Abstract
Background
HVTN 120 is a phase 1/2a randomized double-blind placebo-controlled human immunodeficiency virus (HIV) vaccine trial that evaluated the safety and immunogenicity of ALVAC-HIV (vCP2438) and MF59- or AS01B-adjuvanted bivalent subtype C gp120 Env protein at 2 dose levels in healthy HIV-uninfected adults.
Methods
Participants received ALVAC-HIV (vCP2438) alone or placebo at months 0 and 1. At months 3 and 6, participants received either placebo, ALVAC-HIV (vCP2438) with 200 μg of bivalent subtype C gp120 adjuvanted with MF59 or AS01B, or ALVAC-HIV (vCP2438) with 40 μg of bivalent subtype C gp120 adjuvanted with AS01B. Primary outcomes were safety and immune responses.
Results
We enrolled 160 participants, 55% women, 18–40 years old (median age 24 years) of whom 150 received vaccine and 10 placebo. Vaccines were generally safe and well tolerated. At months 6.5 and 12, CD4+ T-cell response rates and magnitudes were higher in the AS01B-adjuvanted groups than in the MF59-adjuvanted group. At month 12, HIV-specific Env-gp120 binding antibody response magnitudes in the 40 μg gp120/AS01B group were higher than in either of the 200 μg gp120 groups.
Conclusions
The 40 μg dose gp120/AS01B regimen elicited the highest CD4+ T-cell and binding antibody responses.
Clinical Trials Registration . NCT03122223.
Keywords: HIV, vaccine, dose, adjuvant
We report results of HIV vaccine trial HVTN 120, which evaluated ALVAC-HIV (vCP2438) boosted with MF59 or AS01B adjuvanted with gp120 at 2 doses. The highest CD4+ T-cell and binding antibody responses were in the lower dose gp120/AS01B regimen.
In 2009, the RV144 trial concluded in a modified intention to treat analysis that there was modest efficacy of a preventive human immunodeficiency virus (HIV) vaccine regimen (vaccine efficacy 31.2%, 95% confidence interval [CI], 1.1–52.1; P = .04). The regimen comprised a canarypox vector vaccine plus an adjuvanted protein vaccine: ALVAC-HIV (vCP1521) plus subtype B/E glycoprotein 120 (gp120) Env protein (AIDSVAX B/E) formulated with aluminum hydroxide adjuvant [1]. Among the correlates of protection were binding antibodies to V1V2 antigens [2]. Thereafter, vaccine candidates were manufactured to match more closely the world's most prevalent HIV subtype, subtype C. One of those vaccine concepts—a canarypox vector vaccine with subtype C inserts and a subtype C protein vaccine adjuvanted with MF59—was tested in the HVTN 702 trial. In 2020, this trial did not demonstrate efficacy in the South African population where HIV subtype C dominates [3].
In parallel, the post-RV144 subtype C vaccine research program investigated the immunological profiles elicited by various combinations and doses of subtype C vaccine candidates and different adjuvants to optimize the magnitude and duration of immune responses [4]. RV135 evaluated immune responses to a regimen identical to the one used in RV144 but randomized participants to a higher or lower dose of the Env protein vaccine: 200 μg or 600 μg total (100 μg or 300 μg each of MN and A244 proteins). When compared to participants who received the higher Env protein dose, those who received the lower dose had lower anti-MN and anti-A244 antibody response rates, lower geometric mean titers of antibodies to MN and A244, and lower neutralization antibody response rates [5].
Adjuvants are known modifiers of the potency, quality, and longevity of antigen-specific immune responses [6]. The MF59 adjuvant, which was used in the HVTN 702 trial, is an oil-in-water emulsion licensed for influenza vaccines in certain countries. MF59 has demonstrated recruitment of antigen-presenting cells in preclinical models; upregulation of cytokines, chemokines, and receptors [7]; improvement of antibody affinity maturation epitope breadth and binding affinity [8]; and balancing of the T-helper 1 and T-helper 2 responses and proliferation of T cells [9].
AS01B belongs to a liposome-based class of adjuvants and contains 2 immunostimulants. The first is 3-O-desacyl-4′-monophosphoryl lipid A (MPL), a nontoxic derivative of the lipopolysaccharide from Salmonella minnesota, a Toll-like receptor 4 (TLR4) agonist, and a stimulant of nuclear factor-κB (NF-ĸB) transcriptional activity and subsequent cytokine production [10]. MPL directly activates antigen-presenting cells such as dendritic cells to produce cytokines and express elevated levels of costimulatory molecules [11–13]. The second is QS-21, a natural saponin molecule extracted from the bark of the South American tree Quillaja saponaria Molina [14–16], which elicits high antigen-specific antibody responses in humans [16, 17]. AS01B is an MPL, QS-21, and liposome based adjuvant system (50 mg MPL and 50 µg QS-21) that is also part of the licensed herpes zoster vaccine (Shingrix; GSK) and AS01E is also part of the licensed RSV vaccine (Arexvy; GSK). AS01E (containing 25 µg MPL, 25 µg QS-21, and liposome) is part of the candidate M72 tuberculosis vaccine that demonstrated partial efficacy [18] and is part of the RTS,S malaria vaccine given to children in Kenya, Malawi, and Ghana [19].
Currently, no studies have evaluated immune responses with varying doses of Env proteins in the context of ALVAC prime-boost and protein adjuvanted with MF59 or AS01B. Here we describe the outcome of HIV Vaccine Trials Network 120 (HVTN 120), which compared the human safety profiles and immune responses to the vaccine products that did not demonstrate efficacy in HVTN 702—ALVAC-HIV (vCP2438) and MF59-adjuvanted bivalent subtype C gp120—with 2 corresponding regimens containing the AS01B adjuvant, one at the same protein dose (200 μg) as HVTN 702, the other at a lower dose (40 μg).
METHODS
Study Design
This was a multicenter, randomized, placebo-controlled, double-blinded clinical trial conducted from February 2018 to January 2020 at 9 sites in the United States and 1 site each in Tanzania, Zambia, and Zimbabwe (Clinical Trials Registration at https://clinicaltrials.gov/ct2/show/NCT03122223).
Study Population
Eligible participants were healthy adults aged 18 to 40 years who provided written informed consent, demonstrated understanding of the study, were deemed low risk for HIV acquisition, agreed not to enroll in other studies of investigational products, had normal hematology and chemistry panels, and were not infected with HIV-1, HIV-2, hepatitis B, nor hepatitis C. Pregnant women were excluded.
Study Products
Four products were administered in various combinations, all required intramuscular injection. First, ALVAC-HIV (vCP2438) expressed the gene products 96ZM651 gp120 (subtype C strain) linked to the sequences encoding the HIV-1 transmembrane anchor sequence of gp41 (28 amino acids subtype B LAI strain) and gag and pro (subtype B LAI strain). Second, bivalent subtype C gp120, combining subtype C TV1.C gp120 Env and subtype C 1086.C gp120 Env, each at a dose of 100 μg, mixed with MF59 adjuvant (200 µg Pr + MF59). Third, bivalent subtype C gp120, where subtype C TV1.C gp120 Env and subtype C 1086.C gp120 Env are each included at a dose of 20 μg or 100 μg, mixed with AS01B adjuvant. Fourth, there was a placebo of 0.9% sodium chloride. See Supplementary Methods for further information.
Study Procedures
Participants gave written informed consent in their preferred language. At screening, participants underwent safety assessments through medical history, physical examination, and laboratory tests, which included complete blood count, chemistry, pap smear and urinalysis, as well as tests for pregnancy, HIV, syphilis, and hepatitis B and C. HIV antibody testing outside of the study was actively discouraged during participation to avoid potential negative impacts of vaccine-induced positive serology. Participants also underwent risk reduction counseling, pregnancy prevention assessment, and behavioral risk assessment.
Randomization
After confirmation of eligibility, participants were randomized. The randomization sequence was obtained by computer-generated random numbers. Allocation to vaccine or placebo was provided to sites through a web-based randomization system (see Supplementary Methods).
Safety Measures
Standard safety laboratory testing included hematology, serum chemistry, and urinalysis, which were obtained at baseline (during screening) and at each 2-week postvaccination visit plus a month 15 visit (except urinalysis, collected at 2 weeks after the first, fourth, and fifth vaccination). Participants were observed for 30 minutes after vaccinations and recorded solicited local and systemic symptoms (reactogenicity) for 3 days after each vaccination. Adverse events (AEs) were recorded until 30 days after each vaccination, except for AEs leading to early participant withdrawal or early product discontinuation and serious AEs, which were recorded throughout the trial. AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 21.1, and severity was graded using version 2.0 of the DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (November 2014). Safety reviews were conducted by the protocol safety review team and the National Institute of Allergy and Infectious Diseases (NIAID) Data and Safety Monitoring Board.
Immunogenicity Assays
All laboratory assays were performed blinded to treatment group with validated and/or qualified methods detailed below and in Supplementary Methods. Measurements included HIV-specific binding antibody, antibody-dependent cell-mediated cytotoxicity, neutralizing antibody in serum, and T-cell responses 2 weeks after the final vaccination (assessing peak immunogenicity, month 6.5) and at 12 months (6 months after the final vaccination, assessing durability, month 12). A list of the specific antigens used in all immunogenicity assays is in Supplementary Table 1.
Binding Antibody Multiplex Assay
HIV-1–specific immunoglobulin G (IgG) binding antibody responses were measured by binding antibody multiplex assay (BAMA) [20–22]. The area under the titration curve (AUTC) was calculated using the trapezoidal rule based on the raw mean fluorescence intensity (MFI) values truncated at zero across log base 10 dilution or as a 1:50 dilution when the linear range could be captured. Tested antigens and assay reagents are in Supplementary Table 1.
Intracellular Cytokine Staining Assay
Peripheral blood mononuclear cells were isolated and cryopreserved from whole blood, as previously described [23]. T-cell responses to vaccine-matched antigens (ENV ZM96.C gp140, 1086.C gp120, TV1.C gp120, and LAI-Gag) were measured by intracellular cytokine staining as described previously [24, 25] (see Supplementary Methods and antibodies listed in Supplementary Table 2).
Antibody-Dependent Cell-Mediated Cytotoxicity
GranToxiLux antibody-dependent cellmediated cytotoxicity (ADCC-GTL) [26] and the ADCC-Luc [27] assays were performed as previously described (see Supplementary Methods).
Approvals
The study was approved by the institutional review boards of Atlanta-Hope Clinic/Emory University, Boston-Brigham/Partners, Boston-Fenway, Case Western University, Vanderbilt University, University of Pennsylvania, University of Rochester, University of California San Francisco, and Fred Hutch Cancer Center in the United States; and Medical Research Council of Zimbabwe, University of Zambia Biomedical Research Ethics Committee, and Mbeya Medical Research and Ethics Committee in Africa.
RESULTS
Study Population
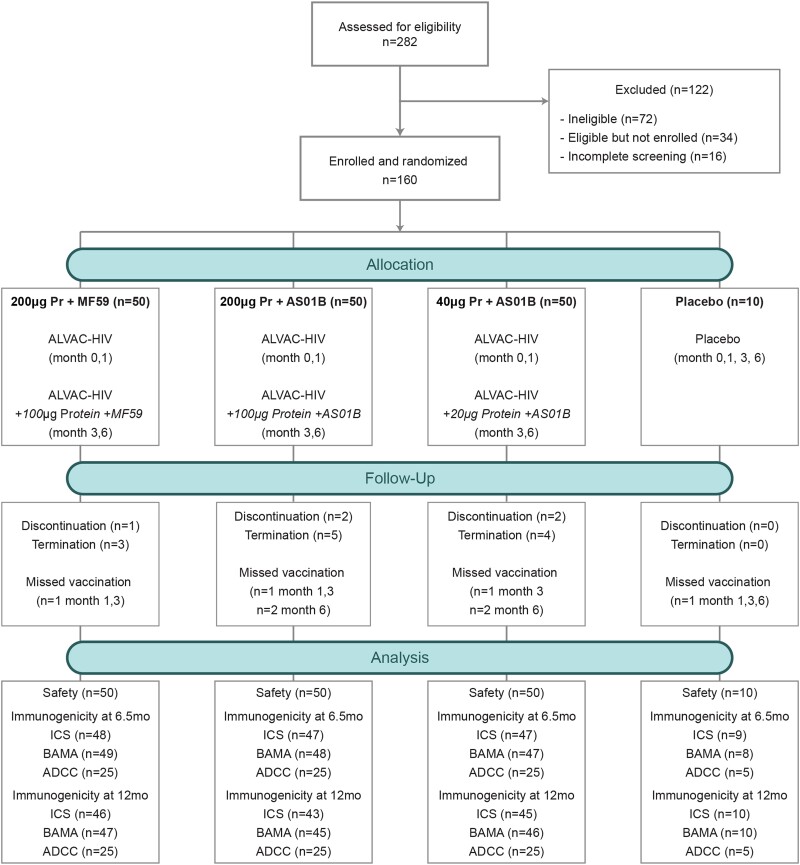
In total, 160 participants enrolled in HVTN 120 between 22 February 2018 and 14 August 2018. Of these, 50 were randomized to each of the 3 groups with active study product: 200 µg protein + MF59 group (200 µg Pr + MF59), 200 μg Env protein with AS01B (200 µg Pr + AS01B), and 40 μg Env protein with AS01B (40 µg Pr + AS01B) (Supplementary Table 3). Ten were randomized to the placebo group. Median age was 24 years (interquartile range, 21–29 years), and 88 (55%) were women (Table 1). Demographics were similar across the 4 groups with overrepresentation of men in the placebo group.
Table 1.
Demographic Information of the 4 Trial Groups
| Characteristic | 200 µg Pr + MF59 | 200 µg Pr + AS01B | 40 µg Pr + AS01B | Placebo | Total |
|---|---|---|---|---|---|
| (n = 50) | (n = 50) | (n = 50) | (n = 10) | (n = 160) | |
| Age, y | |||||
| Median (IQR) | 24 (21–26) | 23.5 (21–28) | 25.5 (22–30) | 24 (22–30) | 24 (21–29) |
| 18–20 | 9 (18) | 9 (18) | 6 (12) | 2 (20) | 26 (16) |
| 21–30 | 30 (60) | 32 (64) | 32 (64) | 6 (60) | 100 (63) |
| 31–40 | 11 (22) | 9 (18) | 12 (24) | 2 (20) | 34 (21) |
| Sex | |||||
| Male | 19 (38) | 20 (40) | 25 (50) | 8 (80) | 72 (45) |
| Female | 31 (62) | 30 (60) | 25 (50) | 2 (20) | 88 (55) |
| Body mass index, kg/m2, median (IQR) | 22.6 (21–24) | 22.3 (21–26) | 22 (22–26) | 23 (21–26) | 22.8 (21–26) |
| Ethnicity | |||||
| Hispanic or Latino/a | 2 (4) | 1 (2) | 1 (2) | 1 (10) | 5 (3) |
| Not Hispanic or Latino/a | 48 (96) | 49 (98) | 49 (98) | 9 (90) | 155 (97) |
| Race | |||||
| United States, Asian | 1 (2) | 0 (0) | 1 (2) | 0 (0) | 2 (1) |
| United States, Black | 0 (0) | 1 (2) | 1 (2) | 1 (10) | 3 (2) |
| United States, White | 18 (36) | 16 (32) | 17 (34) | 3 (30) | 54 (34) |
| SSA, Black | 30 (60) | 30 (60) | 30 (60) | 6 (60) | 96 (60) |
| Mixed | 1 (2) | 3 (6) | 1 (2) | 0 (0) | 5 (3) |
| Vaccination frequencies | |||||
| Day 0 | 50 (100) | 50 (100) | 50 (100) | 10 (100) | 160 (100) |
| Day 28 | 49 (98) | 49 (98) | 50 (100) | 9 (90) | 157 (98) |
| Day 84 | 49 (98) | 49 (98) | 49 (98) | 9 (90) | 156 (98) |
| Day 168 | 49 (98) | 48 (96) | 48 (96) | 9 (90) | 154 (96) |
Data are No. (%) except where indicated.
Abbreviations: IQR, interquartile range; Pr, protein; SSA, sub-Saharan Africa.
Safety and Tolerability
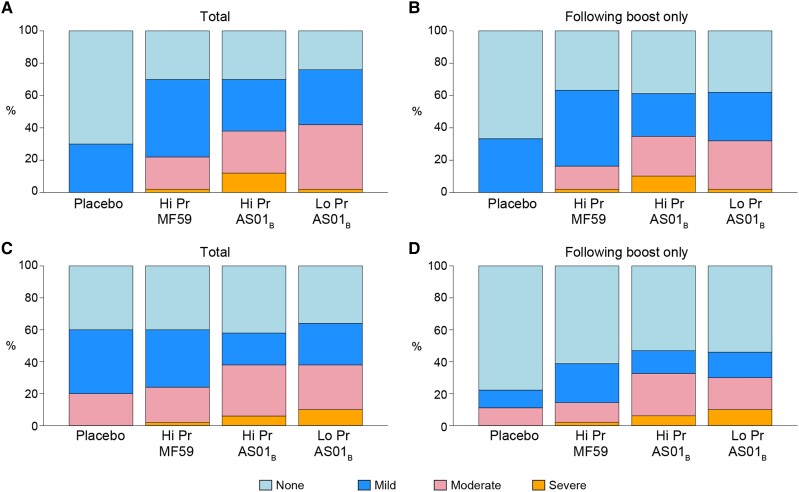
All 160 participants received the first vaccination, 157 received the second, 156 received the third, and 154 received the fourth (Figure 1). Vaccines were generally safe and well tolerated. Five participants discontinued vaccinations: 4 relocated and 1 because of mild to moderate reactogenicity symptoms (grade 1 headache, malaise/fatigue, chills and local pain, grade 2 local tenderness after the third vaccination). We observed a higher trend of severity in maximum local reactogenicity among vaccine recipients in the 200 µg Pr + AS01B arm (Figure 2A and 2B). We also observed a higher trend of severity for maximum systemic reactogenicity among vaccine recipients who received the AS01B adjuvant regardless of protein dose (Figure 2C and 2D). We detected significant differences in reactogenicity between the placebo group and pooled treatment groups for pain, tenderness, and chills (Supplementary Figures 1A and 1C and Supplementary Table 4). We did not observe any significant difference in temperature. We also looked for a difference in reactogenicity symptoms across all vaccinations among the 3 treatment groups and found significant differences in chills and myalgia, which occurred more often and with greater severity in the AS01B arms compared to MF59 (Supplementary Figure 1C). To further investigate differences in reactogenicity, we looked for differences between treatment groups in a pairwise fashion after vaccinations 3 and 4 where adjuvant differed between groups (Supplementary Figures 1B and 1D “boost only”). We found significant differences in chills, headache, and myalgia, which occurred more often in the AS01B adjuvanted groups compared to MF59 but were not significantly different between low- and high-dose protein adjuvanted with AS01B (Supplementary Figure 1D and Supplementary Table 4).
Figure 1.
CONSORT flow diagram of the HVTN 120 trial. Abbreviations: ADCC, antibody-dependent cellmediated cytotoxicity; BAMA, binding antibody multiplex assay; ICS, intracellular cytokine staining; Pr, protein.
Figure 2.
Maximum local and systemic reactogenicity. Stacked bar charts of maximum (A and B) combined local (pain, tenderness, erythema, induration) and (C and D) combined systemic (malaise/fatigue, myalgia, headache, nausea, vomiting, chills, arthralgia, temperature) reactogenicity. A and C, Reactogenicity over all vaccinations among placebo (n = 10), ALVAC-HIV with 200 µg Env protein + MF59 adjuvant boost (Hi Pr + MF59, n = 50), ALVAC-HIV with 200 µg Env protein + AS01B adjuvant boost (Hi Pr + AS01B, n = 50), and ALVAC-HIV with 40 µg Env protein + AS01B adjuvant boost (Lo Pr + AS01B, n = 50). B and D, Reactogenicity over participants receiving 1 or more boost (third and fourth) vaccinations among placebo (n = 9), Hi Pr + MF59 (n = 49), Hi Pr + AS01B (n = 49), and Lo Pr + AS01B (n = 50). Grade 3 (severe), grade 2 (moderate), grade 1 (mild), none.
A total of 222 AEs among 92 participants were reported; 216 (97%) were mild or moderate in severity. Five participants reported 6 grade 3 (severe) AEs, none of which were deemed related to vaccination. Of these, 3 participants experienced 4 episodes of decreased neutrophil count (1 had an episode 2 weeks after vaccination 2, and another 2 weeks after vaccination 3), 1 participant had an increased serum creatinine (while taking creatinine supplements), and 1 participant experienced a migraine (nearly 4 weeks after vaccination 1). Eleven participants had 13 AEs deemed related to vaccination, all resolved within 2 weeks. Seven experienced mild injection site pruritis (1 of these also had corresponding ipsilateral lymph node pain), 1 participant experienced mild pruritis of the medial aspect of the same arm as grade 3 injection site erythema, 1 experienced mild diarrhea 2 days after vaccination 1, and 1 experienced mild insomnia and night sweats 1 day after vaccination 4. One additional participant experienced grade 2 (moderate) shoulder and wrist tenderness of the vaccinated arm. No serious AEs or deaths were reported among vaccine recipients.
Vaccine-Induced Seropositivity
Vaccine-induced seropositivity assessed by commercially available HIV serology kits occurred in 1 vaccine recipient (0.6%). This individual tested reactive with only the Alere Determine HIV-1/2 Ag/Ab Combo test.
Higher Magnitude Antibody Response to Env Following Vaccine Adjuvanted With AS01B Compared to MF59
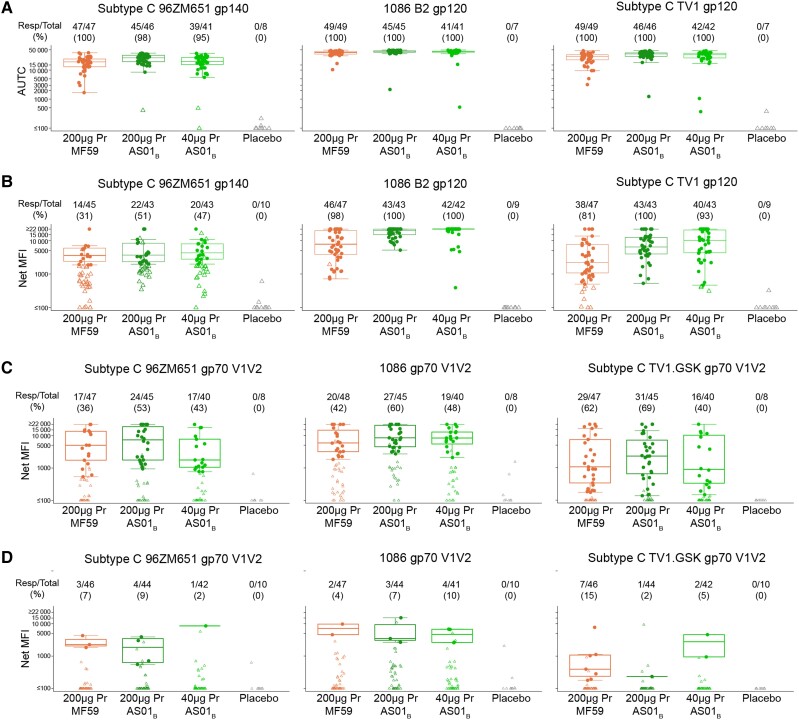
Antibody responses and magnitudes to gp120/gp140 envelope (Figure 3A and 3B) and V1V2 proteins (Figure 3C and 3D) were assessed at 2 weeks (months 6.5) and 6 months (month 12) after the fourth vaccination. At month 6.5 the response magnitude of gp120 IgG binding measured by AUTC among all participants was higher in both the 200 µg Pr + AS01B (P = .002 for antigen 1086.C; P < .001 for antigen ZM96; P < .001 for antigen TV1.C) and 40 µg Pr + AS01B (P = .015 for antigen 1086.C; P = .617 for antigen ZM96; P = .015 for antigen TV1.C) groups compared to that in the 200 µg Pr + MF59 group. At month 12, the binding antibody response net MFI magnitude of the 200 µg Pr + AS01B (P < .001 for all antigens 1086.C, ZM96, antigen TV1.C) and 40 µg Pr + AS01B group (P < .001 for antigen 1086.C; P = .002 for antigen ZM96; P < .001 for antigen TV1.C) remained higher than that of the 200 µg Pr + MF59 group.
Figure 3.
Binding antibody response against gp120 and V1V2. Samples from the participants collected at (A) 2 weeks after the last vaccination (month 6.5) and (B) 6 months after the last vaccination (month 12) were tested against HIV1 subtype C 96ZM651 gp120, 1086 B2 gp120, and subtype C TV1 gp120. C, Month 6.5 and (D) month 12 samples were also tested against HIV1 subtype C 96ZM651 gp70 V1V2, 1086 B2 gp70, and subtype C TV1 GSK gp70 V1V2. Graphs show (A) nonparametric AUTC and (B–D) net MFI. Numbers above indicate responders/total and percent. The median and boxplots (which display the first and third quartiles, whiskers indicate variability) are based on positive responders only (shown as filled circles); negative responders (below background) are shown as open symbols. Treatment groups are 200 µg Pr + MF59 (orange), 200 µg Pr + AS01B, 40 µg Pr + AS01B, and placebo. Abbreviations: AUTC, area under the baseline subtracted curve; gp, glycoprotein; MFI, mean fluorescence intensity; Pr, protein.
Overall, irrespective of vaccine formulation, low V1V2 responses were observed. Similar to the gp120 response, the V1V2 IgG magnitude was highest in the 200 µg Pr + AS01B group at month 6.5 (Figure 3C). While nearly 100% of vaccine recipients produced gp120/gp140 responses at month 6.5, only 68.9% of vaccine recipients produced V1V2 IgG responses. By month 12, the response to V1V2 had waned to <16%, whereas 100% of responders still had gp120/gp140 IgG responses regardless of treatment group.
Env Adjuvanted 40 µg With AS01B Induces Durable CD4+ T-Cell Responses
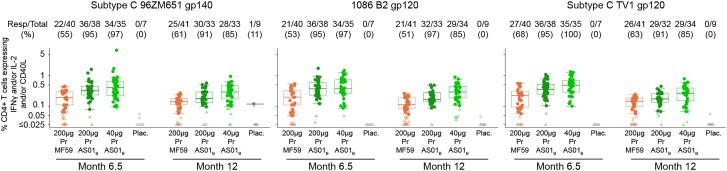
We next assessed the CD4+ T-cell response to the vaccine-matched Env peptide pools (TV1.C gp120, 1086.C gp120, and ZM96 gp140) by intracellular cytokine staining (Supplementary Figure 2). Vaccine-specific responses were assessed by enumerating the frequency of T cells expressing interferon-γ (IFN-γ) and/or interleukin 2 (IL-2) and /or CD40L (also known as CD154). The response rates and magnitudes to all 3 Env proteins were significantly higher in the AS01B-adjuvanted groups compared to the MF59-adjuvanted group at month 6.5 and month 12 (P < .05; Figure 4). In addition, the 40 µg Pr + AS01B group had comparable response rates to the 200 µg Pr + AS01B regimen at both month 6.5 and 12, but significantly higher response magnitudes to all the Env peptide pools at month 12 (P < .05). Overall, the 40 µg Pr + AS01B consistently had the highest response magnitude compared to the other regimens. Neither adjuvant was able to enhance the response to poorly immunogenic antigens LAI Gag or LAI gp41 TM peptide pools at both time points (<10%, data not shown). Generally, the CD4+ T-cell response rates in all 3 groups were durable and were maintained at similar levels at month 12 to those observed at month 6.5 for all 3 envelope peptide pools. Of note, the response magnitudes decreased significantly from month 6.5 to month 12 in all the groups, for all the antigens tested (P < .05; Supplementary Figure 3).
Figure 4.
CD4+ T-cell responses as measured by intracellular cytokine staining. The CD4+ T-cell responses rate (numbers above graph) and magnitude (boxplots) 2 weeks after (month 6.5) and 6 months after (month 12) the final immunization for each treatment arm for the following vaccine-matched antigens: subtype C 96ZM651 gp120, 1086 B2 gp120, and subtype C TV1 gp120. Numbers above indicate responders/total and percent. The median and boxplots (which display the first and third quartiles, whiskers indicate variability) are based on positive responders only (shown as filled circles), negative responders (below background) are shown as open symbols. Treatment groups are 200 µg Pr + MF59, 200 µg Pr + AS01B, 40 µg Pr + AS01B, and placebo. Abbreviations: gp, glycoprotein; IFN-γ, interferon-γ; IL-2, interleukin 2; Pr, protein.
We then examined CD4 T-cell polyfunctionality scores (PFS) using the COMPASS method [28] and found they were higher in the AS01B-adjuvanted groups versus the MF59 group for all Env antigens assayed at month 6.5 and 12 (P < .05; Supplementary Figure 4). No significant differences were observed between the low- and high-dose protein groups. The PFS decreased from month 6.5 to 12 in the AS01B adjuvanted groups to all the antigens, whereas in the MF59 adjuvanted group, a temporal decrease was only observed to 1086 gp120 (P < .05) (Supplementary Figure 4). Heatmaps of PFS show that the highest posterior probabilities at months 6.5 and 12 were found in cells coexpressing 2 markers (IFN-γ or IL-2 and CD40L), 3 markers (IL-2, tumor necrosis factor [TNF], and CD40L), and 4 markers (IFN-g, IL-2, TNF, and CD40L) (Supplementary Figure 5).
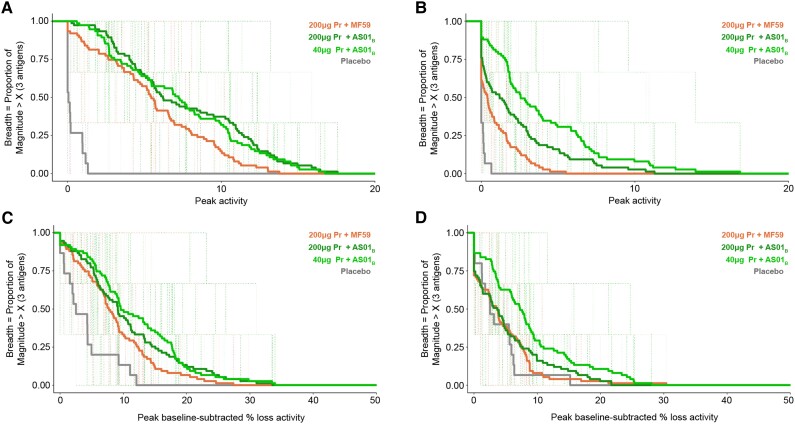
Potent ADCC Response Following Low Protein Env Adjuvanted With AS01B
Lastly, we assessed the ADCC response following vaccination (Figure 5 and Supplementary Figure 6). We observed that response rates and magnitude-breadth of the ADCC responses were overall higher in the participants who received the AS01B adjuvant compared to MF59, although the response rates were not significantly different between groups (Supplementary Figure 6). The magnitude-breadth was significantly higher in the vaccinees who received the AS01B adjuvant regardless of protein dose (Figure 5).
Figure 5.
Higher magnitude-breadth in vaccinees who received the AS01B adjuvant. Magnitude-breadth of antibody-dependent T-cell-mediated cytotoxicity responses measured by (A and B) GranToxiLuc and (C and D) luciferase. Samples from the participants collected at 2 weeks after the last vaccination (month 6.5; A and C) and 6 months after the last vaccination (month 12; B and D) were tested against HIV1 subtype C 96ZM651 gp120, 1086 B2 gp120, and subtype C TV1 gp120. Treatment groups are 200 µg Pr + MF59, 200 µg Pr + AS01B, 40 µg Pr + AS01B, and placebo. Abbreviations: gp, glycoprotein; Pr, protein.
Using the ADCC-GTL assay, we observed that at month 6.5, the magnitude-breadth of the responses were similar between the different doses administered with AS01B, but the 200 µg Pr + AS01B group developed significantly higher magnitude-breadth than the group administered 200 µg Pr + MF59 (P = .022; Figure 5A). Of note, at month 12, the magnitude-breadth of the responses in the 40 µg Pr + AS01B group was significantly higher than that of the 200 µg Pr + AS01B group (P = .007), and both high and low Pr + AS01B groups developed significantly higher magnitude-breadth than the 200 µg Pr + MF59 group (P = .013 and P < .001, respectively; Figure 5B). For the responses detected with the ADCC-Luc assay, at month 6.5, 40 µg Pr + AS01B induced a higher magnitude-breadth compared to 200 µg Pr + MF59, and this was statistically significant, albeit only marginally (P = .052; Figure 5C). At month 12, both 40 µg Pr + AS01B and 200 µg Pr + AS01B induced significantly higher magnitude-breadth compared to 200 µg Pr + MF59 (200 µg Pr + AS01B vs 40 µg Pr + AS01B, P = .010; 200 µg Pr + MF59 vs 40 µg Pr + AS01B, P = .0017; Figure 5D).
DISCUSSION
Our study has four major findings. First, all regimens were generally safe and well tolerated in healthy volunteers in the United States and sub-Saharan Africa. Second, we found that AS01B-adjuvanted groups, regardless of protein dose, induced higher CD4+ T-cell and binding antibody responses compared to the MF59-adjuvanted group. Of significance, the lower protein dose also tended to elicit stronger responses than the higher dose when both were adjuvanted with AS01B. Third, the low-protein AS01B-adjuvanted dose elicited higher HIV-specific Env-gp120 binding antibody response magnitudes and higher CD4+ T-cell response rates and magnitudes to all 3 Env proteins than the MF59-adjuvanted dose. Fourth, none of the regimens elicited persistent high-level responses to V1V2 envelope, a major correlate in the RV144 study, and, additionally, was associated with HIV-1 risk in the HVTN 702 study when combined with CD4+ T-cell responses [29].
HVTN 702 demonstrated that 64% of vaccine recipients made Env-specific CD4+ T-cell responses, which was a significantly higher proportion than the 40% observed in RV144 (P = .03) [29] and similar to the 73% observed here. We found that the CD4+ T-cell responses were durable up to 6 months after the completion of the vaccine regimen (month 12), but that the response magnitudes and polyfunctionality had decreased significantly by then. At month 6.5, the response rates and magnitudes of gp120 IgG binding antibody to gp120/gp140 envelope and V1V2 proteins were higher in both AS01B-adjuvanted groups compared to the MF59-adjuvanted group. However, we observed only 69.9% response rate to V1V2 compared to nearly 100% against gp120/gp140. At month 12, the antibody response magnitude of the AS01B adjuvanted low-dose group remained higher than either the AS01B or MF59 high-dose groups.
Three decades of HIV vaccine efficacy trials suggest that simply demonstrating that a vaccine antigen can bind to certain antibodies or elicit specific cellular responses does not necessarily signify protection against HIV infection. Recently, the 4-dose primary regimen adjuvanted with MF59 presented here was also tested with a month 12 booster in another early-phase trial (HVTN 100), which demonstrated its humoral and cellular immunogenicity [30]. However, the advanced phase trial (HVTN 702) found no HIV preventive efficacy, even with month 12 and 18 boosters [3]. HVTN 120 adapted the previous regimen with AS01B adjuvant and found a significant boosted in both CD4+ T-cell responses and IgG responses to V1V2, even at a protein dose that was one-fifth of that used in the HVTN 100 and HVTN 702 studies. The decision to include AS01B as an adjuvant in this regimen was driven by data showing that it had the ability to enhance and contribute to the induction of durable immune responses, both humoral and cellular, and in some studies this correlated with protection [31].
The only trial to show partial efficacy, RV144, showed the inverse correlation between HIV incidence and IgG bound to V1V2 and the direct correlation between HIV incidence and plasma Env-specific binding IgA [2]. IgG antibodies to vaccine-matched V1V2 at 2 weeks after the fourth vaccination were observed in 100% of RV144 vaccine recipients compared to 67% of HVTN 702 vaccine recipients and 69% of participants receiving the low dose of gp120/AS01B in our study [29]. It is unclear if such comparisons are valid because they extrapolate the IgG correlate from RV144 to other vaccine regimens tested in populations with different races, ethnicity, and genetics.
One limitation of our study is the assessment of positive responders only to estimate the magnitude of B- and T-cell responses. This is a helpful metric to understand the scale of positive responses, although this introduces bias into the interpretation as it excludes negative data.
Protein vaccines have been studied in 6 of the 9 HIV-1 vaccine efficacy trials conducted; none were adjuvanted by AS01B [32]. Our findings about the increased CD4 T-cell and binding antibody responses as well as dose-sparing effect of AS01B suggest that adjuvants may have beneficial effects for a gp120 vaccine. However, the V1V2 envelope responses, that were associated with a lower risk of HIV acquisition during RV144, remain low and not persistent, supporting the requirement for an immunologically effective antigen. In the quest for an effective prophylactic HIV vaccine, these data warrant further studies, including adjuvant comparisons together with a better understanding of correlate of protection for HIV.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Zvavahera Mike Chirenje, Department of Obstetrics and Gynecology, University of California San Francisco, San Francisco, California, USA; Faculty of Medicine and Health Science, University of Zimbabwe Clinical Trials Research Centre, University of Zimbabwe, Harare, Zimbabwe.
Fatima Laher, Perinatal HIV Research Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
One Dintwe, Cape Town HIV Vaccine Trials Network Immunology Laboratory, Cape Town, South Africa; Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Monde Muyoyeta, Centre for Infectious Diseases Research in Zambia, Livingstone, Zambia.
Allan C deCamp, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Zonglin He, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Nicole Grunenberg, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Faatima Laher Omar, Cape Town HIV Vaccine Trials Network Immunology Laboratory, Cape Town, South Africa.
Kelly E Seaton, Center for Human Systems Immunology, Duke University School of Medicine, Durham, North Carolina, USA; Department of Surgery, Duke University Medical Center, Durham, North Carolina, USA.
Laura Polakowski, Vaccine Research Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Amanda S Woodward Davis, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Lucas Maganga, National Institute for Medical Research-Mbeya Medical Research Centre, Mbeya, Tanzania.
Lindsey R Baden, Department of Medicine, Brigham and Women's Hospital, Boston, Massachusetts, USA.
Kenneth Mayer, Beth Israel Deaconess Medical Center, Harvard University, Boston, Massachusetts, USA; The Fenway Institute, Fenway Health, Boston, Massachusetts, USA.
Spyros Kalams, Division of Infectious Diseases, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Michael Keefer, Department of Medicine, University of Rochester, Rochester, NewYork, USA.
Srilatha Edupuganti, Department of Medicine, Emory University, Atlanta, Georgia, USA.
Benigno Rodriguez, Division of Infectious Diseases and HIV Medicine, Department of Medicine, Case Western Reserve University/University Hospitals, Cleveland Medical Center, Cleveland, Ohio, USA.
Ian Frank, School of Medicine, University of Pennsylvania, Philadelphia, USA.
Hyman Scott, SanFrancisco Department of Public Health, San Francisco, California, USA.
Lynda Stranix-Chibanda, Faculty of Medicine and Health Science, University of Zimbabwe Clinical Trials Research Centre, University of Zimbabwe, Harare, Zimbabwe.
Sanjay Gurunathan, Sanofi Pasteur, Swiftwater, Pennsylvania, USA.
Marguerite Koutsoukos, GSK, Wavre, Belgium.
Olivier Van Der Meeren, GSK, Rixensart, Belgium.
Carlos A DiazGranados, Sanofi Pasteur, Swiftwater, Pennsylvania, USA.
Carmen Paez, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Erica Andersen-Nissen, Cape Town HIV Vaccine Trials Network Immunology Laboratory, Cape Town, South Africa; Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
James Kublin, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Lawrence Corey, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Guido Ferrari, Department of Surgery, Duke University Medical Center, Durham, North Carolina, USA; Duke Human Vaccine Institute, Duke University Medical Center, Durham, North Carolina, USA.
Georgia Tomaras, Center for Human Systems Immunology, Duke University School of Medicine, Durham, North Carolina, USA; Department of Surgery, Duke University Medical Center, Durham, North Carolina, USA.
M Juliana McElrath, Cape Town HIV Vaccine Trials Network Immunology Laboratory, Cape Town, South Africa; Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Notes
Acknowledgments. The authors thank the trial participants and staff, the study teams, community members, the HVTN core staff, the Statistical Center for HIV/AIDS Research and Prevention, the HVTN laboratories, the product developers and the DAIDS/NIAID Vaccine Research Program. We thank Nicole Na for assistance with manuscript development, Meg Brandon for regulatory assistance, Philipp Mann, Nicole Frahm, and Simba Takuva; Yong Lin, Jack Heptinstall, Lu Zhang, Sheetal Sawant of the HVTN Laboratory Center. We thank the following people at Cape Town HVTN Immunology Laboratory for performing the intracellular cytokine staining assays: Stephany Wilcox, Saleha Omarjee, Shamiska Rohith, Mahlodi Montlha, Boitumelo Mosito, and Asiphe Besethi (Research Technologists). AS01B and AS01E are trademarks owned by or licensed to GSK.
Author contributions. All authors contributed to drafting the manuscript or revising it critically for important intellectual content and provided final approval for it to be published. Z. M. C., F. L., A. D., and G. F. were involved in the design, analysis, and interpretation of the data. O. D., Z. E., K. E. S., A. W. D., and E. A. N. helped with analysis and interpretation. M. M. contributed to design, data acquisition, analysis, and interpretation. L. S. C. helped with design and data acquisition. L. M., L. R. B., K. M., S. K., M. K., S. E., F. L. O., B. R., I. F., and H. S. helped with data acquisition. N. G., L. P., M. Ko., O. V. D. M., C. D., C. P., and S. G. were involved in design and interpretation. J. K. participated in conception of the study. L. C., G. T., and M. J. M. contributed to interpretation, design, and funding acquisition.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, or the Bill and Melinda Gates Foundation.
Data availability. Data are available in a public database (https://atlas.scharp.org/cpas/project/HVTN%20Public%20Data/HVTN%20120/begin.view).
Financial support. This work was supported by National Institute of Allergy and Infectious Disease (NIAID), National Institutes of Health (grant numbers UM1 AI068614 to HIV Vaccine Trials network (HVTN) Leadership and Operations Center, UM1 AI068635 to HVTN Statistical and Data Management Center, and UM1 AI068618 to HVTN Laboratory Center); US Public Health Service (grant number UM1 AI069453 to Soweto-Bara Clinical Research Site); NIAID (grant numbers HHSN272201300033C and HHSN272201600012C to Novartis Vaccines and Diagnostics, now part of the GlaxoSmithKline Biologicals SA, for the selection and process development of the 2 gp120 envelope proteins TV1.C and 1086.C); and NIAID and Bill and Melinda Gates Foundation Global Health Grant (grant number OPP1017604 for the manufacture and release of the gp120 clinical grade material). M. K. was supported by NIAID (grant number UM1 AI069511 to University of Rochester HVTN Clinical Research Site). F. L. was supported by NIAID U.S. Public Health Service Grant UM1 AI069453 (Soweto-BaraClinical Research Site).
References
- 1. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. . Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. New Engl J Med 2009; 361:2209–20. [DOI] [PubMed] [Google Scholar]
- 2. Haynes BF, Gilbert PB, McElrath MJ, et al. . Immune-correlates analysis of an HIV-1 vaccine efficacy trial. New Engl J Med 2012; 366:1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gray GE, Bekker L-G, Laher F, et al. . Vaccine efficacy of ALVAC-HIV and bivalent subtype C gp120–MF59 in adults. New Engl J Med 2021; 384:1089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Laher F, Bekker L-G, Garrett N, Lazarus EM, Gray GE. Review of preventative HIV vaccine clinical trials in South Africa. Arch Virol 2020; 165:2439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nitayaphan S, Pitisuttithum P, Karnasuta C, et al. . Safety and immunogenicity of an HIV subtype B and E prime-boost vaccine combination in HIV-negative Thai adults. J Infect Dis 2004; 190:702–6. [DOI] [PubMed] [Google Scholar]
- 6. Gregorio ED, Caproni E, Ulmer JB. Vaccine adjuvants: mode of action. Front Immunol 2013; 4:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mosca F, Tritto E, Muzzi A, et al. . Molecular and cellular signatures of human vaccine adjuvants. Proc National Acad Sci 2008; 105:10501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khurana S, Verma N, Yewdell JW, et al. . MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med 2011; 3:85ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol 2008; 180:5402–12. [DOI] [PubMed] [Google Scholar]
- 10. Baldridge J, Myers K, Johnson D, Persing D, Cluff C, Hershberg R. Monophosphoryl lipid A and synthetic lipid A mimetics in TLR4-based adjuvants and immunomodulators. In: Hackett C, Harn D, eds. Vaccine adjuvants: immunological and clinical principles. Totowa, NJ: Humana Press Inc, 2008:235–55. [Google Scholar]
- 11. De Becker G, Moulin V, Pajak B, et al. . The adjuvant monophosphoryl lipid A increases the function of antigen-presenting cells. Int Immunol 2000; 12:807–15. [DOI] [PubMed] [Google Scholar]
- 12. Didierlaurent AM, Morel S, Lockman L, et al. . AS04, An aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol 2009; 183:6186–97. [DOI] [PubMed] [Google Scholar]
- 13. Ismaili J, Rennesson J, Aksoy E, et al. . Monophosphoryl lipid A activates both human dendritic cells and T-cells. J Immunol 2002; 168:926–32. [DOI] [PubMed] [Google Scholar]
- 14. Kensil CR, Patel U, Lennick M, Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J Immunol 1991; 146:431–7. [PubMed] [Google Scholar]
- 15. Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines 2014; 10:471–86. [DOI] [PubMed] [Google Scholar]
- 16. Ragupathi G, Gardner JR, Livingston PO, Gin DY. Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer. Expert Rev Vaccines 2014; 10:463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Evans TG, McElrath MJ, Matthews T, et al. . QS-21 promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immmunization in humans. Vaccine 2001; 19:2080–91. [DOI] [PubMed] [Google Scholar]
- 18. Van Der Meeren O, Hatherill M, Nduba V, et al. . Phase 2b controlled trial of M72/AS01E vaccine to prevent tuberculosis. New Engl J Med 2018; 379:1621–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Partnership R S Clinical Trials, Agnandji ST, Lell B, et al. . First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. New Engl J Med 2011; 365:1863–75. [DOI] [PubMed] [Google Scholar]
- 20. Tomaras GD, Yates NL, Liu P, et al. . Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol 2008; 82:12449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yates NL, deCamp AC, Korber BT, et al. . HIV-1 Envelope glycoproteins from diverse clades differentiate antibody responses and durability among vaccinees. J Virol 2018; 92:e01843-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yates NL, Liao H-X, Fong Y, et al. . Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 2014; 6:228ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bull M, Lee D, Stucky J, et al. . Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods 2007; 322:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DeRosa SC, Carter DK, McElrath MJ. OMIP-014: validated multifunctional characterization of antigen-specific human T-cells by intracellular cytokine staining. Cytom Part A 2012; 81A:1019–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horton H, Thomas EP, Stucky JA, et al. . Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T-cells induced by vaccination. J Immunol Methods 2007; 323:39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pollara J, Hart L, Brewer F, et al. . High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry A 2011; 79:603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trkola A, Matthews J, Gordon C, Ketas T, Moore JP. A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J Virol 1999; 73:8966–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin L, Finak G, Ushey K, et al. . COMPASS identifies T-cell subsets correlated with clinical outcomes. Nat Biotechnol 2015; 33:610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moodie Z, Dintwe O, Sawant S, et al. . Analysis of the HIV vaccine trials network 702 phase 2b–3 HIV-1 vaccine trial in South Africa assessing RV144 antibody and T-cell correlates of HIV-1 acquisition risk. J Infect Dis 2022; 226:246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bekker L-G, Moodie Z, Grunenberg N, et al. . Subtype C ALVAC-HIV and bivalent subtype C gp120/MF59 HIV-1 vaccine in low-risk, HIV-uninfected, South African adults: a phase 1/2 trial. Lancet Hiv 2018; 5:e366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Braeckel EV, Bourguignon P, Koutsoukos M, et al. . An adjuvanted polyprotein HIV-1 vaccine induces polyfunctional cross-reactive CD4+ T-cell responses in seronegative volunteers. Clin Infect Dis 2011; 52:522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim J, Vasan S, Kim JH, Ake JA. Current approaches to HIV vaccine development: a narrative review. J Int Aids Soc 2021; 24(Suppl 7):e25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.