Abstract
Background
Trophoblast migration into maternal decidua is essential for normal pregnancy. It occurs in a defined time window, is spatially highly restricted, and is aberrant in some pathological pregnancies, but the control mechanisms are as yet ill-defined. At the periphery of the placenta, chorionic villi make contact with decidua to form specialised anchoring sites that feed interstitially migrating cytotrophoblast into the placental bed.
Results
Explants of first trimester mesenchymal villi on collagen type I developed cytotrophoblast outgrowths from the villous tips. However, in medium changed daily, cells did not progress to a migratory phenotype, remaining instead as a contiguous multi-layered sheet. This suggested the need for another migration stimulus. To test the possibility that this might arise from mesenchymal cells, serum-free conditioned medium from first trimester placental fibroblasts was added to explant cultures. Cytotrophoblasts were stimulated to migrate in streams across the gel. Affinity depletion of Insulin-like growth factor from fibroblast medium reduced streaming activity, while the addition of exogenous IGF-I (10 ng/ml) to serum-free medium produced a streaming phenotype. IGF receptor type 1 (IGFR1) was present on cells in the columns, and streaming could be inhibited by antibody to this receptor. IGF-II and activin, known stimulators of cytotrophoblast migration, were also active in this model.
Conclusions
These data suggest a paracrine interaction between villous mesenchyme and the cytotrophoblast in anchoring sites that stimulates trophoblast infiltration of decidua. Such a signal would be self-limiting since it diminishes with distance from the placenta. This is a novel mechanism in placental development.
Background
The placenta is the first organ to develop, vascularised chorionic villi being already evident during the third week of gestation. Indeed, rapid early development is a prerequisite for normal progression of the embryo. From these early stages, spatial segregation gives rise to two populations of placental villi, one that floats freely in the intervillous space, and a second population (anchoring villi) that acts to stabilise the mechanical integrity of the placental-maternal interface [1-4]. The surface of both types of structure is covered by trophoblast. The floating villi exhibit a bilayered epithelium consisting of cytotrophoblast with overlying syncytium, while the anchoring sites lack a continuous syncytium, featuring instead villous tips with multilayered columns of largely mononuclear cytotrophoblast [1,5]. Villous cytotrophoblasts act as stem cells for both pathways. The syncytium functions as a transporting epithelium, while extravillous cytotrophoblasts in the distal columns provide physical anchorage of the villous tree, failure of which is likely to lead to miscarriage. The distal columns also act as feeder sites for a large population of migratory cytotrophoblasts that infiltrate the decidua and inner myometrium, where they colonise and transform the spiral arteries to allow increased blood flow to the intervillous space [6]. Impaired transformation of the myometrial arterial segments is associated with pregnancy pathologies including pre-eclampsia [7,8]. Such considerations underline the importance of understanding the mechanisms that control trophoblast differentiation and entry to the migratory or syncytial pathways.
Investigations of events that occur during formation of anchoring sites and the signals that lead to extravillous trophoblast differentiation require experimental systems that replicate as faithfully as possible placental development in vivo. We have used an explant model [5,9-11] in which stem villous cytotrophoblast at the tips of mesenchymal villi is stimulated by attachment to an extracellular matrix (ECM) to undergo a proliferative burst. This is followed by cell reorganisation into a radial outgrowth that resembles the cytotrophoblast columns and shell seen in vivo, both in terms of morphology and the progressive expression of molecular markers of the extravillous pathway [9,10]. The process depends on interaction between fibronectin deposited by the cytotrophoblasts and its receptor, integrin α5β1, at the cell surface [10]. However, cell detachment from the distal column, migration and infiltration of the surrounding ECM as seen in vivo are not observed in vitro.
These findings suggest that whilst contact with a permissive ECM triggers the development of cytotrophoblast columns that function effectively in tissue anchorage, it is insufficient to stimulate the acquisition of a migratory phenotype. Thus, cell migration apparently requires a further stimulus. Using a placenta-decidua coculture system we have tested the possibility that this might arise from maternal decidual cells, but the results were remarkably similar to those seen in explants on cell-free ECM, suggesting that decidua may not be the source of a paracrine stimulus to trophoblast migration [9,12]. In the light of these findings we have now investigated another potential source of paracrine effectors, namely the mesenchymal cell population that lies beneath the villous cytotrophoblast. At the base of cytotrophoblast columns, mesenchymal cells extend processes that approach and even make direct contact with the trophoblast basement membrane [4]. In the current investigation we show that first trimester fibroblasts produce signals that activate cytotrophoblasts to migrate from the periphery of nascent columns, and that one component of the paracrine repertoire is insulin-like growth factor (IGF).
Results
Trophoblast migration in explant culture is modulated by growth factors
Trophoblast cell lines, primary cell isolates and cells from placental explants have been previously observed to exhibit altered migratory activity in the presence of growth factors under various assay conditions. Activin [13] and IGF-II [14,15] have both been reported to stimulate migration, while TGF-β1 has inhibitory effects mediated by alteration of the integrin repertoire expressed by these cells [14,16].
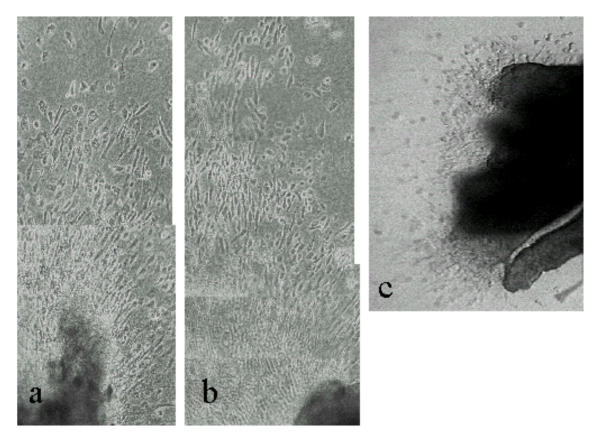
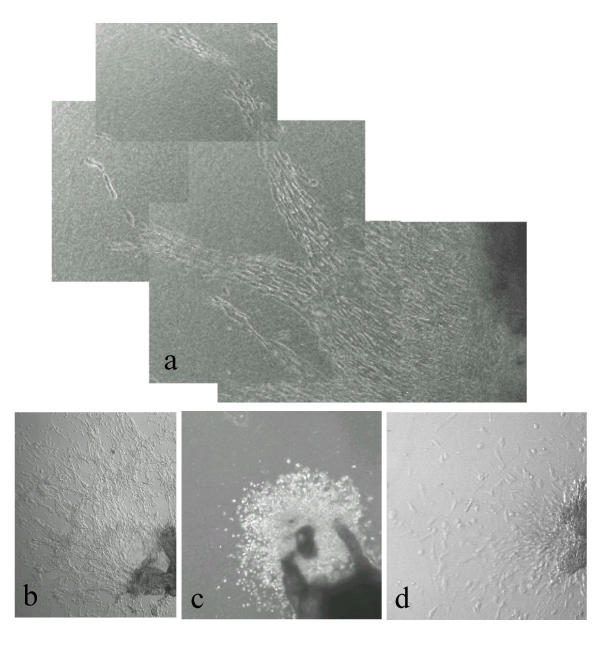
Explant cultures of first trimester villous tissue on a collagen gel substrate were tested to establish that responses to migration-inducing or -inhibiting stimuli could be observed. Outgrowth of multilayered sheets of cytotrophoblast occurred specifically from the attached tips of mesenchymal villi under control conditions, as previously reported [10]. The pattern of cell migration was strongly affected by the presence of specific growth factors in the medium: thus IGF-II (Fig. 1a), and activin (Fig. 1b) both stimulated extensive cell migration, with individual cells detaching from the outgrowing sheet and becoming radially oriented. In contrast, TGF-β1 showed strong inhibition of migration, the extent of sheet outgrowth being reduced as well as little evidence of elongated single cells migrating from the periphery (Fig. 1c).
Figure 1.
First trimester villous explants cultured on a collagen gel support in (a) IGF-II (6 days) or (b) activin AA (6 days) showing stimulation of cytotrophoblast migration across the gel surface. Note the separated bipolar cells oriented in the direction of migration. In (c) is shown an explant cultured for 7 days in the presence of TGF-β1. Cells are mainly rounded and migration is very limited.
Placental fibroblasts secrete factors that stimulate trophoblast migration
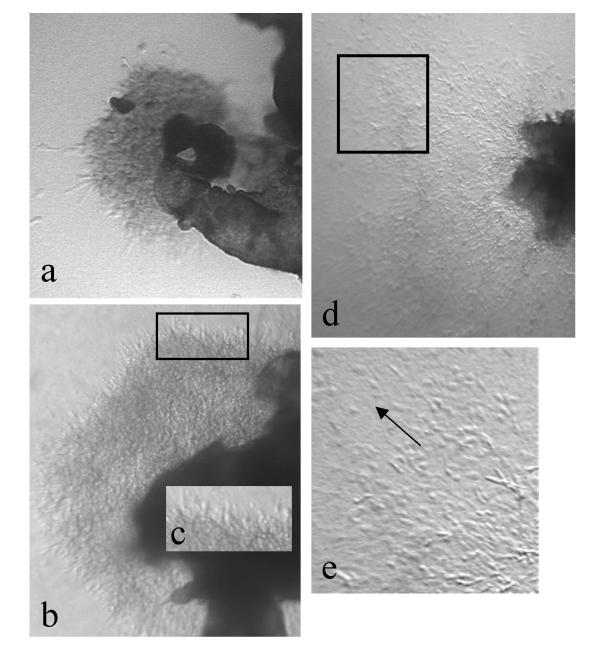
Cultures in which the medium was changed daily, with no serum or growth factor additions, showed radial multi-layered outgrowth of cytotrophoblasts after 24 h, increasing in extent over several days (Fig. 2a,2b,2c). The outgrowths were confined to the gel surface. Cells in these sheets were densely packed and exhibited a largely rounded morphology. Although a few scattered single cells could be observed at the periphery, the vast majority remained in contiguity with the sheet, as previously described [10]. However, when the culture medium was left unchanged, an increase in migratory activity was observed over several days (Fig. 2d,2e). Cells at the periphery of the sheet migrated in streams, usually in a radial direction away from the tissue. Streaming cytotrophoblasts were largely bipolar. This suggested that an autocrine or paracrine stimulus within the explant might be important in trophoblast migration. Such a signal might arguably arise either from the trophoblast or mesenchymal cells present in the system.
Figure 2.
First trimester villous explants after (a) 3 and (b) 5 days of culture on a collagen gel support in serum-free medium with daily medium change. (c) shows an enlargement of the boxed area in (b). In (d) the medium was left unchanged for 5 days. (e) shows an enlargement of the boxed area in (d). Note that both explants show elongated bipolar cytotrophoblasts at the periphery. However when the medium is unchanged, cells detach from the edge of the outgrowing sheet and migration is more extensive.
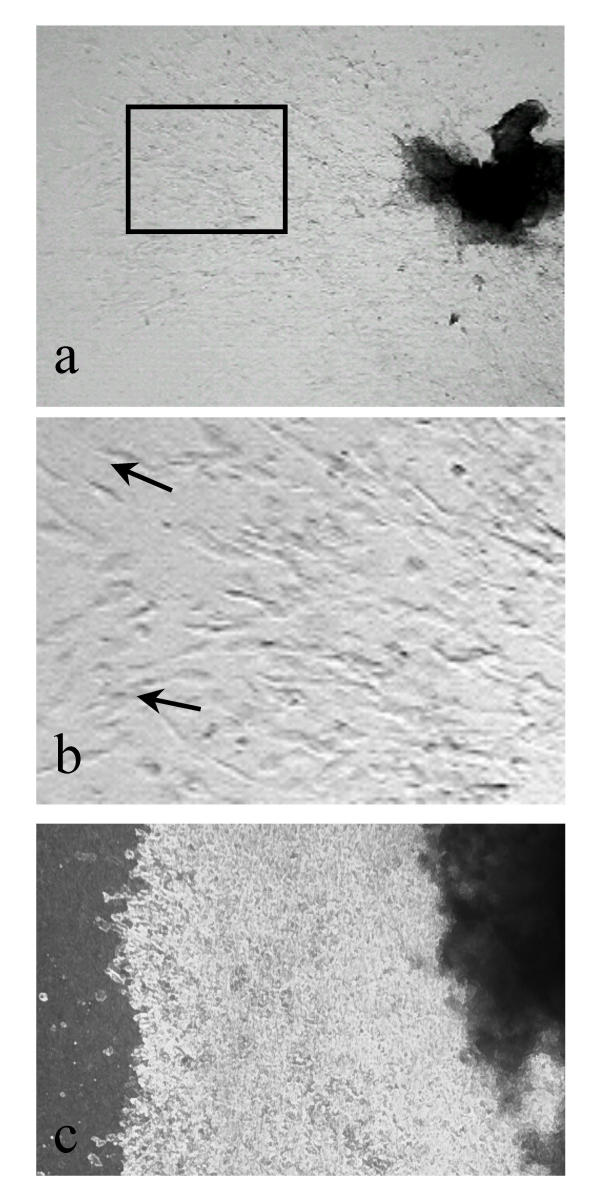
In order to test the possibility that a stimulus of mesenchymal origin might modulate trophoblast behaviour, serum-free conditioned medium from confluent first trimester fibroblasts was added to villous explants on collagen gels 24 h post-attachment, with further daily replacement with fresh batches of conditioned medium. This significantly increased streaming-type migratory behaviour in the cytotrophoblast (Fig. 3a,3b). This observation suggested that the low migratory activity seen when medium was changed daily was due to removal of a paracrine mediator originating in villous fibroblasts.
Figure 3.
First trimester villous explants after 7 days in serum-free conditioned medium from first trimester placental fibroblasts. (b) is an enlargement of the boxed area in (a) showing streaming-type migration by trophoblast, with cells adopting a bipolar morphology oriented in the direction of migration. (c) shows a control experiment in which unconditioned medium was added daily. Tissue is on the right, with collagen gel to the left and a dense multilayered sheet of phase-bright cytotrophoblast in between.
Placental mesenchymal cells produce IGF in vivo
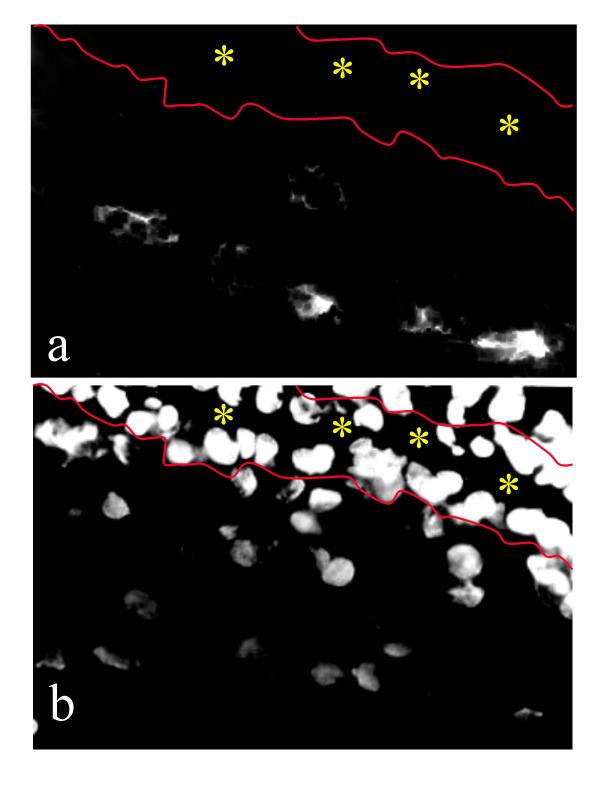
First trimester placenta was assayed to investigate the possibility that an IGF-mediated signal might arise from cells in the mesenchyme adjacent to cytotrophoblast columns. Immunostaining indicated that a subpopulation of placental villous mesenchymal cells indeed produces IGF-I (Fig. 4).
Figure 4.
Immunofluorescence of first trimester mesenchymal villi with antibody to IGF. (b) DAPI staining to show nuclei in the same section. Note that immunoreactivity is associated with a subpopulation of mesenchymal cells. The yellow asterisks mark the intervillous space, bordered by cyto- and syncytiotrophoblast. The red lines indicate the position of the sub-trophoblastic basement membrane in two adjacent chorionic villi.
IGF-I stimulates streaming-type migration of cytotrophoblasts
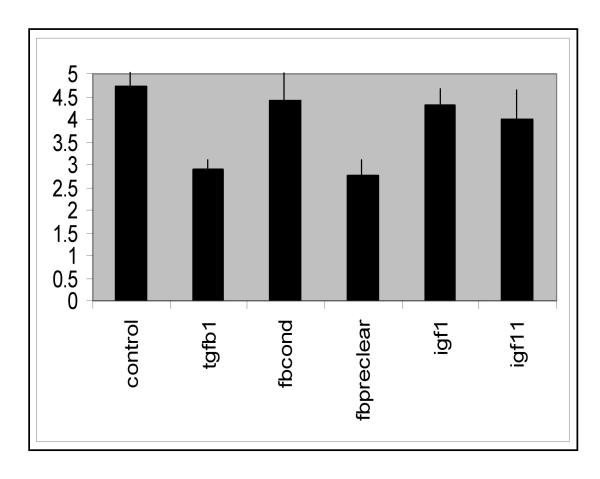
Exogenous IGF-I (10 ng/ml) was added to explants after a 24 h attachment period. IGF-I produced a potent increase in streaming-type migratory activity, with large numbers of bipolar cells radiating from the tissue to the edges of the collagen (Fig. 5a,5b). Fig. 6 shows the results of a comparative semi-quantitative analysis of the various experimental conditions. Various criteria for the assessment of trophoblast outgrowth were combined to produce a point score as described in Methods. IGF-I, IGF-II and fibroblast conditioned medium produced strong activity, while outgrowth in the presence of TGF-β1 was significantly reduced.
Figure 5.
First trimester mesenchymal villous explants cultured in the presence of IGF-I (a, high magnification; b, lower magnification), which produces cell streaming activity. (c) An explant in fibroblast conditioned medium after IGF-1 depletion. Cells in the outgrowth are rounded and clustered together, showing little migratory activity. (d) An explant cultured in IGF-1 and a function-inhibitory monoclonal antibody to the receptor IGF-R1. Migration is inhibited though not completely abolished by the antibody.
Figure 6.
Semi-quantitative evaluation of outgrowth under various experimental conditions. Migration is comparably extensive in the presence of IGF-I, IGF-II or fibroblast conditioned medium (fbcond), while it is significantly less after removal of IGF-I from the medium (fbpreclear; P < 0.05) or in the presence of TGFβ1 (P < 0.001). The control bar represents experiments in which no medium change was carried out. The vertical axis represents migration units as defined in the methods section.
Depletion of IGF from fibroblast medium diminishes the migratory stimulus
Fibroblast conditioned medium was assayed for the presence of IGF-I using RIA. Levels of IGF-I in explant conditioned medium ranged from 2.8–7.2 ng/ml. In order to test the possibility that IGF-I action might stimulate trophoblast migration, the fibroblast conditioned medium was depleted of IGF-I using an affinity column, and screened post-depletion for migration-promoting activity in the explant model. The result was a remarkable reduction in cytotrophoblast streaming behaviour (Fig. 5c, Fig. 6). Cells lost their characteristic bipolar morphology and appeared mostly rounded.
The effect of IGF-I is mediated by the type 1 IGF receptor
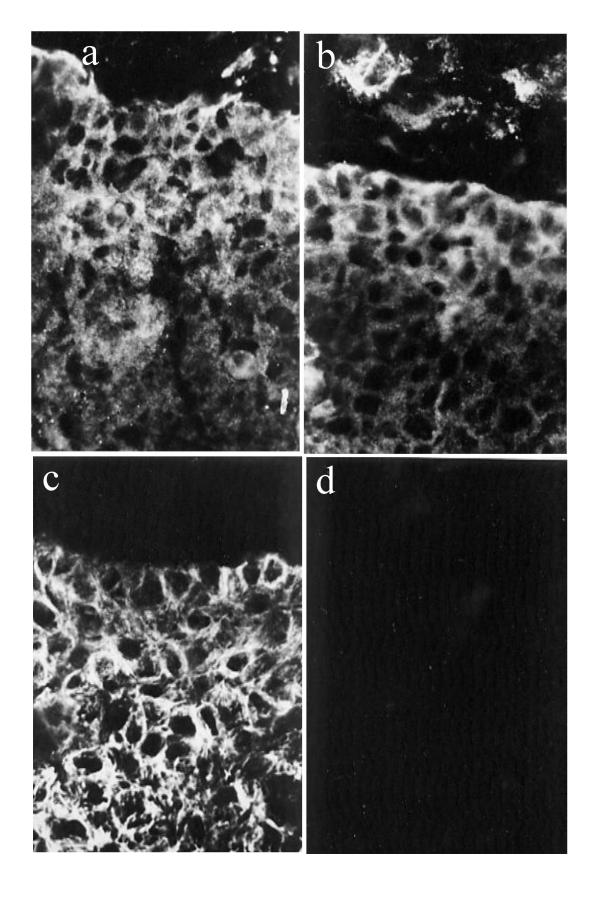
Immunolocalisation experiments demonstrated the presence of the IGF1R within proximal column cytotrophoblast cells of explanted villi (Fig. 7a). The anti-receptor antibody also detected IGFR1 within some mesenchymal cells. Insulin receptor showed a similar distribution (Fig. 7b). The insulin receptor substrate 1 (IRS-1) was also strongly immunopositive in the cell columns. In order to test the functional role of IGFR1, cultures were carried out using IGF-I (or fibroblast-conditioned SFM) to stimulate cytotrophoblast migration in the presence or absence of a function-blocking antibody to the type 1 receptor. The added antibody reduced streaming-type migration (Fig. 5d). Cells grew in a scattered pattern with varied orientation across the collagen surface, a type of behaviour not observed under other conditions.
Figure 7.
Immunofluorescence of first trimester anchoring villi showing reactivity for (a) IGFR-1, (b) insulin receptor, and (c) IRS-1. Staining is prominent in cytotrophoblast columns that occupy the lower part of the field in each case, and is also seen in (a) and (b) in some mesenchymal cells above the column. (d) IRS-2 is negative in a similar field.
Discussion
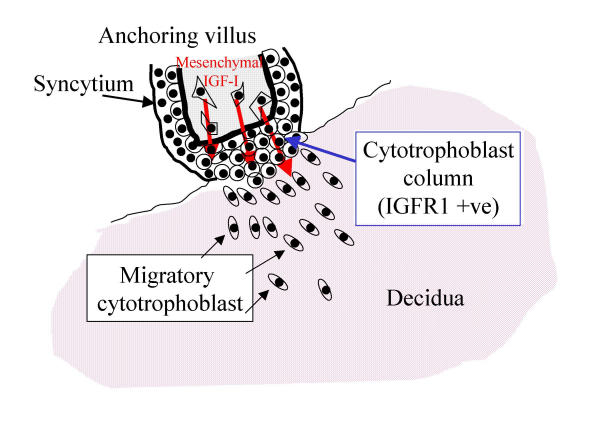
In previous work, we showed that contact between the tips of first trimester mesenchymal villi and a permissive ECM stimulates the development of anchoring columns containing cytotrophoblasts that initially proliferate, and then differentiate in a pattern that resembles closely that seen in columns in vivo [9,10]. However, the cells did not advance to a migratory pattern of behaviour, suggesting that a further stimulus is required. The present results identify placental mesenchyme as one source of this signal (Fig. 8). Developmentally, this is appropriate, as the investment of primary villi by extraembryonic mesenchyme occurs within the first few days after implantation, a time at which trophoblast migration is beginning [4]. The need for mesenchymal stimuli to the trophoblastic epithelium during placental development has also been identified in the mouse, where the gcm1 gene product, which is needed for formation of the labyrinth, is stimulated in basal chorionic trophoblast by contact with the allantoic mesoderm [17].
Figure 8.
Model showing paracrine influence of IGF-I on the migration of extravillous cytotrophoblast from the distal edge of an anchoring villus. In order to generate a pool of extravillous cells, initial anchorage to the decidual ECM is required. These cells then respond to local stimuli to detach and infiltrate the decidua.
The presence of IGFs at the maternal-fetal interface has been documented both in humans and a variety of animal species [18-20]. The importance of the IGF system to pregnancy outcome has been clearly demonstrated by the severe fetal growth restriction that occurs in mice lacking either IGF-I or IGF-II [19]. There is evidence that fetal growth is dependent on IGF in humans [18]. We have now identified IGF-I as an important component in placental mesenchymal-epithelial signalling. A role for the IGF system in promoting cytotrophoblast migration was suggested by the discovery that the extravillous cytotrophoblast of first trimester express mRNA encoding IGF-II [20,21], together with the demonstration that IGF-II stimulates trans-filter migration in cultured cytotrophoblast [14,15]. From these findings arose the idea of an autocrine loop in which trophoblast might enhance its own migration.
We have used an explant model in which de novo development of columns occurs specifically at the distal tips of first trimester mesenchymal villi [10,11]. Columns retain normal polarity, with a stromal compartment beneath the villous basement membrane. In this model IGF-II and activin AA [13] stimulate cytotrophoblast migration, providing further evidence in favour of autocrine effects. Addition of IGF-1 however produces a striking streaming pattern of cytotrophoblast migration. The production of IGF-I in vitro by fibroblasts is consistent with low but significant levels of IGF-I mRNA in this location in vivo [19] as well as immunoreactivity indicating that the factor is present. IGF-I appears not to be produced by trophoblasts. However cytotrophoblast in columns expresses the receptors IGFR1 and IR as well as their intracellular target IRS-1. A fraction of placental IGFR1 heterodimerises with IR [22]. IGFs can act through the insulin receptor [23].
We therefore postulate the existence of a paracrine mechanism that stimulates trophoblast migration (Fig. 8). Further work will be required to investigate the part played by the IGF/insulin receptor family in trophoblast migration, including other intracellular targets and the phenotypic alterations that confer increased motility. There are suggestions that other receptor species including the IGFR2/M6P receptor may be capable of signal transduction and effects on trophoblast motility [15,24].
In this model, as trophoblast migrates more deeply into the decidua and myometrium, it progresses farther away from the villous mesenchyme where IGF-I originates, so the magnitude of the signal must clearly diminish. This suggests the possibility of a self-limiting mechanism for migration, in which local inhibitory stimuli eventually outweigh the stimulatory signal. The reason for the presence of both autocrine (IGF-II) and paracrine (IGF-I) stimuli is as yet unclear, though IGF-I produces a more notable streaming phenotype. An alternative interpretation is that the paracrine trigger might act simply as a switch to a migratory pattern of behaviour. The two IGFs differ in their effects on proliferation and differentiation of rat trophoblast [25]. The role of IGF binding proteins and in particular IGFBP-1, which is a major secretory product of decidual cells [18,20], in regulation of the IGF system at the maternofetal interface remains to be resolved [14,18,26].
Conclusions
Extravillous cytotrophoblast differentiation requires at least two distinct steps (Fig. 8). In the first step, a column forms as a result of attachment to ECM. In the second step, IGF-I from placental mesenchymal cells stimulates progression to a fully migratory phenotype. This leads to a model in which, as the trophoblast migrates further away form its site of origin at the anchoring villus, through the decidua and into the myometrium, the migratory stimulus becomes diluted. Thus a mechanism is suggested whereby trophoblast migration might cease in the inner myometrium. Escape from paracrine regulation is likely to be a feature of trophoblastic tumours. Whether the IGF system also plays a role in the normal colonisation and transformation of maternal spiral arteries, and whether it may be affected in the major pregnancy pathologies of pre-eclampsia, IUGR and spontaneous abortion, remains to be investigated.
Materials and Methods
Collagen type I (rat tail tendon) was obtained from Beckton Dickenson (Oxford, UK) and Matrigel from Stratech Scientific (Luton, UK). Dulbecco's Eagle's Medium (DMEM), Ham's F-12 and fetal calf serum (FCS) were from Gibco (Life Technologies, Paisley, UK). Human Activin AA was a kind gift from the National Hormone and Pituitary Program, NIDDK (Dr Parlow). Rabbit anti-mouse streptavidin-FITC and biotinylated rabbit anti-mouse-Ig were from Dako (High Wycombe, UK). Human recombinant IGF-I was obtained from Genentech (San Francisco, USA), recombinant human TGFβ1 from R&D Systems (Abingdon, UK). Monoclonal anti-cytokeratin 7 was from Dako (Ely, UK). Monoclonal antibody αIR3 to IGFR1 was from Calbiochem (Nottingham, UK). Monoclonal Ab-1 to IGFR1 was a kind gift from Prof K Siddall, University of Cambridge, UK. Monoclonal antibody 15C9 to IGF-I was as described previously [27].
Tissue collection
Normal first trimester (8–12 week) placenta was obtained by elective surgical or medical termination of pregnancy. Tissue was collected in PBS supplemented with antibiotic and antimycotic solution (AAM; Sigma, Poole, UK) prior to dissection. The results obtained using the explant system were independent of collection method.
Explant culture
The explant culture method was as described in Aplin et al[10]. Dissected mesenchymal villous tissue was arranged radially on 80 μl drops of collagen type I gel, covered with 20 μl of serum-free medium (SFM; an equal mixture of DMEM and Ham's F12 (Gibco, Paisley, UK), supplemented with 1% AAM) and incubated overnight at 37°C, 5% CO2 to allow attachment. Wells were then carefully flooded with 1 ml SFM and incubated at 37°C, 5% CO2.
After an initial period of 24 h during which cytotrophoblast proliferation occurs at the villous tips to produce anchoring cell columns [10], growth factors or fibroblast conditioned SFM were added to the culture medium. IGF-I (10 ng/ml), TGF-β1 (4 ng/ml), activin AA (10 ng/ml) or IGF-II (10 ng/ml) were added daily to the culture medium and subsequent growth characteristics monitored by light microscopy. In addition, explants were cultured with monoclonal antibody Ab-1 (1 μg/ml) to human IGF receptor 1 (IGFR1) added daily (with or without 10 μg/ml IGF-I), or in the presence of fibroblast conditioned SFM depleted of IGF-I using immunoaffinity chromatography (see below). Cultures were continued up to 7 days.
This assay has the significant advantage of allowing migratory behaviour to be observed in living trophoblast, because migration occurs largely across the surface of the gel. Thus whole mount examination can be undertaken without the need to produce thin sections. Outgrowths were examined qualitatively taking into account size, distance, the morphology of the constituent cells and their inter-relationships including cell-cell adhesion and sheet integrity. Semi-quantitative evaluation of cytotrophoblast outgrowths from explants was carried out by analysing individual sites at villous tips. Images of live cultures were captured using a an imaging system (KS400, Kontron Elektronik GmbH, Eching, Germany) coupled to a Leitz Diavert microscope. Outgrowth distance was evaluated on a scale of 0 (no outgrowth) to 3 (outgrowth > 1 mm from tissue edge). Cell morphology was described on a scale of 0 (tightly packed cells throughout the outgrowth) to 3 (majority elongated and radially-oriented cells). Note that the number of cells present is not evaluated, as this varies widely between sites and is critically dependent on the size of the area of contact between tissue and gel at the start of the explant, a parameter that varies with the shape of the villus. The two estimates were added together to produce a value in the range 1–6 for each site. At least 3 experimental series with replicates from different placentas were used for each treatment.
Placental fibroblasts
Fibroblasts were isolated and characterised from first trimester tissue as described previously [28] and routinely passaged in monolayer. In order to produce conditioned medium, cells just below confluence were incubated in serum-free medium for 3 days. IGF levels in this medium were measured using our previously reported RIA [29]. Serum-free fibroblast conditioned medium was depleted of IGF-I by affinity chromatography. 1 ml of anti-IGF-I antibody 15C9 (1 mg/ml [27]) was coupled to activated Sephacryl, then the solid phase incubated with conditioned SFM for 16 h at 4°C. IGF-depleted medium was harvested by centrifugation at 2800 rpm,10 min, and the supernatant was filter-sterilised, aliquotted and stored at -20°C. RIA was used to confirm removal of IGF.
Immunocytochemistry
Fresh placental tissue and 4–7 day old explant cultures were snap frozen in OCT (Tissue-Tek, Cambridge, UK) and stored at -80°C. 6 μm cryosections were cut and mounted on poly-L-lysine-coated slides for immunocytochemical analysis. Cryosections were stained essentially as described previously [10]. IGFR1 was localised with antibody αIR-3 (5 μg/ml). Antibody to cytokeratin 7 was used as a positive control marker to verify the identity of trophoblast [28].
Immunoassays
IGF-I was measured by a previously reported modification of the functional separation method [30] in which excess IGF-II blocks the interference from IGFBPs [29]. Briefly, samples were diluted 1:100 in acidic sample buffer and then incubated with 100 μg/l IGF-II, 125I-IGF-I (20 000 cpm) and anti-IGF-I antibody (1:4000 dilution of clone BPL-M23) at 4C, pH 7.8 for 16 h. Separation of bound and free radioligand was performed with anti-mouse IgG linked to cellulose (Sac-Cel; IDS, Tyne & Wear, UK) for 1 h at 37C, followed by centrifugation at 1000 g for 10 minutes at 4C. The detection limit, defined as 10% displacement of the binding at zero dose of IGF-I was 0.08 ng/tube and the inter- and intra-assay coefficients of variation were 5.2–7.4% and 4.0–5.7% respectively. Cross reactivity with IGF-II and insulin was <2% for both peptides.
Acknowledgments
Acknowledgements
We thank WellBeing for funding this work and Anne White for support and stimulating discussions. M Westwood is a Dorothy Hodgkin Research Fellow of the Royal Society.
Contributor Information
Helen Lacey, Email: Helen.Lacey@man.ac.uk.
Teresa Haigh, Email: Teresa.Haigh@Prime-Medica.com.
Melissa Westwood, Email: Melissa.Westwood@man.ac.uk.
John D Aplin, Email: john.aplin@man.ac.uk.
References
- Enders AC. Fine structure of anchoring villi of the human placenta. Am J Anat. 1968;122:419–452. doi: 10.1002/aja.1001220302. [DOI] [PubMed] [Google Scholar]
- Boyd JD, Hamilton WJ. The Human Placenta. The Macmillan Press, London. 1970.
- Aplin JD, CJP Jones, Haigh T, Vicovac Lj, Church HJ. Anchorage in the developing placenta: an overlooked determinant of pregnancy outcome? Human Fertility. 1998;1:75–79. doi: 10.1080/1464727982000198161. [DOI] [PubMed] [Google Scholar]
- Aplin JD, Haigh T, Lacey H, Chen C-P, Jones CJP. Tissue interactions in the control of trophoblast invasion. J Reprod Fertil suppl. 2000;1:57–64. [PubMed] [Google Scholar]
- Vicovac Lj, Aplin JD. Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat. 1996;156:202–216. doi: 10.1159/000147847. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1:3–19. doi: 10.1016/s0143-4004(80)80012-9. [DOI] [PubMed] [Google Scholar]
- Robertson WB, Brosens I, Landells WN. Abnormal placentation. In: RM Wynn, editor. Obstetrics and Gynecology Annual. Vol. 14. Appleton-Century Crofts, Norwalk, Connecticut; 1985. pp. 411–426. [PubMed] [Google Scholar]
- Pijnenborg R. Trophoblast invasion. Reprod Med Rev. 1994;3:53–73. [Google Scholar]
- Vicovac Lj, Jones CJP, Aplin JD. Trophoblast differentiation during anchoring villus formation in a coculture model of the human implantation site in vitro. Placenta. 1995;16:41–56. doi: 10.1016/0143-4004(95)90080-2. [DOI] [PubMed] [Google Scholar]
- Aplin JD, Haigh T, Jones CJP, Church HJ, Vicovac Lj. Development of cytotrophoblast columns from explanted first trimester placental villi: role of fibronectin and integrin α5β1. Biol Reprod. 1999;60:828–838. doi: 10.1095/biolreprod60.4.828. [DOI] [PubMed] [Google Scholar]
- Aplin JD, Lacey H, Haigh T, Jones CJP, Chen C-P, Westwood M. Growth factor-extracellular matrix synergy in the control of trophoblast invasion. Biochem Soc Transac. 1999;28:199–202. doi: 10.1042/bst0280199. [DOI] [PubMed] [Google Scholar]
- Vicovac Lj, Papic N, Aplin JD. Tissue interactions in first trimester trophoblast-decidua cocultures. Trophoblast Res. 1993;7:223–236. [Google Scholar]
- Caniggia I, Lye SJ, Cross J. Activin is a local regulator of human cytotrophoblast cell differentiation. Endocrinology. 1997;138:3976–3986. doi: 10.1210/endo.138.9.5403. [DOI] [PubMed] [Google Scholar]
- Irving JA, Lala PK. Functional role of cell surface integrins on human trophoblast cell migration: regulation by TGF, IGF-II and IGFBP-1. Exp Cell Res. 1995;217:419–427. doi: 10.1006/excr.1995.1105. [DOI] [PubMed] [Google Scholar]
- Hamilton GS, Lysiak JJ, Han VK, Lala PK. Autocrine-paracrine regulation of human trophoblast invasiveness by insulin-like growth factor (IGF)-II and IGF-binding protein (IGFBP)-1. Exp Cell Res. 1998;244:147–156. doi: 10.1006/excr.1998.4195. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Taylor CV, Knox Ritchie JW, Lye SJ, Letarte M. Endoglin regulates trophoblast differentiation along the invasive pathway in human placental villous explants. Endocrinology. 1997;138:4977–4988. doi: 10.1210/endo.138.11.5475. [DOI] [PubMed] [Google Scholar]
- Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini RA, Cross JC. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet. 2000;25:311–314. doi: 10.1038/77076. [DOI] [PubMed] [Google Scholar]
- Westwood M. Role of insulin-like growth factor binding protein 1 in human pregnancy. Rev Reprod. 1999;4:160–167. doi: 10.1530/ror.0.0040160. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Han VKM, Carter AM. Spatial and temporal expression of mRNAs for IGFs and their binding proteins in the placenta of man and laboratory animals. Placenta. 2000;21:289–305. doi: 10.1053/plac.1999.0498. [DOI] [PubMed] [Google Scholar]
- Ohlsson R, Holmgren L, Glaser A, Szpecht A, Pfeifer-Ohlsson S. Insulin-like growth factor 2 and short-range stimulatory loops in control of human placental growth. EMBO J. 1989;8:1993–1999. doi: 10.1002/j.1460-2075.1989.tb03606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailyes EM, Nave BT, Soos MA, Orr SR, Hayward AC, Siddle K. Insulin receptor/ IGF-I receptor hybrids are widely distributed in mammalian tissues: quantification of individual receptor species by selective immunoprecipitation and immunoblotting. Biochem J. 1997;327:209–215. doi: 10.1042/bj3270209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciacca L, Costantino A, Pandini G, Mineo R, Frasca F, Scalia P, Sbraccia P, Goldfine ID, Vigneri R, Belfiore A. Insulin receptor activation by IGF-II in breast cancers: evidence for a new autocrine/paracrine mechanism. Oncogene. 1999;18:2471–2479. doi: 10.1038/sj/onc/1202600. [DOI] [PubMed] [Google Scholar]
- McKinnon T, Chakraborty C, Gleeson LM, Chidiac P, Lala PK. Stimulation of human extravillous trophoblast migration by IGF-II is mediated by IGF type 2 receptor involving inhibitory G protein(s) and phosphorylation of MAPK. J Clin Endocrinol Metab. 2001;86:3665–3674. doi: 10.1210/jcem.86.8.7711. [DOI] [PubMed] [Google Scholar]
- Kanai-Azuma M, Kanai Y, Kurohmaru M, Sakai S, Hayashi Y. Insulin-like growth factor (IGF)-I stimulates proliferation and migration of mouse ectoplacental cone cells, while IGF-II transforms them into trophoblastic giant cells in vitro. Biol Reprod. 1993;48:252–261. doi: 10.1095/biolreprod48.2.252. [DOI] [PubMed] [Google Scholar]
- Irwin JC, Suen LF, Martina NA, Mark SP, Giudice LC. Role of the IGF system in trophoblast invasion and pre-eclampsia. Hum Reprod. 1999;14:90–96. doi: 10.1093/humrep/14.suppl_2.90. [DOI] [PubMed] [Google Scholar]
- Crosby SR, Tsigos C, Anderton CD, Gordon C, Young RJ, White A. Elevated plasma insulin-like growth factor binding protein-1 levels in type 1 (insulin-dependent) diabetic patients with peripheral neuropathy. Diabetologia. 1992;35:868–872. doi: 10.1007/BF00399934. [DOI] [PubMed] [Google Scholar]
- Haigh T, Chen C-P, CJP Jones, Aplin JD. Studies of mesenchymal cells from 1st trimester human placenta: expression of cytokeratin outside the trophoblast lineage. Placenta. 1999;20:615–626. doi: 10.1053/plac.1999.0441. [DOI] [PubMed] [Google Scholar]
- Gill MS, Whatmore AJ, Tillmann V, White A, Addison GM, Price DA, Clayton PE. Urinary IGF-I and IGF binding protein-3 in children with disordered growth. Clin Endocrinol Oxf. 1997;46:483–492. doi: 10.1046/j.1365-2265.1997.1670981.x. [DOI] [PubMed] [Google Scholar]
- Blum WF, Breier BH. Radioimmunoassys for IGFs and IGFBPs. Growth Regul. 1994;4:11–19. [PubMed] [Google Scholar]