TB [29, 34]. While this study was not powered to assess treatment outcomes, future studies examining whether pyrazinamide resistance, or shorter-course therapy with the second-line regimen examined here, are associated with differential clinical outcomes for DR-TB is warranted.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Kayvan Zainabadi, Center for Global Health, Weill Cornell Medicine, NewYork, New York.
Stalz Charles Vilbrun, Les Centres GHESKIO, Port-au-Prince, Haiti.
Laurent Daniel Mathurin, Les Centres GHESKIO, Port-au-Prince, Haiti.
Kathleen Frances Walsh, Center for Global Health, Weill Cornell Medicine, NewYork, New York; Division of General Internal Medicine, Department of Medicine, Weill Cornell Medicine, New York, New York.
Jean William Pape, Center for Global Health, Weill Cornell Medicine, NewYork, New York; Les Centres GHESKIO, Port-au-Prince, Haiti.
Daniel W Fitzgerald, Center for Global Health, Weill Cornell Medicine, NewYork, New York.
Myung Hee Lee, Center for Global Health, Weill Cornell Medicine, NewYork, New York.
Notes
Acknowledgments. The authors are grateful to the subjects who volunteered for this study, as well as the clinical, research, and administrative staff of GHESKIO who made this study possible. We thank the Foundation Mérieux for helping to build and maintain the biosafety level 3 facility at GHESKIO.
Author contributions. Conceptualization, funding acquisition, and methodology: K. Z., K. F. W., and D. W. F. Supervision and project administration: S. C. V., L. D. M., K. F. W., and J. W. P. Investigation: K. Z. Formal analysis: K. Z. and M. H. L. Writing of original draft, review, and editing: All authors.
Disclaimer. The views expressed are solely those of the authors and do not necessarily represent the views of the National Institutes of Health.
Financial support. This work was supported by the National Institutes of Health (grant numbers U19AI111143 and K24AI098627 to D. F. W.). K. Z. was supported by a Vanderbilt-Emory-Cornell-Duke Global Health Fellowship, funded by the Fogarty International Center of the National Institutes of Health (grant number D43 TW009337). Funding to pay the Open Access publication charges for this article was provided by Weill Cornell Medical College.
References
- 1. World Health Organization (WHO) . World tuberculosis report 2022. Geneva, Switzerland: WHO, 2022. [Google Scholar]
- 2. World Health Organization (WHO) . WHO consolidated guidelines on tuberculosis. Module 4: treatment—drug-resistant tuberculosis treatment, 2022 update. Geneva, Switzerland: WHO, 2022. [PubMed] [Google Scholar]
- 3. Ndjeka N, Schnippel K, Master I, et al. . High treatment success rate for multidrug-resistant and extensively drug-resistant tuberculosis using a bedaquiline-containing treatment regimen. Eur Respir J 2018; 52:1801528. [DOI] [PubMed] [Google Scholar]
- 4. Diacon AH, Pym A, Grobusch MP, et al. . Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med 2014; 371:723–32. [DOI] [PubMed] [Google Scholar]
- 5. Pym AS, Diacon AH, Tang S-J, et al. . Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. Eur Respir J 2016; 47:564–74. [DOI] [PubMed] [Google Scholar]
- 6. Ahmad N, Ahuja SD, Akkerman OW, et al. . Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment—2017. Lancet 2018; 392:821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schnippel K, Ndjeka N, Maartens G, et al. . Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med 2018; 6:699–706. [DOI] [PubMed] [Google Scholar]
- 8. Guglielmetti L, Le Du D, Jachym M, et al. . Compassionate use of bedaquiline for the treatment of multidrug-resistant and extensively drug-resistant tuberculosis: interim analysis of a French cohort. Clin Infect Dis 2015; 60:188–94. [DOI] [PubMed] [Google Scholar]
- 9. Olayanju O, Limberis J, Esmail A, et al. . Long-term bedaquiline-related treatment outcomes in patients with extensively drug-resistant tuberculosis from South Africa. Eur Respir J 2018; 51:1800544. [DOI] [PubMed] [Google Scholar]
- 10. Borisov SE, Dheda K, Enwerem M, et al. . Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR-and XDR-TB: a multicentre study. Eur Respir J 2017; 49:1700387. [DOI] [PubMed] [Google Scholar]
- 11. Zhang S-J, Yang Y, Sun W-W, et al. . Effectiveness and safety of bedaquiline-containing regimens for treatment on patients with refractory RR/MDR/XDR-tuberculosis: a retrospective cohort study in east China. BMC Infect Dis 2022; 22:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reuter A, Tisile P, Von Delft D, et al. . The devil we know: is the use of injectable agents for the treatment of MDR-TB justified? Int J Tuberc Lung Dis 2017; 21:1114–26. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization (WHO) . Rapid сommunication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). Geneva, Switzerland: WHO, 2018. [Google Scholar]
- 14. Zainabadi K, Walsh KF, Vilbrun SC, et al. . Characterization of differentially detectable Mycobacterium tuberculosis in the sputum of subjects with drug-sensitive or drug-resistant tuberculosis before and after two months of therapy. Antimicrob Agents Chemother 2021; 65:e0060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zainabadi K, Saito K, Mishra S, et al. . Transcriptional biomarkers of differentially detectable Mycobacterium tuberculosis in patient sputum. mBio 2022; 13:e0270122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zainabadi K, Lee MH, Walsh KF, et al. . An optimized method for purifying, detecting and quantifying Mycobacterium tuberculosis RNA from sputum for monitoring treatment response in TB patients. Sci Rep 2022; 12:17382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ocheretina O, Escuyer VE, Mabou MM, et al. . Correlation between genotypic and phenotypic testing for resistance to rifampin in Mycobacterium tuberculosis clinical isolates in Haiti: investigation of cases with discrepant susceptibility results. PLoS One 2014; 9:e90569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Institute of Allergy and Infectious Diseases . Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events. Bethesda, MD: National Institutes of Health, 2014. [Google Scholar]
- 19. Saito K, Warrier T, Somersan-Karakaya S, et al. . Rifamycin action on RNA polymerase in antibiotic tolerant Mycobacterium tuberculosis results in differentially detectable populations. Proc Natl Acad Sci U S A 2017; 114:E4832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McAulay K, Saito K, Warrier T, et al. . Differentially detectable Mycobacterium tuberculosis cells in sputum from treatment-naive subjects in Haiti and their proportionate increase after initiation of treatment. mBio 2018; 9:e02192-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harris PA, Taylor R, Minor BL, et al. . The REDCap consortium: building an international community of software partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hesseling A, Walzl G, Enarson D, et al. . Baseline sputum time to detection predicts month two culture conversion and relapse in non-HIV-infected patients. Int J Tuberculosis Lung Dis 2010; 14:560–70. [PubMed] [Google Scholar]
- 24. Parikh R, Nataraj G, Kanade S, Khatri V, Mehta P. Time to sputum conversion in smear positive pulmonary TB patients on category I DOTS and factors delaying it. J Assoc Physicians India 2012; 60:6. [PubMed] [Google Scholar]
- 25. Shenai S, Ronacher K, Malherbe S, et al. . Bacterial loads measured by the Xpert MTB/RIF assay as markers of culture conversion and bacteriological cure in pulmonary TB. PLoS One 2016; 11:e0160062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Peters JS, McIvor A, Papadopoulos AO, et al. . Differentially culturable tubercle bacteria as a measure of tuberculosis treatment response. Front Cell Infect Microbiol 2023; 12:1064148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aung K, Van Deun A, Declercq E, et al. . Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis 2014; 18:1180–7. [DOI] [PubMed] [Google Scholar]
- 28. Bastos ML, Hussain H, Weyer K, et al. . et al . Treatment outcomes of patients with multidrug-resistant and extensively drug-resistant tuberculosis according to drug susceptibility testing to first- and second-line drugs: an individual patient data meta-analysis. Clin Infect Dis 2014; 59:1364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tweed CD, Dawson R, Burger DA, et al. . Bedaquiline, moxifloxacin, pretomanid, and pyrazinamide during the first 8 weeks of treatment of patients with drug-susceptible or drug-resistant pulmonary tuberculosis: a multicentre, open-label, partially randomised, phase 2b trial. Lancet Respir Med 2019; 7:1048–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Esmail A, Oelofse S, Lombard C, et al. . An all-oral 6-month regimen for multidrug-resistant tuberculosis: a multicenter, randomized controlled clinical trial (the NExT study). Am J Respir Crit Care Med 2022; 205:1214–27. [DOI] [PubMed] [Google Scholar]
- 31. Donald P, Diacon A. The early bactericidal activity of anti-tuberculosis drugs: a literature review. Tuberculosis 2008; 88:S75–83. [DOI] [PubMed] [Google Scholar]
- 32. Tasneen R, Garcia A, Converse PJ, et al. . Novel regimens of bedaquiline-pyrazinamide combined with moxifloxacin, rifabutin, delamanid and/or OPC-167832 in murine tuberculosis models. Antimicrob Agents Chemother 2022; 66:e0239821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tasneen R, Li SY, Peloquin CA, et al. . Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother 2011; 55:5485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paton NI, Cousins C, Suresh C, et al. . Crook AM; TRUNCATE-TB trial team. Treatment strategy for rifampin-susceptible tuberculosis. N Engl J Med 2023; 388:873–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.